Abstract
Gas transfer in the female lung varies over the menstrual cycle in parallel with the cyclic angiogenesis that occurs in the uterine endometrium. Given that vessels form and regress in the uterus under the control of hormones, angiogenic factors and pro-angiogenic circulating bone marrow-derived progenitor cells, we tested the possibility that variation in pulmonary gas transfer over the menstrual cycle is related to a systemic cyclic pro-angiogenic state that influences lung vascularity. Women were evaluated over the menstrual cycle with weekly measures of lung diffusing capacity and its components, the pulmonary vascular capillary bed and membrane diffusing capacity, and their relation to circulating CD34+CD133+ progenitor cells, hemoglobin, factors affecting hemoglobin binding affinity, and pro-angiogenic factors. Lung diffusing capacity varied over the menstrual cycle, reaching a nadir during the follicular phase following menses. The decline in lung diffusing capacity was accounted for by ~25% decrease in pulmonary capillary blood volume. In parallel, circulating CD34+CD133+ progenitor cells decreased by ~24%, and were directly related to angiogenic factors, and to lung diffusing capacity and pulmonary capillary blood volume. The finding of greater number of lung microvessels in ovariectomized female mice receiving estrogen as compared to placebo verified that pulmonary vascularity is influenced by hormonal changes. These findings suggest that angiogenesis in the lungs may participate in the cyclic changes in gas transfer that occur over the menstrual cycle.
Keywords: Gas transfer, endothelial progenitor cell, angiogenesis, menstrual cycle
Introduction
The first breath of the newborn marks the transition from fluid to air respiration. From that time on, the primary essential function of the respiratory system is gas exchange. Oxygen diffuses along a pressure gradient from the inhaled air across the surfaces of the terminal bronchioles, alveolar ducts and alveoli into the rich vascular network of capillaries, and upon entering the bloodstream readily crosses the erythrocyte membrane and binds to the ferrous heme of hemoglobin (10). Thus, gas transfer in the lung is modeled as a series of resistances: the molecular diffusion of the gas across the terminal bronchiolo-alveolar-capillary membranes, the diffusion within the blood, and the chemical reaction of the gas with hemoglobin. The Roughton and Forster (31) equation summarizes these concepts:
DL represents the total lung diffusing capacity; Dm the diffusing capacity of the membrane that separates the air from the blood in the terminal ventilatory unit; Vc the pulmonary capillary bed available for gas transfer; and θ is the rate of reaction of the gas with the red cell (31). Dm is a function of the diffusion coefficient of the gas in the alveolar membrane, the thickness and the area of the membrane, the solubility of the gas in the lung tissue and its molecular weight and is independent of lung blood volume in this model. The second element on the right side of the equation represents the diffusion of the gas within the blood and its reaction with hemoglobin; however, it can be simplified to θVc for gases such as carbon monoxide, because the rate at which the gas is removed from the plasma by combining to the hemoglobin molecule is faster relative to that at which it is removed by circulating blood. As such when measuring carbon monoxide lung diffusing capacity, the effect of cardiac output can be neglected.
During postnatal development, gas transfer capacity increases as body mass increases. The increase in gas transfer is attributed to an increase in alveoli number and size allowing the respiratory system to increase overall lung diffusing capacity to meet the rising metabolic demands of larger and more active tissues (38). Metabolic demands also increase as a normal physiologic process in women during reproduction (8), and gas transfer has been noted to vary cyclically in women during their reproductive years (33, 35). The mechanism of menses-related changes in gas transfer, which are typified by a maximal diffusing capacity for carbon monoxide (DLCO) just prior to menses that rapidly drops and reaches a nadir on the third day of menstruation, is not clear (33). Here, we hypothesized that the increase in gas transfer over the course of the menstrual cycle may be related to increase of the surface area of pulmonary capillaries available for gas exchange, Vc, through the process of new growth of vessels, which subsequently regress over the time of menstruation.
Angiogenesis is routine during the reproductive cycle, with vessels forming and regressing in the endometrium and ovaries during the menstrual cycle under the control of hormones and angiogenic factors, such as vascular endothelial growth factor (VEGF) (2, 24, 29). VEGF levels vary over the menstrual cycle with two peaks, around ovulation and during the luteal phase (2, 24). Although tissue resident endothelial cells participate in the new vessel formation, bone marrow-derived endothelial progenitor cells (EPC) also actively promote the formation of new blood vessels and the maintenance of vascular homeostasis (16, 17, 28). