Abstract
The skin barrier function is indispensable for terrestrial animals to avoid dehydration. The function is achieved by a hydrophobic cornified layer consisting of dead keratinocytes and lipids, and by an intercellular junction barrier formed among differentiated keratinocytes. A recent report demonstrated that TRPV4, one of the temperature-sensitive cation channels, contributes to the formation and maintenance of the intercellular junction-dependent barrier in the skin. TRPV4 associates with the E-cadherin complex via β-catenin, and thereby participates in the promotion of cell-cell junction development. TRPV4 allows influx of Ca2+ ions from the extracellular space at physiological skin temperatures. The Ca2+ influx induces Rho activation and promotes actin fiber organization and junction formation, thereby augmenting barrier integrity. Indeed, the intercellular junction structures and the skin barrier function were impaired in TRPV4-deficeint mice. This novel role of TRPV4 in keratinocytes may explain the significant correlation between temperature and the condition of skin.>
Key words: TRP channel, skin barrier, Ca2+ ion
Environmental temperature affects all organisms and their styles of living. Recently, a unique group of temperature-sensitive ion channels, named thermosensitive TRP channels (thermoTRPs), has been recognized as primary bio-thermometers in a range of species.1,2 Several thermoTRPs are expressed in cells other than primary sensory neurons, and serve different roles under physiological body temperatures. For example, TRPM2 expressed in pancreatic β cells, is involved in insulin secretion in a NAD metabolite-dependent manner, and the channel activity is maximized at core body temperaure.3 TRPM5, which is expressed in taste cells, contributes to perception of sweet substances, which is enhanced by warm temperatures.4 TRPV4, which is expressed in the hippocampus, is constitutively active under core brain temperature, and participates in the positive shift of resting membrane potential.5 Thus, the roles of these channels are affected by changes in body temperature through their thermosensitivty.
Skin keratinocytes reportedly express at least two types of thermoTRPs, TRPV3 and TRPV4.6,7 Both channels are activated by warm temperatures around 33°C, which is close to physiological skin temperature. Since genetically modified mice lacking either of the channels showed abnormal behavior in thermotaxis,8,9 these channels were considered to be thermosensors of ambient temperatures. A recent study reported a mechanism for TRPV3-dependent transmission of temperature information to neurons, in which ATP was released from keratinocytes downstream of warmth activation of TRPV3.10 This finding raised the possibility that TRPV4 might play another role in skin although TRPV4 could also be required for temperature sensation. Another recent study proved that TRPV4 is activated under physiological skin temperature, thereby regulating skin barrier function.11 Thus, two thermoTRPs in keratinocytes play distinct roles in spite of their capability to sense a similar range of temperatures.
The barrier functions of the skin are indispensable in at least two respects. First, the hydrophobic cornified layer, which is composed of a continuous sheet of protein-enriched cells embedded in an extracellular non-polar lipid layer, is important to prevent infection and invasion of bacteria and hazardous substances.12 Second, intercellular junctions including adherens-junctions (AJs) and tight-junctions (TJs) among differentiated keratinocytes restrict diffusion and evaporation of water from inside the body. The importance of these junction-based barriers was shown by mice lacking E-cadherin or claudin-1,13,14 as both defects were lethal within 7–24 hr of birth because of excess dehydration, although the cornified layer was normally formed. Therefore, in addition to a cornified layer, formation and maintenance of intercellular junctions among keratinocytes is crucial for survival.
TRPV4 is involved in junction-based barrier formation.11 TRPV4 binds to β-catenin, which is an adaptor protein linking intercellular adhesion molecules (E-cadherin) and the cytoskeleton (actin fibers). There are dynamic arrangements of adhesion molecules and cytoskeletal components in both early and late stages of cell-cell junction development along with keratinocyte differentiation.15–17 Addition of Ca2+ to primary cultures of keratinocytes induces E-cadherin-dependent formation of early cell-cell contacts anchored by actin stress fiber. Consequently, cell stratification is accompanied by maturation of intercellular junctions and cortical actin formation. All these processes were significantly delayed and immature in TRPV4-deficient keratinocytes. In keratinocyte differentiation, signals mediated by the Rho family of small GTPases are involved in actin organization, formation of cell-cell contacts, and in cortical actin formation for the maintenance of E-cadherin-based adhesion.18,19 Indeed, Rho activation was increased after addition of Ca2+ in wild-type cells, which was almost negligible in TRPV4-deficient cells.
