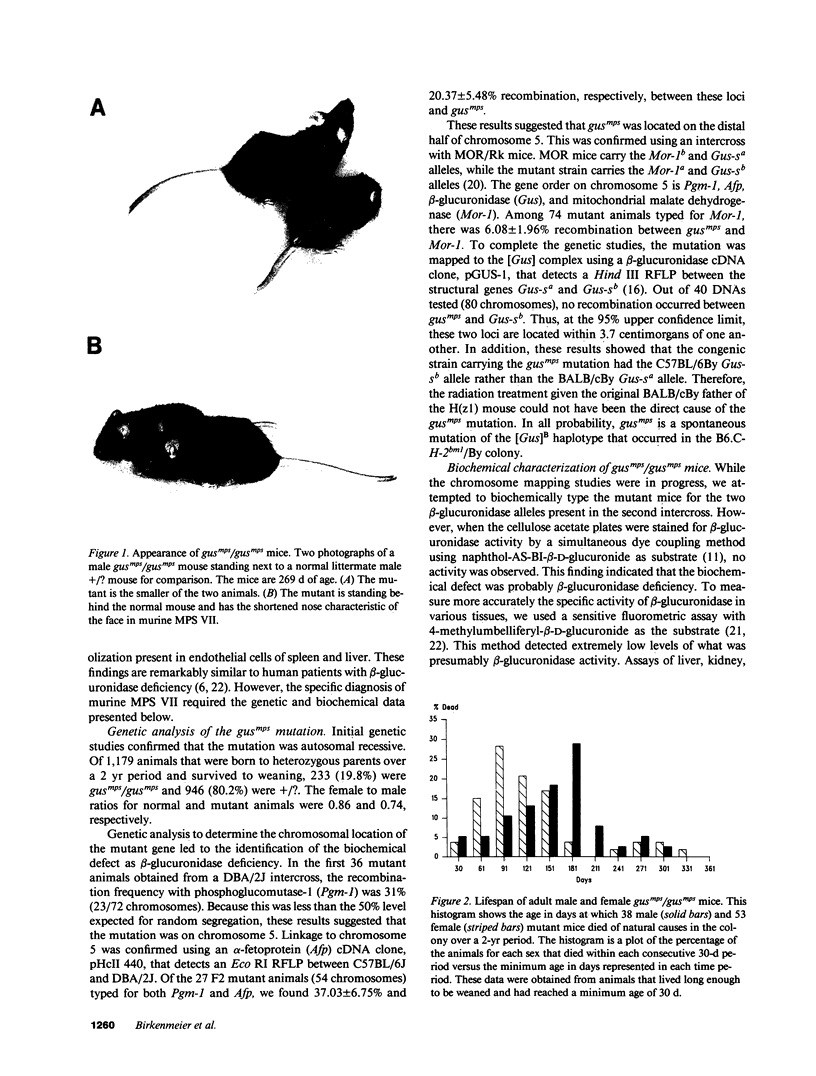
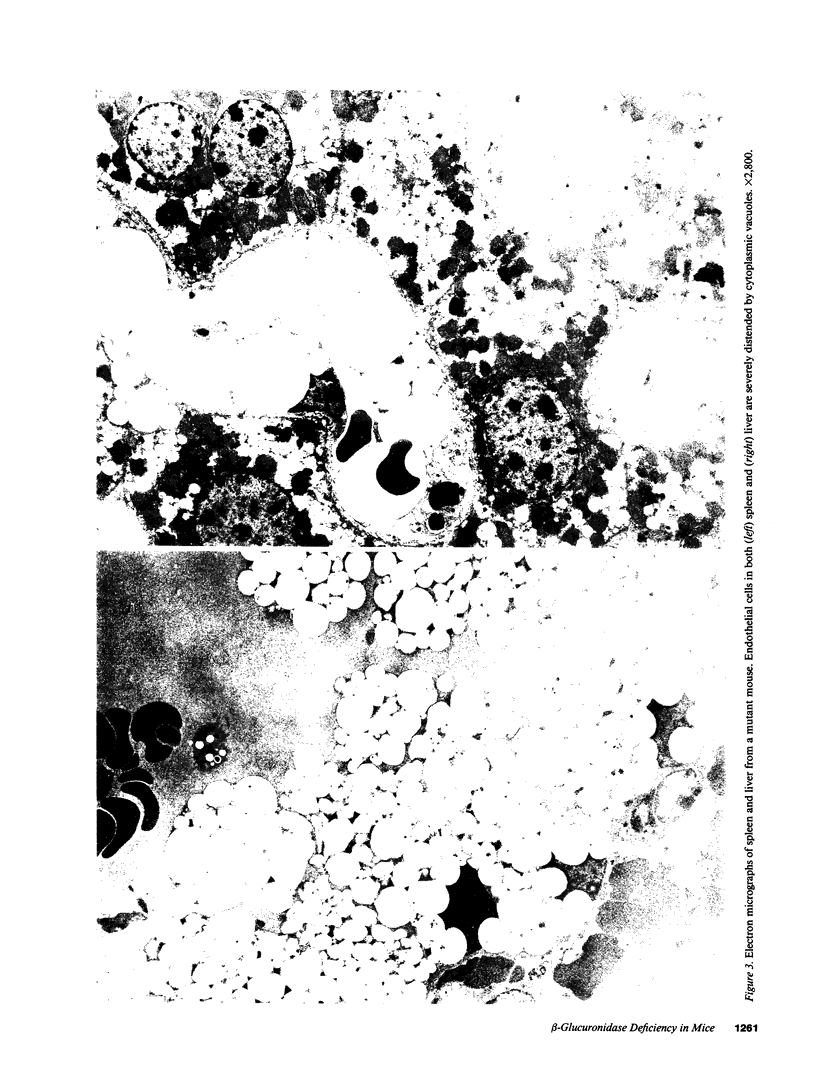
Abstract
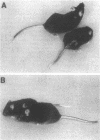
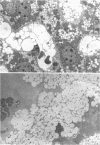
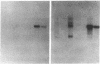
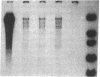
We have characterized a new mutant mouse that has virtually no beta-glucuronidase activity. This biochemical defect causes a murine lysosomal storage disease that has many interesting similarities to human mucopolysaccharidosis type VII (MPS VII; Sly syndrome; beta-glucuronidase deficiency). Genetic analysis showed that the mutation is inherited as an autosomal recessive that maps to the beta-glucuronidase gene complex, [Gus], on the distal end of chromosome 5. Although there is a greater than 200-fold reduction in the beta-glucuronidase mRNA concentration in mutant tissues, Southern blot analysis failed to detect any abnormalities in the structural gene, Gus-sb, or in 17 kb of 5' flanking and 4 kb of 3' flanking sequences. Surprisingly, a sensitive S1 nuclease assay indicated that the relative level of kidney gusmps mRNA responded normally to androgen induction by increasing approximately 11-fold. Analysis of this mutant mouse may offer valuable information on the pathogenesis of human MPS VII and provide a useful system in which to study bone marrow transplantation and gene transfer methods of therapy.
Full text
PDF

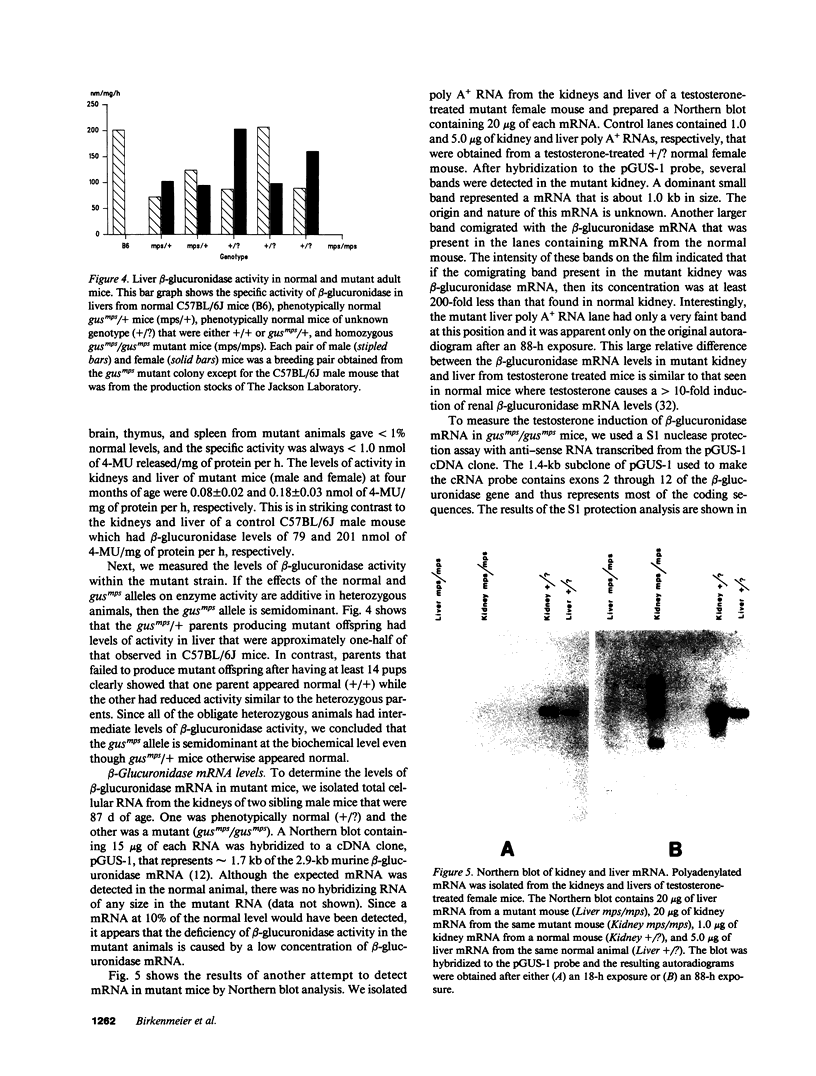
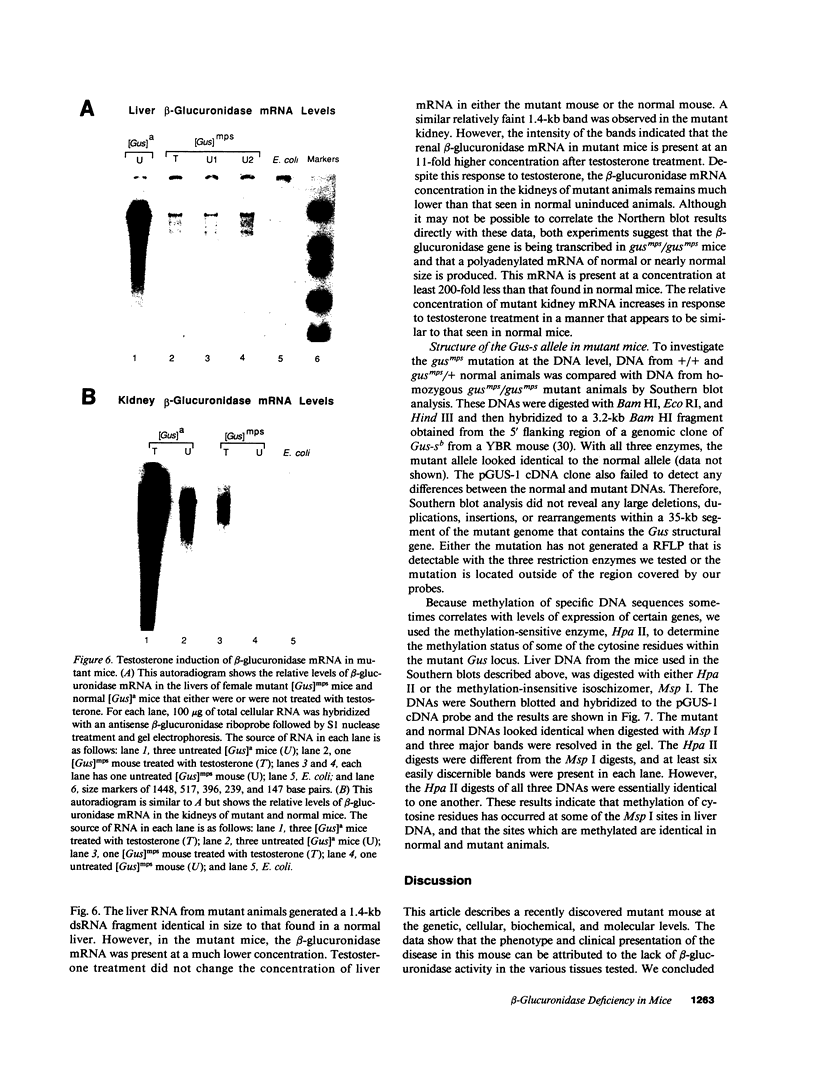



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey D. W., Kohn H. I. Inherited histocompatibility changes in progeny of irradiated and unirradiated inbred mice. Genet Res. 1965 Nov;6(3):330–340. doi: 10.1017/s0016672300004225. [DOI] [PubMed] [Google Scholar]
- Bernsen P. L., Wevers R. A., Gabreëls F. J., Lamers K. J., Sonnen A. E., Stekhoven J. H. Phenotypic expression in mucopolysaccharidosis VII. J Neurol Neurosurg Psychiatry. 1987 Jun;50(6):699–703. doi: 10.1136/jnnp.50.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dorfman A., Matalon R. The mucopolysaccharidoses (a review). Proc Natl Acad Sci U S A. 1976 Feb;73(2):630–637. doi: 10.1073/pnas.73.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gallagher P. M., D'Amore M. A., Lund S. D., Elliott R. W., Pazik J., Hohman C., Korfhagen T. R., Ganschow R. E. DNA sequence variation within the beta-glucuronidase gene complex among inbred strains of mice. Genomics. 1987 Oct;1(2):145–152. doi: 10.1016/0888-7543(87)90006-1. [DOI] [PubMed] [Google Scholar]
- Ganschow R., Paigen K. Glucuronidase phenotypes of inbred mouse strains. Genetics. 1968 Jul;59(3):335–349. doi: 10.1093/genetics/59.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehler J., Cantz M., Tolksdorf M., Spranger J., Gilbert E., Drube H. Mucopolysaccharidosis. VII. Beta-glucuronidase deficiency. Humangenetik. 1974 Jul 15;23(2):149–158. doi: 10.1007/BF00282212. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., Sly W. S. Beta-glucuronidase deficiency mucopolysaccharidosis: methods for enzymatic diagnosis. J Lab Clin Med. 1973 Dec;82(6):969–977. [PubMed] [Google Scholar]
- Haskins M. E., Desnick R. J., DiFerrante N., Jezyk P. F., Patterson D. F. Beta-glucuronidase deficiency in a dog: a model of human mucopolysaccharidosis VII. Pediatr Res. 1984 Oct;18(10):980–984. doi: 10.1203/00006450-198410000-00014. [DOI] [PubMed] [Google Scholar]
- Heuckeroth R. O., Birkenmeier E. H., Levin M. S., Gordon J. I. Analysis of the tissue-specific expression, developmental regulation, and linkage relationships of a rodent gene encoding heart fatty acid binding protein. J Biol Chem. 1987 Jul 15;262(20):9709–9717. [PubMed] [Google Scholar]
