Abstract
Specialized neuronal structures namely growth cones, filopodia and spines are important entities by which neurons communicate with each other, integrate multiple signaling events, consolidate interacting structures and exchange synaptic information. Recent studies confirmed that Transient Receptor Potential Vanilloid sub type 1 (TRPV1), alternatively known as capsaicin receptor, forms a signaling complex at the plasma membrane and integrate multiple exogenous and endogenous signaling cues there. This receptor localizes in the neuronal growth cones and also in filopodial tips. In addition, TRPV1 is endogenously present in synaptic structures and located both in pre- and post-synaptic spines of cortical neurons. Being nonselective Ca2+-channel, TRPV1 regulates the morphology and the functions of these structures by various mechanisms. Our studies indicated that physical interaction with signaling and structural molecules, modulation of different cytoskeleton, synaptic scaffolding structures and vesicle recycling by Ca2+-dependent and -independent events are the key mechanisms by which TRPV1 regulates growth cone, filopodia and spines in a coordinated manner. TRPV1 not only regulates the morphology, but also regulates the functions of these entities. Thus TRPV1 is important not only for the detection of noxious stimuli and transmission of pain signaling, but also are for the neuronal communications and network formation.
Key words: TRPV1, synapse, filopodia, synaptic vesicle, vesicle recycling, growth cone, cytoplasmic transport packet, microtubule, NADA, pain
Transient Receptor Potential Vanilloid sub type 1 (TRPV1), alternatively known as capsaicin receptor, is the founding member of the TRPV subfamily.1 TRPV1 acts as a non-selective cation channel and is important for detection of several physical and chemical stimuli.2 Since its discovery, a large number of studies have been done to characterize this receptor, mostly in the context of ionic response, diseases and pain signaling.3–8 Although the expression of TRPV1 was initially considered to be limited to peripheral neurons only, subsequent studies confirmed that the expression of TRPV1 is ubiquitous. It is now well-established that TRPV1 in peripheral neurons can be activated by several noxious physical (like high heat, low pH) as well as endogenous and/or exogenous chemical stimuli. However, several studies suggested that importance of TRPV1 goes beyond detection and integration of noxious stimuli, and further transmission of these pain signals. Indeed, apart from peripheral neurons, TRPV1 has been detected in different cells and/or in tissues where TRPV1 expression is important and relevant for many physiological functions. Recent studies confirmed that TRPV1 is involved in several cellular functions and signaling events, metabolic disorders and development of diseases including cancers.3–8
Our previous studies demonstrated that TRPV1 interacts with the tubulin as well as with polymerized microtubules.9 The C-terminal cytoplasmic domain of TRPV1 contains two novel tubulin-binding motif sequences which are characterized by the presence of positively charged amino acid residues.10 The C-terminal cytoplasmic domain of TRPV1 not only interacts with tubulin dimer, it modulates the physico-chemical properties of microtubules in vitro too.9 Moreover, microtubule cytoskeleton acts as a key regulator of TRPV1 localization, function and signaling events.11 Further studies indicated that TRPV1-tubulin complex can also determine other signaling events. For example, interaction of γ-aminobutyric acid receptor-associated protein (GABA-RAP) with TRPV1 is dependent on the tubulin interaction.12 From several studies, it is evident that TRPV1 and microtubule cytoskeleton not only interact but also forms a scaffold at the sub-membranous region.11–13 Both TRPV1 and microtubule cytoskeleton regulate each other and thus share a multi-directional crosstalk which is important for several cellular signaling and functions.
