Abstract
Adherent cells migrate on extracellular -matrices (ECM) by repeated spreading and contraction of the cell body. Focal adhesions (FAs) play a major role in the adherence of cells to the ECM and in the generation of the cellular forces that maintain morphology and allow cells to move. FAs also mediate bidirectional transmembrane signals in conjunction with growth factor receptors and signaling molecules. Although the mechanisms that regulate cell migration are not yet fully understood, the regulation of the formation and turnover of FAs is a key factor determining the rate and direction of cell migration. We recently identified a component of FAs termed ZF21, which is a member of a family of proteins characterized by the presence of a conserved phosphoinositide-binding motif. ZF21 promotes dephosphorylation of FAK at Tyr397 upon microtubule extension to FAs and thereby regulates the disassembly of FAs in a microtubules-dependent manner. To obtain further insight into the regulation of cell adhesion by ZF21, we analyzed proteins associated with ZF21 by proteomic analysis. We identified 45 proteins including FA-related proteins and multiple RNA binding proteins that have been shown recently to be components of the spreading initiation center (SIC). SICs are cell adherent structures that can be observed only in the early stages of cell spreading and have been implicated in regulating the rate of cell spreading. In this article, we report new ZF21-binding proteins identified by proteomic analysis and discuss the potential functions of ZF21 in regulating disassembly of FAs.
Key words: cell adhesion, cell spreading, focal adhesion, spreading initiation center, LC/MS
ZF21 is an Integral New Component of FAs
Cells in tissue are usually surrounded by an extracellular matrix (ECM) and interaction between the cells and this ECM plays a pivotal role not only in maintaining cell morphology and tissue structure but also in mediating signals that regulate a variety of cellular functions, such as proliferation, motility, survival and differentiation.1–3 Integrins serve as major receptors for ECM proteins.4 Binding of cells to the ECM induces clustering of integrins and recruitment of multiple cellular proteins to the cytoplasmic portions of the integrins.4 These proteins include scaffold proteins, such as paxillin, vinculin, α-actinin and talin and signal proteins such as FAK and c-Src.5,6 These structures formed at the cell-ECM interface are called focal adhesions (FAs). FAs can be easily observed during cell spreading on a rigid ECM surface, such as ECM-coated culture dishes (2D culture conditions) or in cells adhering to the basement membrane. In contrast, FAs do not form readily visible structures when cells are cultured in collagen gel (3D culture conditions).7 Nevertheless, components of FAs play pivotal roles in cell migration even under 3D growth conditions.8 Presumably, FAs are too small in size and/or too short-lived to be observed under 3D growth conditions. Although the structural relevance of FAs that arise during 2D growth to the cell adhesion machinery present during 3D culture conditions is not clear, the physical and functional interactions between the components of FAs are presumably preserved, even during 3D conditions so as to regulate migration speed and the extension of protrusions along the direction of movement. Thus, the analysis of FAs during 2D growth conditions still provides useful clues to the understanding of cell migration during both 2D and 3D growth conditions.
We recently showed that the ZF21 protein is a component and regulator of FAs.9 ZF21 contains a FYVE domain, which was originally identified as a domain conserved among Fab1p, YOPB, Vps27p and EEA1 proteins that interacts with phosphoinositides in the lipid layers of membranes.10 Many FYVE domain-containing proteins are conserved from yeast to mammals and they are thought to play roles in membrane trafficking by associating with vesicles, although the precise functions of most such proteins, including ZF21, remain unknown. As the FYVE domain is the sole conserved motif among such proteins, members of this family of protein are expected to have unique functions. ZF21 is expressed nearly ubiquitously in most tissues and adherent cell lines. During 2D culture conditions, ZF21 localizes to vesicles that contain the early endosomal marker, EEA1, which is also an FYVE domain-containing protein. However, ZF21 also localizes to FAs under 2D growth conditions.9
ZF21 Regulates the Turnover of FAs and Cell Motility
Constitutive knockdown of the expression of ZF21 does not affect the viability or growth of cells in culture, but alters cell morphology by enhancing adherence to components of the ECM, such as fibronectin, type-I collagen and vitronectin.9 We also observed accumulation of integrin β1 on the surface of the cell and an increase in the size and number of FAs. Depletion of ZF21 suppressed cell motility presumably as a consequence of the enhanced cell adhesion to ECM.
During cell migration, there is a continuous dynamic process of formation and disassembly of FAs and these two steps are regulated by different mechanisms.11,12 The roles of ZF21 during formation and disassembly of FAs was dissected using nocodazole, which disrupts microtubules (MTs). The extension of MTs to FAs is critical for the disassembly of FAs.13,14 Treatment of cells with nocodazole stabilizes FAs by preventing their disassembly. Depletion of ZF21 increases the number of FAs in control, but not nocodazole-treated cells.9 Washing out of the nocodazole from the culture media causes a synchronous disassembly of FAs that leads to a progressive reduction in the number of FAs. However, the decrease of FAs after washing out of the nocodazole was delayed in ZF21-depleted cells, even though the MTs were able to reform.9 These results indicate that ZF21 is specifically required during disassembly but not formation of FAs. However, localization of ZF21 at FAs was not affected by nocodazole treatment.
ZF21 Associates with Multiple Proteins Regulating Disassembly of FAs
Extension of MTs to FAs is essential to induce disassembly of FAs.13 m-Calpain, a calcium-dependent endopeptidase, has been implicated in the disassembly of FAs via cleavage of integrin, FAK, talin and α-actinin.15–17 Dephosphorylation of FAK, paxillin and p130CAS by protein tyrosine phosphatases, such as PTP-PEST, SHP-2 or PTP-1B, also occur during disassembly of FA complexes.18–20 At the final stage of FA disassembly, integrins are internalized in a dynamin-dependent manner and incorporated into early endosomes.13
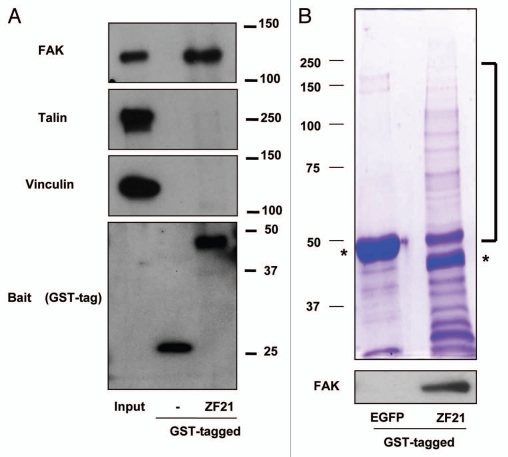
To explore possible functions of ZF21 during FA disassembly, we analyzed ZF21 binding proteins that are linked to FAs.9 A recombinant ZF21 protein fused to glutathione S-transferase (GST) was used to capture ZF21 binding proteins for western blot analysis. We found that GST-ZF21 bound to FAK, but not to other FA components such as talin or vinculin (Fig. 1A). In the previous study, we also demonstrated that GST-ZF21 did not bind to paxillin and zyxin.9 Thus, the interaction between ZF21 and FAK is specific, though it may not be a direct one. We further observed that multiple proteins associated with GST-ZF21 by SDS-polyacrylamide gel analysis (Fig. 1B). Therefore, we carried out an analysis of these associating proteins using LC-MS. Repeated analysis of the pulled-down samples identified 45 proteins classified into FA disassembly-related, RNA-binding, motor, chaperon, cell cycle-related, proteasome-related, nuclear-related and other proteins as summarized in Table 1. FAK was not identified by the mass spectrometry analysis even though it was detected by western blot analysis. This is probably due to either the relatively low level of binding of FAK to GST-ZF21 compared to the other proteins or to a low ionization efficiency of its fragments.
Figure 1.
Analysis of proteins associating with ZF21. (A) ZF21 fused to GST was used to pull down proteins from whole cell lysates of HeLa cells as described previously.9 Proteins specifically bound to GST-ZF21 were analyzed by western blot using antibodies against the indicated proteins (FAK, talin, vinculin or GST). (B) The proteins pulled down using GST-ZF21 or GST-EGFP were subjected to SDS-PAGE and stained with Coomassie Brilliant Blue R-250. The protein bands that appeared to bind specifically to GST-ZF21 but not to GST-EGFP were cut out and these bands were subjected to in-gel digestion with trypsin and analyzed by LC/MS. Controls were the corresponding gel fragments in GST-EGFP column. Asterisks indicate GST-EGFP and GST-ZF21 proteins.
Table 1.
List of ZF21-binding proteins identified in Fig. 1B
| FA disassembly-related (3) | Alpha-tubulin (CAA25855) | Chaperon (7) | HSP90 beta (NP_031381) |
| Beta-tubulin (AAB59507) | HSP90AA1 (AAI08696) | ||
| m-Calpain (NP_001739) | HSP70B' (NP_002146) | ||
| RNA-binding (18) | DDX1 (NP_004930) | HSP8A (NP_006588) | |
| DDX3X (NP_001347) | GRP78/Bip (NP_005338) | ||
| DDX5 (NP_004387) | TCP1 (CAA52808) | ||
| DDX17 (CAG30318) | TRAP1 (AAC24722) | ||
| hnRNP H1 (NP_005511) | Cell cycle-related (3) | APC-7 (NP_057322) | |
| hnRNP K (NP_112553) | CTF18 (NP_071375) | ||
| hnRNP M (NP_005959) | Spermatogenesis associated 5 (EAX05213) | ||
| hnRNP U (CAA46472) | Proteasome-related (2) | 26S subunit, ATPase, 1 (AAH16368) | |
| PABP1 (EAW91819) | CAND1 (AAH26220) | ||
| PABP4 (NP_003810) | Nuclear-related (4) | Matrin-3 (NP_061322) | |
| PAI1 RNA-binding protein 1 (NP_001018077) | Importin-9 (NP_060555) | ||
| NSUN2 (NP_060225) | Lamin A/C (NP_733821) | ||
| PTB-associated splicing factor (NP_005057) | NuMA1 (BAD92078) | ||
| TAF15 (NP_003478) | Other (5) | Vimentin (NP_003371) | |
| EWS (NP_005234) | SNX-9 (NP_057308) | ||
| FUS/TLS protein (AAC35284) | Plakophilin 3 (NP_009114) | ||
| Motor (3) | Myosin-9 (NP_002464) | ATAD3A (NP_060658) | |
| Myosin-10 (NP_005955) | Alkaline phosphatase 1 (AAA51700) | ||
| Myosin-14 (NP_001070654) | Total | 45 proteins |
Forty-five proteins were identified by the analyses of protein bands indicated in Fig. 1B and they were classified into FA disassembly-related, RNA-binding, motor, chaperon, cell cycle-related, proteasome-related, nuclear-related and other proteins. Accession numbers assigned by NCBI for each protein are shown in parentheses after the protein names.
Possible Mechanisms Whereby ZF21 Regulates Disassembly of FAs
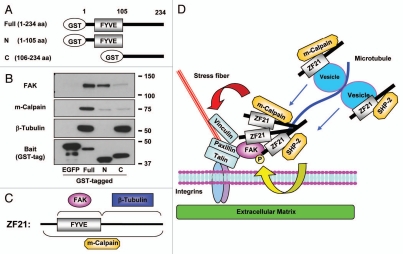
Extension of MTs to FAs is critical to induce disassembly of the latter. According to earlier works by Small et al. exposure of adherent cells to nocodazole, which induces depolymerization of MTs, leads to an increase in the number of enlarged FAs.21 In addition, they also reported that kinesin-1, which is a conventional motor protein that conveys vesicles on MTs, is necessary for the disassembly of FAs.22 Inhibition of kinesin-1 activity with a blocking antibody or forced expression of a dominant negative mutant kinesin-1 in cells induces a dramatic increase in the size and number of FAs. Thus, extension of MTs to FAs appears to deliver one or a set of key unidentified factors that are necessary for disassembly of FAs. Dynamin, which is important for endocytosis of membrane proteins at the final stage of FA turnover, localizes to FAs prior to extension of MTs to FAs through an interaction with FAK that is phosphorylated at Tyr397.13 After extension of MTs to FAs, the dissociation of dynamin from FAK is observed concomitant with dephosphorylation of FAK at pTyr397, followed by disassembly of FAs.13 Therefore, dephosphorylation of FAK at Tyr397 is important for completion of the disassembly of FAs by freeing dynamin from FAK and thereby promoting endocytosis of integrin-β1. We have shown previously that SHP-2, which is a tyrosine phosphatase implicated in the dephosphorylation of FAK, can bind ZF21.9 In addition to FAK and SHP-2, the ZF21-associating proteins listed in Table 1 include m-calpain and components of MTs (α-tubulin and β-tubulin) that have been implicated in the disassembly of FAs. We performed a pull-down assay using ZF21 derivatives fused to GST so as to identify the portion of ZF21 that interacts with these proteins (Fig. 2A). The results are summarized in Figure 2B and C. A fusion of GST to an N-terminal fragment of the protein containing the FYVE domain bound FAK whereas a fusion of GST to a C-terminal fragment of the protein bound β-tubulin. Neither of these fusion proteins was sufficient to bind m-calpain.
