To the Editors
HIV-1 infection is associated with several immunological disturbances including a reduction in circulating CD34+ hematopoietic progenitor cells 1. Depletion of CD34+ cells may have far-reaching consequences, given their role in immune reconstitution and their association with vascular repair and cardiovascular disease 2,3. Although CD34+ cells are generally resistant to HIV-1 infection 4, uninfected CD34+ cells from AIDS patients demonstrate a commitment to apoptosis 5. This adverse effect is likely due in large part to direct cytotoxic interactions with HIV-1 and, in particular, the envelope glycoprotein gp120 6.
The advent of highly-active antiretroviral therapy (HAART) in the treatment of HIV-1 infection has dramatically slowed the rate of progression of HIV-1 to AIDS and improved patient outcomes 7. As HIV-1-infected patients are living longer, HIV-1-related metabolic and cardiovascular complications are growing in this population 8, which may be linked to numerical or functional deficits in circulating progenitors. Treatment with protease inhibitors, an important component of many HAART regimens, generally results in improved viability of CD34+ cells from HIV-1-infected individuals 9,10. However, this may be largely driven by the antiviral activity of these medications, while the direct effects on CD34+ cells are less clear. One prior investigation reported that ritonavir had anti-apoptotic effects on CD34+ cells 9. Accordingly, we hypothesized that other common protease inhibitors would also reduce CD34+ cell apoptosis at physiological concentrations (i.e. typical maximum plasma levels) in vitro.
Freshly-isolated CD34+ cells from healthy G-CSF-treated human donors (n=3) were obtained from an independent supplier (Allcells, LLC, Berkeley, CA). Donors tested negative for HIV-1, hepatitis B and hepatitis C. Viability of cells determined by trypan blue exclusion tests was 95.7% ± 0.4%. Purity, as assessed by flow cytometric analysis with propidium iodide, was 97.7% ± 0.4%.
CD34+ cells were cultured in serum-free medium (StemCell Technologies Inc., Vancouver, Canada) supplemented with 100ng/mL of thrombopoietin, Flt3 ligand and stem cell factor. Cells were were treated for 48 h with either 1 μg/mL ritonavir (RTV) 11, 5.2 μg/mL atazanavir (ATV) 11, or 9.8 μg/mL lopinavir (LPV) 11 (obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH). Positive control experiments were performed using 100 ng/mL HIV-1Bal gp120 (R5 trophic) (AIDS Research and Reference Reagent Program) and HIV-1Lav gp120 (X4 trophic) 12 (Protein Sciences Corporation, Meriden, CT).
Activation of caspase-3 was induced in CD34+ cells by incubating with staurosporine (1 μmol/L for 3 h; Sigma Aldrich, St. Louis, MO). The concentration of active caspase-3 large subunit in cell lysates was determined using enzyme immunoassays (R&D Systems, Minneapolis, MN). The intra- and inter-assay coefficients of variation for this assay in our laboratory are <10%.
Experimental points were performed in duplicate with three independent experiments. Differences between treatments were determined by analysis of variance. Where indicated by a significant F value, post hoc tests, with Bonferroni’s correction for multiple comparisons, were performed. Results are expressed as mean ± SEM. Statistical significance was set at p<0.05.
Upon stimulation with staurosporine, both R5 (6.0±0.4 ng/mL) and X4 (6.0±0.1 ng/mL) gp120 resulted in a 65% greater activation of caspase-3 compared with the control condition (3.6±0.4 ng/mL; P<0.05) (Fig 1A). Within the protease inhibitor group, the capacity of staurosporine to induce an apoptotic response was 40% higher in cells treated with ATV (4.9±0.7 ng/mL) and LPV (5.1±0.2 ng/mL) compared with the control condition (P<0.05) (Fig 1B). In contrast, treatment with RTV (4.1±0.5 ng/mL) did not significantly alter intracellular active caspase-3.
Figure.
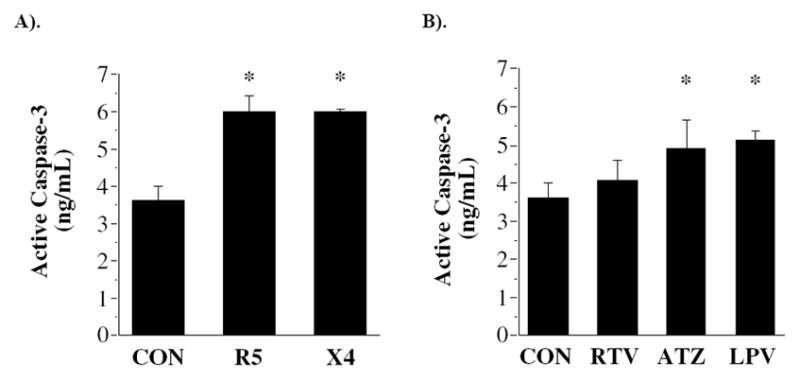
Active caspase-3 concentration following incubation with gp120 envelope proteins from R5 and X4 strains of HIV-1 virus (A); and protease inhibitors ritonavir (RTV), atazanavir (ATV) and lopinavir (LPV) (B). *P<0.05 vs. control.
The novel findings of the present study are that the protease inhibitors atazanavir and lopinavir reduce the resistance of CD34+ cells to an apoptotic stimulus. To our knowledge, this is the first study to demonstrate the pro-apoptotic effects of protease inhibitors on CD34+ cells from healthy adults.
CD34+ cells from AIDS patients demonstrate a marked predisposition towards apoptosis 5. Zauli et al. 13 showed that virus isolates from HIV-1-seropositive patients induced a dose-dependent inhibition on the growth of CD34+ cells in vitro, an effect blocked by pre-treatment with an anti-gp120 antibody. Subsequent investigations further implicated gp120 (in concentrations ranging from 0.01 to 20 ug/mL) in the induction of CD34+ cell apoptosis 14,15. Our results extend these findings to broadly physiological concentrations of gp120 from two strains of the HIV-1 virus, HIV-1Bal and HIV-1Lav, and add to the growing evidence base that indicates a direct cytotoxic activity of HIV-1 gpl20 on human CD34+ cells.
HAART has been shown to restore CD34+ cell function in HIV-1-infected patients. For example, increased CD34+ cell colony formation has been documented following 3–6 months of HAART 10,16. In addition, the protease inhibitor ritonavir lowered apoptosis in CD34+ cells from HIV-1 patients in vitro, but only at a concentration of 5 nmol/L; no differences were recorded at concentrations between 5–20 nmol and a decrease was observed at concentrations > 20 nmol 9. Interestingly, these effects have only been documented in CD34+ cells from HIV-1-infected patients and only following short-term HAART administration. Our results show that the protease inhibitors atazanavir and lopinavir reduce resistance to apoptosis in CD34+ cells from healthy adults. While ritonavir had no effect in the present investigation, it should be noted that the concentraqtion of ritonavir used in this study was that which is achieved in vivo to boost lopinavir levels through inhibition of cytochrome P450 CYP3A; although higher concentrations of ritonavir that mediate direct antiretroviral activity are no longer used clinically, an important question for future studies is whether higher concentrations of ritonavir and therapeutic doses of other protease inhibitors also induce a pro-apoptotic phenotype. It is plausible, therefore, that the pro-survival effects of protease inhibitors on CD34+ cells are secondary to their antiviral activity, and that direct interaction with healthy cells is actually harmful, albeit less harmful than exposure to gp120. This information may have clinically relevant implications in the context of longer-term treatment given its potential to explain, at least in part, why some patients do not reconstitute their CD4+ T cell population despite HAART and the accelerated cardiovascular morbidity observed in patients on HAART.
Although we endeavored to confer physiological relevance on our study by using concentrations of protease inhibitors and gp120 found in the plasma of HIV-1-positive patients 11,12, it is important to be conservative in any attempt to generalize our results to an in vivo context. CD34+ cells are exposed to these agents continuously in the circulation in addition to the full complexity of vascular milieu. In addition, although our findings were similar in all subjects, our sample was small and further experiments will be required to delineate the mechanisms of the PI-induced apoptotic effect.
In conclusion, our results suggest that typical plasma concentrations of atazanavir and lopinavir can reduce resistance to apoptosis in healthy CD34+ cells, suggesting a role for protease inhibitors in poor immune reconstitution and accelerated cardiovascular disease in some HAART-treated patients.
Acknowledgments
Support: National Institutes of Health (HL088911); American Heart Association (0840167N).
