Abstract
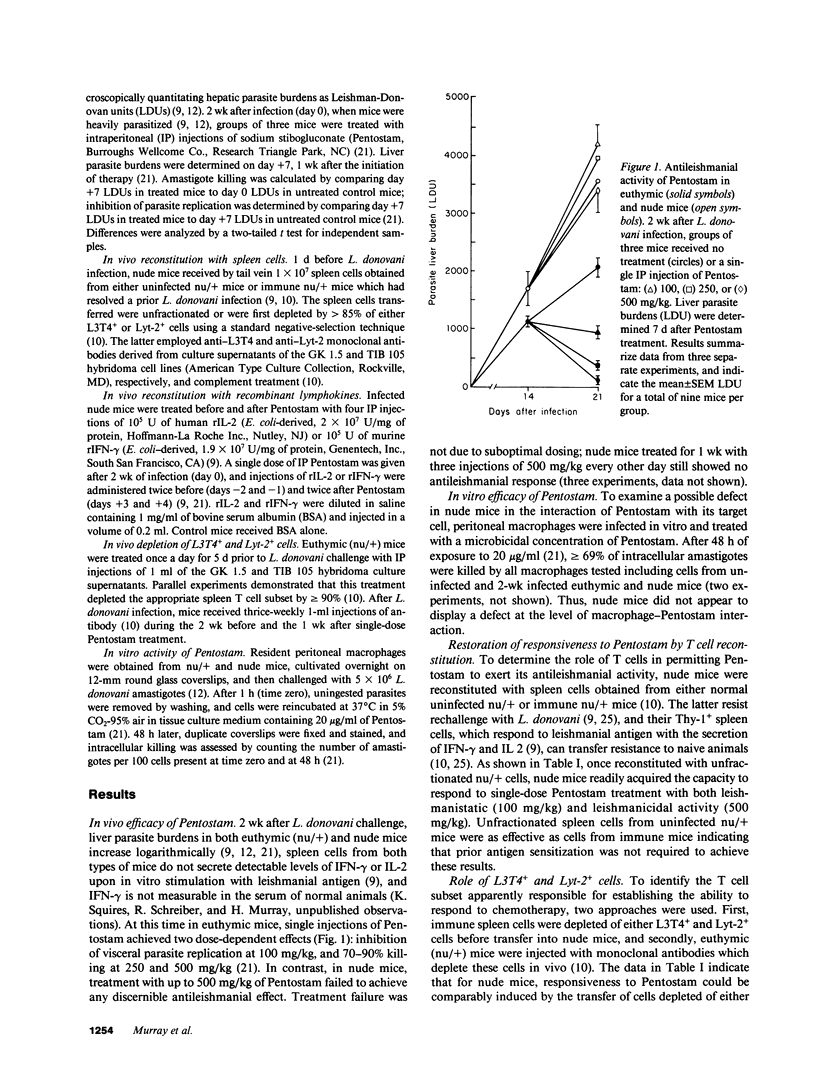
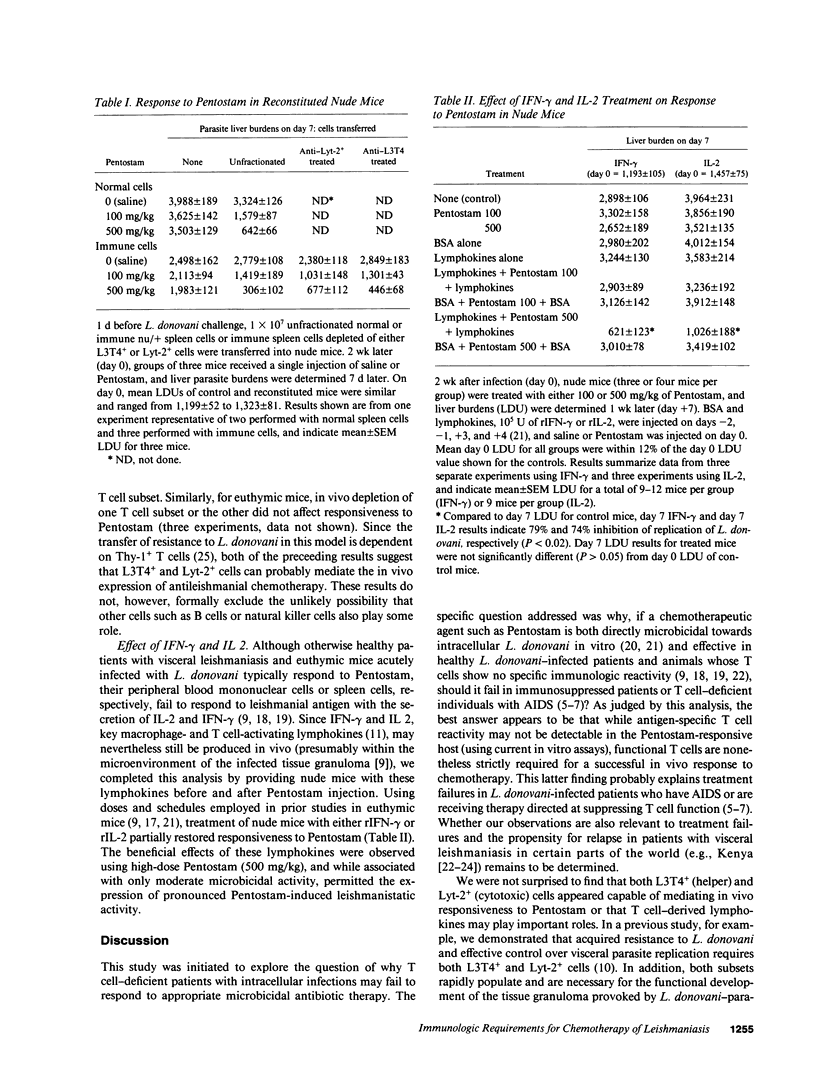
Although directly microbicidal, pentavalent antimony has failed as treatment for visceral leishmaniasis in patients who also have AIDS or are receiving immunosuppressive therapy. To define the role of T cells in the successful host response to chemotherapy, we examined the efficacy of pentavalent antimony (sodium stibogluconate, Pentostam) in normal and T cell-deficient BALB/c mice infected with Leishmania donovani. In euthymic (nu/+) mice, single injections of 250 and 500 mg/kg of Pentostam induced the killing of 67% and 89% of intracellular liver amastigotes, respectively. In contrast, in athymic nude (nu/nu) mice, up to three injections of 500 mg/kg achieved no L. donovani killing and did not retard visceral parasite replication. Once nude mice were reconstituted with nu/+ spleen cells, however, Pentostam exerted strong leishmanicidal activity, an effect that appeared to be transferred by either L3T4+ or Lyt-2+ cells. Responsiveness to chemotherapy could also be induced by providing nude mice with either interferon-gamma or interleukin 2 alone. The absence of this T cell- and probably lymphokine-dependent mechanism is a likely explanation for treatment failures in immunocompromised patients infected with L. donovani and perhaps other systemic intracellular pathogens as well.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Berman J. D., Chulay J. D., Hendricks L. D., Oster C. N. Susceptibility of clinically sensitive and resistant Leishmania to pentavalent antimony in vitro. Am J Trop Med Hyg. 1982 May;31(3 Pt 1):459–465. doi: 10.4269/ajtmh.1982.31.459. [DOI] [PubMed] [Google Scholar]
- Carvalho E. M., Badaró R., Reed S. G., Jones T. C., Johnson W. D., Jr Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Invest. 1985 Dec;76(6):2066–2069. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chulay J. D., Bhatt S. M., Muigai R., Ho M., Gachihi G., Were J. B., Chunge C., Bryceson A. D. A comparison of three dosage regimens of sodium stibogluconate in the treatment of visceral leishmaniasis in Kenya. J Infect Dis. 1983 Jul;148(1):148–155. doi: 10.1093/infdis/148.1.148. [DOI] [PubMed] [Google Scholar]
- Fernández-Guerrero M. L., Aguado J. M., Buzón L., Barros C., Montalbán C., Martín T., Bouza E. Visceral leishmaniasis in immunocompromised hosts. Am J Med. 1987 Dec;83(6):1098–1102. doi: 10.1016/0002-9343(87)90948-x. [DOI] [PubMed] [Google Scholar]
- Handa K., Suzuki R., Matsui H., Shimizu Y., Kumagai K. Natural killer (NK) cells as a responder to interleukin 2 (IL 2). II. IL 2-induced interferon gamma production. J Immunol. 1983 Feb;130(2):988–992. [PubMed] [Google Scholar]
- Hefeneider S. H., Conlon P. J., Henney C. S., Gillis S. In vivo interleukin 2 administration augments the generation of alloreactive cytolytic T lymphocytes and resident natural killer cells. J Immunol. 1983 Jan;130(1):222–227. [PubMed] [Google Scholar]