Figure 2.
A possible mechanism of ZF21-mediated disassembly of FAs. (A) Domain structure of GST-ZF21 derivatives used for the pull-down assays in (B). Full (1–234 aa), full-length; N (1–105 aa), an N-terminal domain fragment including FYVE domain; C (106–234 aa), a C-terminal domain mutant. The amino acid positions are indicated by aa. (B) Pull-down experiments were carried out as described in Figure 1A. The proteins bound to GST-ZF21 derivatives were subjected to western blot analysis using antibodies against FAK, m-calpain, b-tubulin or GST. (C) Binding regions of ZF21 with FAK, m-calpain or b-tubulin are summarized based on the results in (B). (D) A model proposed for ZF21-mediated disassembly of FAs, though association of proteins with ZF21 may not be direct. ZF21 resides in FAs even when MT extension to the FA is disrupted by nocodazole treatment. ZF21 binds to FAK in FAs via its FYVE domain. The C-terminal region of the pre-existing ZF21 in FAs may bind MTs that have extended to the FAs. ZF21 may also reside in the transport vesicles via the FYVE domain. Since ZF21 can bind SHP-2 and m-calpain, these factors might also be transported to FAs by MTs if ZF21 interacts with the vesicles transported by kinesin-1 on MTs. Binding of m-calpain to ZF21 requires the entire region of the protein, but the binding site of ZF21 to SHP-2 is not known. The pre-existing ZF21 in FAs may form oligomers with the ZF21 transported to the FAs by vesicles. The ability of ZF21 to form oligomers in FAs may bring phosphorylated FAK in closer proximity to SHP-2. m-Calpain cleaves integrin, FAK, talin, paxillin and vinculin. The ZF21 oligomer also acts as a platform to accumulate factors for executing the disassembly of FAs. Thus, MT extension to FAs causes accumulation of factors responsible for the initiation of the disassembly of FAs. Finally, the remains of the disassembled FAs are internalized in a dynamin-dependent manner.
MTs are likely to convey some critical factors together with vesicles transported by kinesin-1 to FAs so as to induce FA disassembly. ZF21 itself appears insufficient to induce disassembly of FAs because ZF21 remains localized to FAs during nocodazole treatment. However, since we have observed that ZF21 can form oligomers (unpublished data), ZF21 in FAs may present as oligomers and act as acceptor sites for MTs via binding of its C-terminal portion to α- and β-tubulins. ZF21 can also associate with vesicles via the FYVE domain and it is probably transported to FAs by MTs. Therefore, it is possible that ZF21-associated vesicles act as cargos carrying SHP-2 and m-calpain to FAs through MTs. Indeed, Bhatt et al. observed that µ- or m-calpain was required for MT-mediated turnover of FAs, but not for formation of MTs or their targeting to FAs.23 Thus, depletion of ZF21 may interrupt continuous supply of the disassembly regulators such as SHP-2 and m-calpain by MTs and thereby prevent disassembly of FAs. In addition, ZF21 carried by MTs may bind and form homo-oligomers with the ZF21 proteins that are already present at FAs. Since the resident ZF21 at FAs is presumably binding to pTyr397 FAK, ZF21 associating with SHP-2 may facilitate dephosphorylation of the FAK by forming a homo-oligomer. The possible roles of ZF21 proposed for MT-dependent FA disassembly are illustrated in Figure 2D.