- Hoogerbrugge P. M., Poorthuis B. J., Mulder A. H., Wagemaker G., Dooren L. J., Vossen J. M., van Bekkum D. W. Correction of lysosomal enzyme deficiency in various organs of beta-glucuronidase-deficient mice by allogeneic bone marrow transplantation. Transplantation. 1987 May;43(5):609–614. doi: 10.1097/00007890-198705000-00001. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes. FASEB J. 1987 Dec;1(6):462–468. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- LEVVY G. A. beta-Glucuronidase and related enzymes. Br Med Bull. 1953;9(2):126–130. doi: 10.1093/oxfordjournals.bmb.a074327. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee J. E., Falk R. E., Ng W. G., Donnell G. N. Beta-glucuronidase deficiency. A heterogeneous mucopolysaccharidosis. Am J Dis Child. 1985 Jan;139(1):57–59. doi: 10.1001/archpedi.1985.02140030059029. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Beamer W. G., Coleman D. L., Longcope C. Androgenic and estrogenic metabolites in serum of mice fed dehydroepiandrosterone: relationship to antihyperglycemic effects. Metabolism. 1987 Sep;36(9):863–869. doi: 10.1016/0026-0495(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Lusis A. J., Chapman V. M., Wangenstein R. W., Paigen K. Trans-acting temporal locus within the beta-glucuronidase gene complex. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4398–4402. doi: 10.1073/pnas.80.14.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusis A. J., Tomino S., Paigen K. Isolation, characterization, and radioimmunoassay of murine egasyn, a protein stabilizing glucuronidase membrane binding. J Biol Chem. 1976 Dec 25;251(24):7753–7760. [PubMed] [Google Scholar]
- Meredith S. A., Ganschow R. E. Apparent trans control of murine beta-glucuronidase synthesis by a temporal genetic element. Genetics. 1978 Dec;90(4):725–734. doi: 10.1093/genetics/90.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. J., Paigen K. Genome organization and polymorphism of the murine beta-glucuronidase region. Genomics. 1988 Jan;2(1):25–31. doi: 10.1016/0888-7543(88)90105-x. [DOI] [PubMed] [Google Scholar]
- PAIGEN K. The effect of mutation on the intracellular location of beta-glucuronidase. Exp Cell Res. 1961 Nov;25:286–301. doi: 10.1016/0014-4827(61)90280-4. [DOI] [PubMed] [Google Scholar]
- Paigen K. Acid hydrolases as models of genetic control. Annu Rev Genet. 1979;13:417–466. doi: 10.1146/annurev.ge.13.120179.002221. [DOI] [PubMed] [Google Scholar]
- Palmer R., Gallagher P. M., Boyko W. L., Ganschow R. E. Genetic control of levels of murine kidney glucuronidase mRNA in response to androgen. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7596–7600. doi: 10.1073/pnas.80.24.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shows T. B., Chapman V. M., Ruddle F. H. Mitochondrial malate dehydrogenase and malic enzyme: Mendelian inherited electrophoretic variants in the mouse. Biochem Genet. 1970 Dec;4(6):707–718. doi: 10.1007/BF00486384. [DOI] [PubMed] [Google Scholar]
- Shull R. M., Hastings N. E., Selcer R. R., Jones J. B., Smith J. R., Cullen W. C., Constantopoulos G. Bone marrow transplantation in canine mucopolysaccharidosis I. Effects within the central nervous system. J Clin Invest. 1987 Feb;79(2):435–443. doi: 10.1172/JCI112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly W. S., Brot F. E., Glaser J., Stahl P. D., Quinton B. A., Rimoin D. L., McAlister W. H. Beta-glucuronidase deficiency mucopolysaccharidosis. Birth Defects Orig Artic Ser. 1974;10(12):239–245. [PubMed] [Google Scholar]
- Sly W. S., Quinton B. A., McAlister W. H., Rimoin D. L. Beta glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr. 1973 Feb;82(2):249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Paigen K., Ganschow R. E. Genetic control of glucuronidase induction in mice. J Mol Biol. 1973 Dec 5;81(2):225–243. doi: 10.1016/0022-2836(73)90191-5. [DOI] [PubMed] [Google Scholar]
- Tomino S., Paigen K., Tulsiani D. R., Touster O. Purification and chemical properities of mouse liver lysosomal (L form) beta-glucuronidase. J Biol Chem. 1975 Nov 10;250(21):8503–8509. [PubMed] [Google Scholar]
- Watson C. S., Catterall J. F. Genetic regulation of androgen-induced accumulation of mouse renal beta-glucuronidase messenger ribonucleic acid. Endocrinology. 1986 Mar;118(3):1081–1086. doi: 10.1210/endo-118-3-1081. [DOI] [PubMed] [Google Scholar]