Activation of TRPV1 Results in Cytoskeletal Reorganization
In addition to the physical interaction, TRPV1 regulates cytoskeletal dynamics and activation of TRPV1 results in rapid reorganization of cytoskeleton.14–16 Specifically, activation of TRPV1 by RTX (Resinifera toxin, a potent agonist of TRPV1) induces in rapid disassembly of dynamic microtubules. This results in a sudden loss of the anterograde force which maintains the cellular shape and structure. In contrast, the integrity of the actin filaments and neurofilaments remain intact after TRPV1 activation.14 In agreement with this imbalance in anterograde and retrograde forces, activation of TRPV1 induces morphological changes and results cell retraction. Activation of TRPV1 also results in retraction of growth cones and further varicosity formation all over the neurites, specifically in IB4-positive Dorso Root Ganglion (DRG) neurons developed from embryonic mouse or adult rat. Thus, this kind of rapid reorganization of cytoskeleton is an important mechanism by which TRPV1 expressing neurons and other cells can regulate their structure and motility.15 This rapid reorganization of microtubules seems to be a common mechanism for other TRPVs too as activation of TRPV4 by 4αPDD also results in growth cone retraction.17 However, RTX-induced microtubule disassembly is fast enough in TRPV1 expressing cells when compared to the 4αPDD-induced microtubule disassembly in TRPV4 expressing cells.17 In this context, it is important to mention that expression of TRPV1 in DRG neurons initiates during early developmental stages (even in the embryonic stages) and expression of TRPV1 is involve in the differentiation of DRG neurons to different lineages.15,18,19 Taken together these data suggest that TRPV1 is involved the multiple signaling events and also involved in process of neuronal connection formation.
Role of TRPV1 in Growth Cone Regulation and Axonal Migration
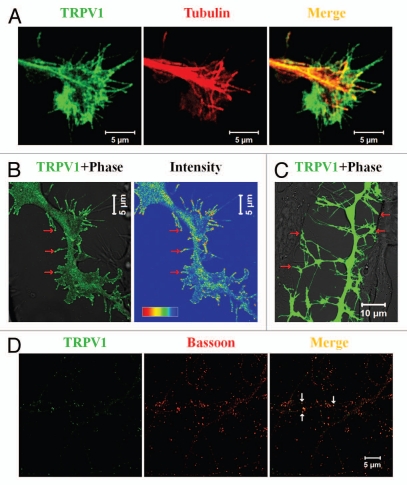
We have detected TRPV at the specialized neuronal structures, namely at the growth cones. A subset of rat DRG neurons, especially the IB4-positive neurons are sensitive to RTX and reveal Ca2+-influx.15 We have demonstrated that activation of TRPV1 results in retraction of growth cones and further development of multiple varicosities due to the rapid loss of microtubule structures in a subset of peripheral neurons, namely in IB4-positive DRG neurons.15 This proofed that endogenous TRPV1 is present in a subset (mostly in IB4-positive) of DRG neurons and primarily located at the growth cones and/or in some regions within the neurites.15 In agreement with the effect (as visualized by Ca2+-influx) of TRPV1 activation noted in primary neurons, a predominant localization of TRPV1 can also be seen in the growth cones and/or in filopodial structures developed from F11 cells, a rat DRG-neuron derived cell line (Fig. 1). When expressed ectopically in this F11 cells, TRPV1 is mainly located at the central zone (C-zone), in the peripheral zone (P-zone) including the filopodial structures and at the membrane of the growth cones. As this central zone represents the microtubule plus end structures, presence of TRPV1 in this region mostly represents dense vesicular distribution of TRPV1 (discussed later).
Figure 1.
TRPV1 co-localizes in specialized neuronal structures. (A) TRPV1 localizes in the growth cone of neurons. Shown are the confocal images of a F11 cell expressing TRPV1 (green) and immunostained for tubulin (red). Scale bar 5 µm. (B) Induction of filopodia and specific localization of TRPV1 at these filopodial structures are common features observed when TRPV1 is expressed ectopically in F11 cells (also in many other cell lines). A large number of these filopodial structures show a distinct bulbous ‘head’ on a thin ‘neck’. TRPV1 is often localized in the stalk and become enriched at the filopodial tips (indicated by arrows), mostly due to active transport to the tips. Intensity profile (shown in a rainbow scale) is shown in right. Scale bar 5 µm. (C) Majority of these filopodial structures are involved in cell-to-cell contact formation. Scale bar 10 µm. (D) TRPV1 is localized at the pre- as well as in post-synaptic structures. Shown are the confocal images of cortical neurons immunostained for TRPV1 (green) and Bassoon (Red). Distinct punctate immunoreactivity of TRPV1 in the synaptic structures is indicated by arrows. Scale bar 5 µm.
Presence of TRPV1 in the growth cone is not only important for the Ca2+-influx, Ca2+-regulation and Ca2+-signaling, but also for sensing of different neuronal guidance cues. Thus TRPV1 helps in chemotaxis and also acts as a key regulator for the axonal guidance. In this context, it is important to mention that so far few other TRP channels has also been reported at the growth cone structures where they play important roles in terms of axonal guidance and migration.20–26
Role of TRPV1 in Filopodia Initiation and Regulation
Recently we have demonstrated the role of TRPV1 in the filopodia initiation, elongation and further regulation of filopodial dynamics.27 Our studies demonstrated that activation of TRPV1 induces massive filopodial structures. Ectopic expression of full-length TRPV1 and/or even a fragment of TRPV1 can also induce cytoskeletal reorganization and can induce multiple filopodial structure from cell body as well as from the neurites. Though the exact molecular mechanisms underlying this filopodia initiation is not clear, non-conventional myosin motors may have a role in this process. It is worth mentioning that F11 cells which ectopically express TRPV1; reveal higher expression and altered distribution of non-conventional myosin motor proteins, namely non-conventional myosin II and myosin III.27 Interestingly, the N-terminal region of TRPV1 seems to be important for filopodia initiation. This is mainly due to the fact that the full-length TRPV1 as well as TRPV1-ΔCt (TRPV1 with the N-terminal cytoplasmic domain and with the transmembrane regions) induce filopodial structures, but TRPV1-ΔNt (TRPV1 with the C-terminal cytoplasmic domain and with the transmembrane regions) fails to induce much of the filopodial structures.27 This result suggested that the N-terminal region of TRPV1 forms a supra-molecular signaling complex at the sub-membranous region which promotes filopodia formation.
Detailed characterization of these TRPV1-induced filopodial structures also revealed some important aspects which indicated the nature as well as the functions of TRPV1 in these structures. First, TRPV1 not only localizes at the filopodial tips but also reveals specific enrichment there (Fig. 1B).27,28 Second, in contrast to the common notion of filopodial structural organization, often these TRPV1-induced filopodial tips lack considerable amount of actin. This indicates either less actin polymerization there and/or more retrograde motion of the actin polymers towards the filopodial base. Third, in agreement with the physical interaction of TRPV1 with the tubulin, a significant number of these filopodial structures reveal presence of polymerized microtubules within the filopodial stalk. Within these structures, polymerized microtubule can even be immunostained by antibodies specific for acetylated tubulin.27 Presence of this post-translationally modified tubulin suggests that the live-span of the microtubule filaments within these filopodial structures are long enough. However, most important aspect of these filopodia is that the tips contain several synaptic vesicular and scaffolding proteins. Some of these filopodial tips even reveal fast uptake of FM4-64 dye there. These tips are also involved in the cell-to-cell contact formation. Taken together, all these results suggest that the TRPV1-induced filopodial tips have synaptic properties and may have a role in the synapse formation (Fig. 1C).
Presence of TRPV1 in the Synapse
Recently, several studies have speculated the involvement of TRPV1 in the synaptic transmission.29,30 In agreement with those observations; we have detected TRPV1 both in pre- and post-synaptic sites.28 This is due to the fact that TRPV1 co-localizes with pre-synaptic marker proteins like Bassoon, post-synaptic protein PSD-95 and with Prosap, a protein exclusively present in the post-synaptic density. In addition, we have demonstrated that TRPV1 is present in the biochemical preparation of synaptic structures, namely in the synaptosomal fraction and also in the post-synaptic density (PSD) fraction.28 This biochemical analysis demonstrated that in the PSD fraction, a portion of TRPV1 is present as SDS-PAGE-resistant dimeric entities suggesting that it forms tight complexes there. Surprisingly, in the synaptosomal fraction, a smeary appearance of TRPV1 is observed. This is highly suggestive for the presence of glycosylated TRPV1 there. Though these results suggest that within the synaptic structures, TRPV1 forms different molecular complexes, detailed characterization of these complexes, and their exact occurrence in the synaptic structures and better understandings about their regulations are still required. We also noted that activation of TRPV1 by N-arachidonoyl-dopamine (NADA, an endogenous agonist of TRPV1) results in rapid elongation of spines and this elongation can be effectively blocked by 5′-iodoresiniferatoxin (5′-I-RTX, an antagonist of TRPV1).28 These results suggest that within spine, TRPV1 has a role in maintenance of spine length and morphology. We also noted that activation of TRPV1 alters vesicular recycling in TRPV1 expressing cell population as well as in the specific structures including filopodia. Using F11 cells as a model system we demonstrated that TRPV1 activation leads to rapid vesicle fusion within the filopodial structures. This result agrees both with the general concept of Ca2+-influx mediated exocytosis and with the previously reported TRPV1-medaited neurosecretion.28
Transportation of TRPV1 in Neurons
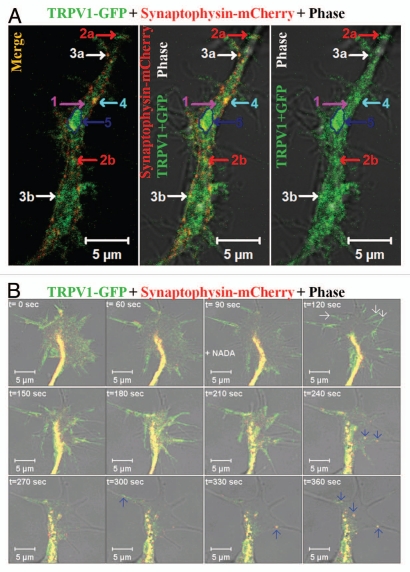
We have noted that TRPV1 is transported to the neuronal ends by different entities (Fig. 2A). Within the neurite, a fraction of TRPV1-GFP has been detected as diffuse staining which recovers fast after photobleaching. Thus, this diffuse pattern of TRPV1-GFP mostly matches well with cytoplasmic and/or small vesicular distribution of TRPV1. However, TRPV1-GFP can also be detected in structures which are different in size, mobility and in recycling properties.28 Many of these entities are relatively bigger than conventional vesicles. Often these structures reveal saltatory movement within the neurites, i.e., they move very fast for a short duration and then remain paused for a long time.28 While at pause, some of these entities can uptake FM4-64 dye rapidly indicating fast recycling events there.28 In addition, most of these entities reveal presence of either pre- or post-synaptic proteins. We have also noted co-movement of TRPV1-GFP with other synaptic proteins, namely synaptophysin-mCherry via these entities. Therefore, some of these entities reveal characteristics which match well with the cytoplasmic transport packets (CTPs) (Fig. 2). We noted that activation of TRPV1 results movement of these CTPs within the filopodial structures.28 We also observed a fast translocation of TRPV1-GFP to the plasma membrane of growth cones immediately after addition of NADA (Fig. 2B).15,28 This is mostly due to the translocation and subsequent fusion of readily available vesicles containing TRPV1-GFP. In addition to this rapid translocation, movement of individual CTPs containing TRPV1-GFP and Synaptophysin-mCherry from the central zone of growth cone towards the filopodial membrane has been observed (Fig. 2B). Though the exact reason is not clear, often we noted that a same trajectory is followed during the movement of these individual CTPs. In a similar manner, we have detected fast fusion of CTPs at the base of the filopodia and further movement of these CTPs within the filopodia. This vesicular fusion seems to be important for the filopodia elongation as this elongation needs rapid supply of more lipid membranes. However, the exact molecular mechanisms which regulate the formation and the movement of these TRPV1 containing CTPs within the growth cone and/or within the filopodia are not well understood and further investigations are required.
Figure 2.
Distribution and movement of TRPV1 via different types of synaptic vesicles. (A) Distribution of TRPV1-GFP in different population within a neurite. Show are the live cell confocal images of a neurite developed from F11 cell expressing TRPV1-GFP (green) and Synaptophysin-mCherry (red). TRPV1-GFP is detected in different groups: (1) A diffused pattern which is mostly cytoplasmic and recovers fast from all sides after photo-bleaching (indicated by pink arrow), (2a and b) medium- and small-sized particles that move uninterruptedly with an average speed and travels long distance (indicated by red arrows respectively), (3a and b) the small particles located at the base of the existing filopodia and can move fast within the filopodial structures after activation (indicated by white arrows respectively), (4) medium-sized (indicated by blue arrow) particle which mostly stay at the inner side of the neuritic membrane but do not move. This population uptake FM4-64 dye rapidly, (5) some of the much bigger entities (indicated by a dark blue arrow and a marked region) generally remain immobile for a long time and show only flickering. These bigger particles can move all of a sudden to a short distance and very fast. Some of these entities can be categorized as cytoplasmic transport packets (CTP) as these entities fit well with the properties of CTPs. Scale bar 5 µm. (B) Shown are the live cell confocal images of a growth cone developed from F11 cell expressing TRPV1-GFP (green) and Synaptophysin-mCherry (red). Note that activation of F11 cells by NADA (at 90 sec time frame), an endogenous stimulus for TRPV1 results in rapid translocation of TRPV1-GFP to the plasma membrane (indicated by white arrows at 120 sec) resulting in sudden increase in the intensity of TRPV1-GFP at the of growth cone membrane. Activation also results in selective co-migration of TRPV1-GFP-containing pre-synaptic vesicles to filopodial structures (indicated by blue arrows). Often the movement of these vesicles follows a distinct route. So far the exact signaling events and the molecular mechanisms which regulate this translocation to membrane and movement of vesicles are not known. Scale bar 5 µm.