Intracellular Ca2+ level was increased after addition of Ca2+ in wild-type cells, which is thought to facilitate Rho activation. However, the drastic increase in intracellular Ca2+ was observed only when cells were kept at physiological skin temperature (33°C), but not at 24°C. TRPV4-deficient cells showed significantly lower intracellular Ca2+ level at 33°C. Since TRPV4 is activated above 33°C,5,7 we propose a model in which skin temperature regulates intercellular junction-dependent barrier formation via TRPV4 activation (Fig. 1). Without extracellular Ca2+, keratinocytes stay in an undifferentiated state and adhesion components are located in the cytoplasm.11 Upon induction by high extracellular Ca2+, the intercellular junction components relocate to the plasma membrane to form a primitive complex. The process is TRPV4-independent, and TRPV4 is expected to associate with β-catenin at this step. When the temperature is high enough to activate TRPV4, Ca2+ influx occurs through the channels. This Ca2+ increase should promote Rho activation and augment actin fiber formation adjacent to E-cadherin complexes, leading to clustering of E-cadherin complexes including TRPV4, which acts like a core molecule.
Figure 1.
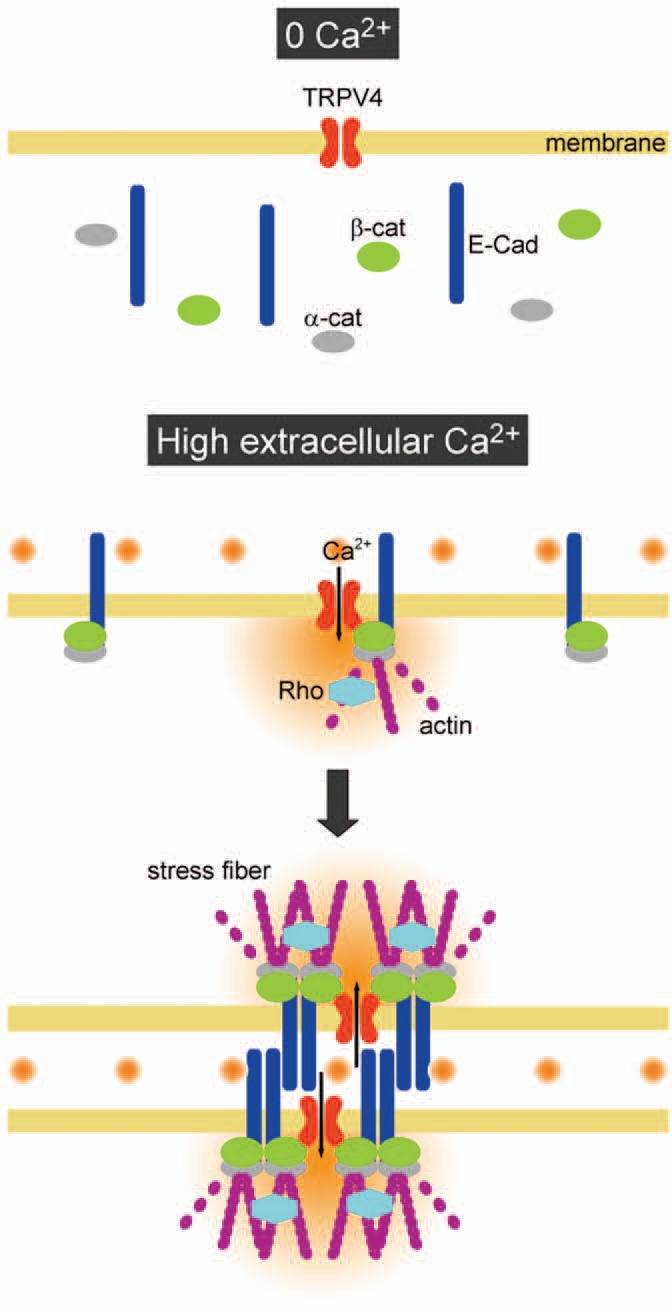
A proposed model for TRPV4-dependent clustering of AJ complexes. In the absence of extracellular Ca2+ (0 Ca2+), keratinocytes do not contact each other. After addition of high extra-cellular Ca2+, adhesion molecules relocate to the plasma membrane. TRPV4 binds to β-catenin, making complexes with adhesion molecules. Under physiological skin temperature, Ca2+ influx via TRPV4 promotes Rho activation, which enhances actin polymerization at cell-cell contact sites. Clustering of AJ complexes is promoted adjacent to TRPV4, leading to maturation of intercellular junction.