Involvement of ZF21 in the Spreading Initiation Center of the Cells
ZF21 was also found to associate with many RNA binding proteins (RBPs) that have been recently shown to be components of the spreading initiation center (SICs) (Table 1).24 Proteomic analyses revealed that these RBPs interact with components of FAs, such as talin, vinculin and paxillin, during the attachment of cells to the ECM. Immunohistochemical analysis of the localization of these proteins showed that they are associated with transient structures that are different from FAs at the periphery of cells during their initial adhesion to the ECM. This structure was termed the SIC by deHoog et al.24 SICs are relatively large cell adhesion structures compared to FAs and contain RBPs and some FA components that are surrounded by actin sheath. As is the case with FAs, proteins associated with the cytoplasmic domain of integrins, such as FAK, talin, paxillin and vinculin, were also found in SICs.24,25 In the early stages of cell spreading, the RBPs, such as hnRNP-K, -E1 and FUS/TLS, co-localize with FA marker proteins in large punctate structures at the periphery of the cells. As the cells exhibit a more flattened morphology, the RBPs disappear from the puncta. These findings have led to the suggestion that SICs are precursors of FAs. Electropolation of an antibody against either hnRNP-K, -E1 or FUS/TLS into cells was reported to increase their spreading and SICs are thought to regulate the rate of cell spreading. However, exact functions of RBPs in SICs during cell attachment to the ECM remains to be characterized more clearly.
Potential interaction between ZF21 and SIC proteins was suggested by the proteomic analysis. To confirm this further, we expressed hnRNPK1, G3BP2, PABP1 in Cos-1 cells as fusions with an mCherry tag and each such protein was confirmed to bind to GST-ZF21 (data not shown). The association of ZF21 with SIC-related RBPs, such as hnRNPK and FUS/TLS, suggests that ZF21 may also be a component of SICs at an early stage of cell adhesion. Interestingly, the phenotype of ZF21-depleted cells, such as increased spreading and stabilization of FAs, resembles to that of cells following electroporation with antibodies against RBPs. ZF21 associating with vesicles on MTs may also act as a carrier for certain cytoplasmic proteins to SICs like it does to FAs and regulate the fate of SICs.
Conclusions
Analysis of ZF21 interacting proteins identified multiple factors involved in cell adhesion, including components of both FAs and SICs. In particular, ZF21 was found to associate with proteins that regulate disassembly of FAs via different portions and we also observed that it is indeed a crucial factor in regulating the disassembly of FAs. ZF21 appears to act as a platform to supply proteins for disassembly to FAs. Formation and disassembly of FAs are regulated by different mechanisms and the FA structure might be maintained by a dynamic equilibrium between its formation and disassembly. Although it is still unclear how extension of MTs to FA triggers the disassembly process, we posit that a continuous supply of regulator proteins for disassembly of FAs is essential to shift the equilibrium towards FA disassembly. Disruption of MTs or depletion of ZF21 may interrupt this supply and thereby stabilize the FA structures. ZF21 may also play a role in recruiting components of SICs through its ability to bind and convey them to the newly formed cell attachment sites through MTs. But roles of ZF21 in SICs are still very speculative.
In a recent paper, Winograd-Katz et al. attempted to identify multiple genes involved in FA formation using an siRNA library.26 Although their study sheds light on the signaling mechanisms regulating the formation of integrin-mediated adhesion to the ECM, our knowledge of the turnover mechanism remains limited. We hope that our analysis of the binding proteins of ZF21 will shed light on the dynamic regulation of FA turnover.
Acknowledgements
This work was supported by the Specific Coordination Fund for Promoting Science to T.S., by a Grant-in-Aid for Scientific Research on Priority Areas, i.e., the “Integrative Research toward the Conquest of Cancer” (M.S.) and by Global COE Program (T.S. and M.S.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations
- ECM
extracellular matrix
- EEA1
early endosome antigen 1
- FYVE
Fab1p YOPB Vps27p, EEA1
- FA
focal adhesion
- MT
microtubule
- RBP
RNA binding protein
- SIC
spreading initiation center
- ZF21
ZFYVE21
References
- 1.Hynes RO, Lander AD. Contact and adhesive specificities in the associations, migrations and targeting of cells and axons. Cell. 1992;68:303–322. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- 2.Meighan CM, Schwarzbauer JE. Temporal and spatial regulation of integrins during development. Curr Opin Cell Biol. 2008;20:520–524. doi: 10.1016/j.ceb.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 4.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 5.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 6.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 8.Fraley SI, Feng Y, Krishnamurthy R, Kim DH, Celedon A, Longmore GD, et al. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol. 2010;12:598–604. doi: 10.1038/ncb2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagano M, Hoshino D, Sakamoto T, Kawasaki N, Koshikawa N, Seiki M. ZF21 protein regulates cell adhesion and motility. J Biol Chem. 2010;285:21013–21022. doi: 10.1074/jbc.M110.106443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenmark H, Aasland R. FYVE-finger proteins—effectors of an inositol lipid. J Cell Sci. 1999;112:4175–4183. doi: 10.1242/jcs.112.23.4175. [DOI] [PubMed] [Google Scholar]
- 11.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 12.Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr Opin Cell Biol. 2008;20:85–90. doi: 10.1016/j.ceb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–590. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- 14.Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–747. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glading A, Lauffenburger DA, Wells A. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/s0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- 16.Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 17.Frame MC, Fincham VJ, Carragher NO, Wyke JA. v-Src's hold over actin and cell adhesions. Nat Rev Mol Cell Biol. 2002;3:233–245. doi: 10.1038/nrm779. [DOI] [PubMed] [Google Scholar]
- 18.Angers-Loustau A, Cote JF, Charest A, Dowbenko D, Spencer S, Lasky LA, et al. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration and cytokinesis in fibroblasts. J Cell Biol. 1999;144:1019–1031. doi: 10.1083/jcb.144.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu DH, Qu CK, Henegariu O, Lu X, Feng GS. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration and focal adhesion. J Biol Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Lin SY, Neel BG, Haimovich B. Phosphorylated alpha-actinin and protein-tyrosine phosphatase 1B coregulate the disassembly of the focal adhesion kinase x Src complex and promote cell migration. J Biol Chem. 2006;81:1746–1754. doi: 10.1074/jbc.M509590200. [DOI] [PubMed] [Google Scholar]
- 21.Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 1999;146:1033–1044. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krylyshkina O, Kaverina I, Kranewitter W, Steffen W, Alonso MC, Cross RA, et al. Modulation of substrate adhesion dynamics via microtubule targeting requires kinesin-1. J Cell Biol. 2002;156:349–359. doi: 10.1083/jcb.200105051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatt A, Kaverina I, Otey C, Huttenlocher A. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J Cell Sci. 2002;115:3415–3425. doi: 10.1242/jcs.115.17.3415. [DOI] [PubMed] [Google Scholar]
- 24.de Hoog CL, Foster LJ, Mann M. RNA and RNA binding proteins participate in early stages of cell spreading through spreading initiation centers. Cell. 2004;117:649–662. doi: 10.1016/s0092-8674(04)00456-8. [DOI] [PubMed] [Google Scholar]
- 25.Andersson MK, Stahlberg A, Arvidsson Y, Olofsson A, Semb H, Stenman G, et al. The multifunctional FUS, EWS and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol. 2008;9:37. doi: 10.1186/1471-2121-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winograd-Katz SE, Itzkovitz S, Kam Z, Geiger B. Multiparametric analysis of focal adhesion formation by RNAi-mediated gene knockdown. J Cell Biol. 2009;186:423–436. doi: 10.1083/jcb.200901105. [DOI] [PMC free article] [PubMed] [Google Scholar]