- Ho M., Koech D. K., Iha D. W., Bryceson A. D. Immunosuppression in Kenyan visceral leishmaniasis. Clin Exp Immunol. 1983 Feb;51(2):207–214. [PMC free article] [PubMed] [Google Scholar]
- Hoover D. L., Finbloom D. S., Crawford R. M., Nacy C. A., Gilbreath M., Meltzer M. S. A lymphokine distinct from interferon-gamma that activates human monocytes to kill Leishmania donovani in vitro. J Immunol. 1986 Feb 15;136(4):1329–1333. [PubMed] [Google Scholar]
- Hoover D. L., Nacy C. A., Meltzer M. S. Human monocyte activation for cytotoxicity against intracellular Leishmania donovani amastigotes: induction of microbicidal activity by interferon-gamma. Cell Immunol. 1985 Sep;94(2):500–511. doi: 10.1016/0008-8749(85)90274-6. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., De Libero G. Listeria monocytogenes-reactive T lymphocyte clones with cytolytic activity against infected target cells. J Exp Med. 1986 Jul 1;164(1):363–368. doi: 10.1084/jem.164.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C. D., Welte K., Murray H. W. Antigen-induced human interferon-gamma production. Differential dependence on interleukin 2 and its receptor. J Immunol. 1987 Oct 1;139(7):2325–2328. [PubMed] [Google Scholar]
- Lamas S., Orte L., Parras F., García Laraña J., Matesanz R., Ortuño J. Non-fatal leishmaniasis in a renal transplant recipient. Nephron. 1987;45(1):71–71. doi: 10.1159/000184076. [DOI] [PubMed] [Google Scholar]
- MANSON-BAHR P. E. East African kalazar with special reference to the pathology, prophylaxis and treatment. Trans R Soc Trop Med Hyg. 1959 Mar;53(2):123–137. doi: 10.1016/0035-9203(59)90060-4. [DOI] [PubMed] [Google Scholar]
- Ma D. D., Concannon A. J., Hayes J. Fatal leishmaniasis in renal-transport patient. Lancet. 1979 Aug 11;2(8137):311–312. doi: 10.1016/s0140-6736(79)90333-7. [DOI] [PubMed] [Google Scholar]
- McElrath M. J., Murray H. W., Cohn Z. A. The dynamics of granuloma formation in experimental visceral leishmaniasis. J Exp Med. 1988 Jun 1;167(6):1927–1937. doi: 10.1084/jem.167.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Berman J. D., Wright S. D. Immunochemotherapy for intracellular Leishmania donovani infection: gamma interferon plus pentavalent antimony. J Infect Dis. 1988 May;157(5):973–978. doi: 10.1093/infdis/157.5.973. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Carriero S. M., Donelly D. M. Presence of a macrophage-mediated suppressor cell mechanism during cell-mediated immune response in experimental visceral leishmaniasis. Infect Immun. 1986 Nov;54(2):487–493. doi: 10.1128/iai.54.2.487-493.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cartelli D. M. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest. 1983 Jul;72(1):32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., DePamphilis J., Schooley R. T., Hirsch M. S. Circulating interferon-gamma in AIDS patients treated with interleukin-2. N Engl J Med. 1988 Jun 9;318(23):1538–1539. doi: 10.1056/NEJM198806093182312. [DOI] [PubMed] [Google Scholar]
- Murray H. W. Interferon-gamma, the activated macrophage, and host defense against microbial challenge. Ann Intern Med. 1988 Apr;108(4):595–608. doi: 10.7326/0003-4819-108-4-595. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Masur H., Keithly J. S. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J Immunol. 1982 Jul;129(1):344–350. [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Murray H. W., Stern J. J., Welte K., Rubin B. Y., Carriero S. M., Nathan C. F. Experimental visceral leishmaniasis: production of interleukin 2 and interferon-gamma, tissue immune reaction, and response to treatment with interleukin 2 and interferon-gamma. J Immunol. 1987 Apr 1;138(7):2290–2297. [PubMed] [Google Scholar]
- Murray H. W., Welte K., Jacobs J. L., Rubin B. Y., Mertelsmann R., Roberts R. B. Production of and in vitro response to interleukin 2 in the acquired immunodeficiency syndrome. J Clin Invest. 1985 Nov;76(5):1959–1964. doi: 10.1172/JCI112194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Prendergast T. J., Wiebe M. E., Stanley E. R., Platzer E., Remold H. G., Welte K., Rubin B. Y., Murray H. W. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984 Aug 1;160(2):600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Wheeler D. A., Harrison L. H., Kay H. D. The immunobiology of leishmaniasis. Rev Infect Dis. 1983 Sep-Oct;5(5):907–927. doi: 10.1093/clinids/5.5.907. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Lal S. L., Shrivastava S. N., Blackwell J., Neva F. A. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987 Feb 1;138(3):908–913. [PubMed] [Google Scholar]
- Sharma S. D., Hofflin J. M., Remington J. S. In vivo recombinant interleukin 2 administration enhances survival against a lethal challenge with Toxoplasma gondii. J Immunol. 1985 Dec;135(6):4160–4163. [PubMed] [Google Scholar]
- Stern J. J., Oca M. J., Rubin B. Y., Anderson S. L., Murray H. W. Role of L3T4+ and LyT-2+ cells in experimental visceral leishmaniasis. J Immunol. 1988 Jun 1;140(11):3971–3977. [PubMed] [Google Scholar]
- Vilcek J., Henriksen-Destefano D., Siegel D., Klion A., Robb R. J., Le J. Regulation of IFN-gamma induction in human peripheral blood cells by exogenous and endogenously produced interleukin 2. J Immunol. 1985 Sep;135(3):1851–1856. [PubMed] [Google Scholar]
- Wallis P. J., Clark C. J. Visceral leishmaniasis complicating systemic lupus erythematosus. Ann Rheum Dis. 1983 Apr;42(2):201–202. doi: 10.1136/ard.42.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yebra M., Segovia J., Manzano L., Vargas J. A., Bernaldo de Quirós L., Alvar J. Disseminated-to-skin kala-azar and the acquired immunodeficiency syndrome. Ann Intern Med. 1988 Mar;108(3):490–491. doi: 10.7326/0003-4819-108-3-490_2. [DOI] [PubMed] [Google Scholar]