Future Outlook and Conclusions
So far several TRP channels including other TRPVs have also been detected at the growth cones and/or in synaptic structures.20–26,31,32 Though the presence of TRP channels in growth cone and in filopodial structures correlates well with several important functions like chemosensation, axonal migration, axonal turning and contact formation; still now several central questions remain unanswered. For example, the exact mechanisms and signaling pathways which regulates the development of filopodial structures from TRPV1 expressing neurites are not clear. The molecular factors and the mechanism by which the typical “head-like” tip of the filopodia is formed and maintained in TRPV1 expressing cells are not known. In addition, the factors that regulate the spacing between two successive filopodia are still unknown. Within these structures, how TRPV1 actually regulates the cytoskeletal reorganization and coordinates vesicle recycling that remain to explore in future.
Acknowledgements
C.G. acknowledges research and/or funding support from Prof. E.D. Gundelfinger and Dr. K.H. Smalla (Magdeburg, Germany), Prof. F. Hucho (FU, Berlin) and Dr. T. Hucho, Dr. N. Rademacher, Dr. V. Kalscheuer and Prof. H.H. Ropers (Max Planck Institute for Molecular Genetics, Berlin). CG is currently supported by National Institute of Science Education and Research, India.
Abbreviations
- TRPV1
transient receptor potential vanilloid sub type 1
- DRG
dorso root ganglion
- NADA
N-arachidonoyl-dopamine
- CTP
cytoplasmic transport packets
- RTX
resinifera toxin
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/13397
References
- 1.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 3.Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- 4.Cortright DN, Krause JE, Broom DC. TRP channels and pain. Biochim Biophys Acta. 2007;1772:978–988. doi: 10.1016/j.bbadis.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Suri A, Szallasi A. The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol Sci. 2008;29:29–36. doi: 10.1016/j.tips.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Jordt SE, Ehrlich BE. TRP channels in disease. Subcell Biochem. 2007;45:253–271. doi: 10.1007/978-1-4020-6191-2_9. [DOI] [PubMed] [Google Scholar]
- 7.Cortright DN, Szallasi A. TRP channels and pain. Curr Pharm Des. 2009;15:1736–1749. doi: 10.2174/138161209788186308. [DOI] [PubMed] [Google Scholar]
- 8.Jara-Oseguera A, Simon SA, Rosenbaum T. TRPV1: on the road to pain relief. Curr Mol Pharmacol. 2008;1:255–269. doi: 10.2174/1874467210801030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goswami C, Dreger M, Jahnel R, Bogen O, Gillen C, Hucho F. Identification and characterization of a Ca2+-sensitive interaction of the vanilloid receptor TRPV1 with tubulin. J Neurochem. 2004;91:1092–1103. doi: 10.1111/j.1471-4159.2004.02795.x. [DOI] [PubMed] [Google Scholar]
- 10.Goswami C, Hucho TB, Hucho F. Identification and characterisation of novel tubulin-binding motifs located within the C-terminus of TRPV1. J Neurochem. 2007;101:250–262. doi: 10.1111/j.1471-4159.2006.04338.x. [DOI] [PubMed] [Google Scholar]
- 11.Goswami C, Hucho T. Submembraneous microtubule cytoskeleton: biochemical and functional interplay of TRP channels with the cytoskeleton. FEBS J. 2008;275:4684–4699. doi: 10.1111/j.1742-4658.2008.06617.x. [DOI] [PubMed] [Google Scholar]
- 12.Laínez S, Valente P, Ontoria-Oviedo I, Estévez-Herrera J, Camprubí-Robles M, Ferrer-Montiel A, et al. GABAA receptor associated protein (GABARAP) modulates TRPV1 expression and channel function and desensitization. FASEB J. 2010;24:1958–1970. doi: 10.1096/fj.09-151472. [DOI] [PubMed] [Google Scholar]
- 13.Goswami C, Hucho T. Novel aspects of the submembraneous microtubule cytoskeleton. FEBS J. 2008;275:4653. doi: 10.1111/j.1742-4658.2008.06613.x. [DOI] [PubMed] [Google Scholar]
- 14.Goswami C, Dreger M, Otto H, Schwappach B, Hucho F. Rapid disassembly of dynamic microtubules upon activation of the capsaicin receptor TRPV1. J Neurochem. 2006;96:254–266. doi: 10.1111/j.1471-4159.2005.03551.x. [DOI] [PubMed] [Google Scholar]
- 15.Goswami C, Schmidt H, Hucho F. TRPV1 at nerve endings regulates growth cone morphology and movement through cytoskeleton reorganization. FEBS J. 2007;274:760–772. doi: 10.1111/j.1742-4658.2006.05621.x. [DOI] [PubMed] [Google Scholar]
- 16.Han P, McDonald HA, Bianchi BR, Kouhen RE, Vos MH, Jarvis MF, et al. Capsaicin causes protein synthesis inhibition and microtubule disassembly through TRPV1 activities both on the plasma membrane and intracellular membranes. Biochem Pharmacol. 2007;73:1635–1645. doi: 10.1016/j.bcp.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 17.Goswami C, Kuhn J, Heppenstall PA, Hucho T. Importance of non-selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PLoS One. 2010;5:11654. doi: 10.1371/journal.pone.0011654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci. 2007;27:2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funakoshi K, Nakano M, Atobe Y, Goris RC, Kadota T, Yazama F. Differential development of TRPV1-expressing sensory nerves in peripheral organs. Cell Tissue Res. 2006;323:27–41. doi: 10.1007/s00441-005-0013-3. [DOI] [PubMed] [Google Scholar]
- 20.Montell C. Exciting trips for TRPs. Nat Cell Biol. 2004;6:690–692. doi: 10.1038/ncb0804-690. [DOI] [PubMed] [Google Scholar]
- 21.Gomez T. Neurobiology: channels for pathfinding. Nature. 2005;434:835–838. doi: 10.1038/434835a. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, et al. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- 23.Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 2005;434:898–904. doi: 10.1038/nature03478. [DOI] [PubMed] [Google Scholar]
- 24.Shim S, Goh EL, Ge S, Sailor K, Yuan JP, Roderick HL, et al. XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nat Neurosci. 2005;8:730–735. doi: 10.1038/nn1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:37–45. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- 26.Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 27.Goswami C, Hucho T. TRPV1 expression-dependent initiation and regulation of filopodia. J Neurochem. 2007;103:1319–1333. doi: 10.1111/j.1471-4159.2007.04846.x. [DOI] [PubMed] [Google Scholar]
- 28.Goswami C, Rademacher N, Smalla KH, Kalscheuer V, Ropers HH, Gundelfinger ED, et al. TRPV1 acts as a synaptic protein and regulates vesicle recycling. J Cell Sci. 2010;123:2045–2057. doi: 10.1242/jcs.065144. [DOI] [PubMed] [Google Scholar]
- 29.Kauer JA, Gibson HE. Hot flash: TRPV channels in the brain. Trends Neurosci. 2009;32:215–224. doi: 10.1016/j.tins.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Alter BJ, Gereau RW., 4th Hotheaded: TRPV1 as mediator of hippocampal synaptic plasticity. Neuron. 2008;57:629–631. doi: 10.1016/j.neuron.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 31.Shibasaki K, Murayama N, Ono K, Ishizaki Y, Tominaga M. TRPV2 enhances axon outgrowth through its activation by membrane stretch in developing sensory and motor neurons. J Neurosci. 2010;30:4601–4612. doi: 10.1523/JNEUROSCI.5830-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibasaki K, Suzuki M, Mizuno A, Tominaga M. Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci. 2007;27:1566–1575. doi: 10.1523/JNEUROSCI.4284-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]