Consistent with this hypothesis, TRPV4 colocalized with β-catenin, E-cadherin and the cortical actin network at the apical side of keratinocytes, where the intercellular junction barrier is formed.11 Transmission electron microscopy revealed that AJ- and TJ-mediated junctions are poorly formed at the apical side, and the permeation of dextran among cells was significantly increased in vitro in TRPV4-deficient cells. Furthermore, TRPV4-deficient mice displayed an impaired skin barrier function, which was attributed to leaky inter-cellular junctions among the topmost cells. One intriguing point is that TRPV3 cannot replace TRPV4's barrier function in keratinocytes.
TRPV3-deficient keratinocytes displayed Ca2+-induced Rho activity, normal formation of intercellular junctions and stratification, and functional intercellular junction barriers in vitro and in vivo, all of which were comparable to wild-type cells and skin. Moreover, TRPV3 did not bind to β-catenin. These findings suggest that TRPV4's modulation of the Ca2+ supply could change local Ca2+ dynamics around AJ complexes, which may stabilize and maintain intercellular junctions in keratinocytes.
TRPV4-deficiency caused a thicker cornified layer, which resembled those observed in E-cadherin- or claudin-deficient mice.13,14 However, the contribution of TRPV4 may be different from those of E-cadherin and claudin, since TRPV4-deficient mice survive and grow like wild-type mice. We speculate that TRPV4 is a skin temperature-dependent regulatory component with a barrier function. When the temperature is raised, the intercellular junction barrier is reinforced in a TRPV4-dependent manner to prevent excess water loss. This implies that manipulating TRPV4 activity could be a better solution for skin damage repair. On the other hand, many questions are not explained by our hypothesis. For example, how does TRPV4-mediated Ca2+ activate Rho? Is TRPV4 activation sustained/required for formation/maintenance of the junction barrier? How does TRPV4-mediated AJ formation augment TJ barriers? These mechanistic links should be clarified in the future. TRPV4 is now considered as a multimodal receptor sensing a variety of stimuli, including artificial and natural substances,20,21 unsaturated fatty acids,22 and mechanical deformation,23–26 as well as temperature.7,24 Investigating how TRPV4 functions under certain circumstances would improve our understanding of this channel and its reactions to a variety of stimuli.
Acknowledgements
This work was supported by grants from the Ministry of Education, Culture, Sport, Science and Technology in Japan (to T.S. and M.T.) and from The Nakatomi Foundation (to M.T.).
Abbreviations
- TRPV4
transient receptor potential vanilloid 4
- AJs
adherens-junctions
- TJs
tight-junctions
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/13461
References
- 1.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 3.Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, et al. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 2006;25:1804–1815. doi: 10.1038/sj.emboj.7601083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, et al. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- 5.Shibasaki K, Suzuki M, Mizuno A, Tominaga M. Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci. 2007;27:1566–1575. doi: 10.1523/JNEUROSCI.4284-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 7.Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 10.Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, et al. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009;458:1093–1102. doi: 10.1007/s00424-009-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sokabe T, Fukumi-Tominaga T, Yonemura S, Mizuno A, Tominaga M. The TRPV4 channel contributes to intercellular junction formation in keratinocytes. J Biol Chem. 2010;285:18749–18758. doi: 10.1074/jbc.M110.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17:1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 13.Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, et al. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 2005;24:1146–1156. doi: 10.1038/sj.emboj.7600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 16.Mege RM, Gavard J, Lambert M. Regulation of cell-cell junctions by the cytoskeleton. Curr Opin Cell Biol. 2006;18:541–548. doi: 10.1016/j.ceb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Bershadsky A. Magic touch: how does cell-cell adhesion trigger actin assembly? Trends Cell Biol. 2004;14:589–593. doi: 10.1016/j.tcb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Braga VM. Cell-cell adhesion and signalling. Curr Opin Cell Biol. 2002;14:546–556. doi: 10.1016/s0955-0674(02)00373-3. [DOI] [PubMed] [Google Scholar]
- 19.Vaezi A, Bauer C, Vasioukhin V, Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev Cell. 2002;3:367–381. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe H, Davis JB, Smart D, Jerman °C, Smith GD, Hayes P, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 21.Smith PL, Maloney KN, Pothen RG, Clardy J, Clapham DE. Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J Biol Chem. 2006;281:29897–29904. doi: 10.1074/jbc.M605394200. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 23.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 24.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu L, Gao X, Brown RC, Heller S, O'Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:1699–1713. doi: 10.1152/ajprenal.00462.2006. [DOI] [PubMed] [Google Scholar]
- 26.Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, et al. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem. 2009;284:21257–21264. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]