Reference List
- 1.Banda NK, Simon GR, Sipple JD, et al. Depletion of CD34+ CD4+ cells in bone marrow from HIV-1-infected individuals. Biol Blood Marrow Transplant. 1999;5:162–172. doi: 10.1053/bbmt.1999.v5.pm10392962. [DOI] [PubMed] [Google Scholar]
- 2.Scadden DT, Shen H, Cheng T. Hematopoietic stem cells in HIV disease. J Natl Cancer Inst Monogr. 2001:24–29. doi: 10.1093/oxfordjournals.jncimonographs.a024253. [DOI] [PubMed] [Google Scholar]
- 3.Fadini GP, de Kreutzenberg SV, Coracina A, et al. Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur Heart J. 2006;27:2247–2255. doi: 10.1093/eurheartj/ehl198. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Scadden DT, Crumpacker CS. Primitive hematopoietic cells resist HIV-1 infection via p21. J Clin Invest. 2007;117:473–481. doi: 10.1172/JCI28971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Re MC, Zauli G, Gibellini D, et al. Uninfected haematopoietic progenitor (CD34+) cells purified from the bone marrow of AIDS patients are committed to apoptotic cell death in culture. AIDS. 1993;7:1049–1055. doi: 10.1097/00002030-199308000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Zauli G, Re MC, Visani G, Furlini G, La PM. Inhibitory effect of HIV-1 envelope glycoproteins gp120 and gp160 on the in vitro growth of enriched (CD34+) hematopoietic progenitor cells. Arch Virol. 1992;122:271–280. doi: 10.1007/BF01317189. [DOI] [PubMed] [Google Scholar]
- 7.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 8.Bradbury RA, Samaras K. Antiretroviral therapy and the human immunodeficiency virus--improved survival but at what cost? Diabetes Obes Metab. 2008;10:441–450. doi: 10.1111/j.1463-1326.2007.00760.x. [DOI] [PubMed] [Google Scholar]
- 9.Sloand EM, Maciejewski J, Kumar P, Kim S, Chaudhuri A, Young N. Protease inhibitors stimulate hematopoiesis and decrease apoptosis and ICE expression in CD34(+) cells. Blood. 2000;96:2735–2739. [PubMed] [Google Scholar]
- 10.Adams GB, Pym AS, Poznansky MC, McClure MO, Weber JN. The in vivo effects of combination antiretroviral drug therapy on peripheral blood CD34+ cell colony-forming units from HIV type 1-infected patients. AIDS Res Hum Retroviruses. 1999;15:551–559. doi: 10.1089/088922299311079. [DOI] [PubMed] [Google Scholar]
- 11.Physicians' Desk Reference. 61. Montvale, NJ: Thomson PDR; 2007. [Google Scholar]
- 12.Oh SK, Cruikshank WW, Raina J, et al. Identification of HIV-1 envelope glycoprotein in the serum of AIDS and ARC patients. J Acquir Immune Defic Syndr. 1992;5:251–256. [PubMed] [Google Scholar]
- 13.Zauli G, Re MC, Visani G, et al. Evidence for a human immunodeficiency virus type 1-mediated suppression of uninfected hematopoietic (CD34+) cells in AIDS patients. J Infect Dis. 1992;166:710–716. doi: 10.1093/infdis/166.4.710. [DOI] [PubMed] [Google Scholar]
- 14.Zauli G, Re MC, Furlini G, Giovannini M, La PM. Human immunodeficiency virus type 1 envelope glycoprotein gp120-mediated killing of human haematopoietic progenitors (CD34+ cells) J Gen Virol. 1992;73 ( Pt 2):417–421. doi: 10.1099/0022-1317-73-2-417. [DOI] [PubMed] [Google Scholar]
- 15.Banda NK, Tomczak JA, Shpall EJ, et al. HIV-gp120 induced cell death in hematopoietic progenitor CD34+ cells. Apoptosis. 1997;2:61–68. doi: 10.1023/a:1026439726053. [DOI] [PubMed] [Google Scholar]
- 16.Dam NS, Kjaer EA, Mathiesen L, Nielsen JO, Hansen JE. Highly active antiretroviral therapy normalizes the function of progenitor cells in human immunodeficiency virus-infected patients. J Infect Dis. 1998;178:1299–1305. doi: 10.1086/314464. [DOI] [PubMed] [Google Scholar]


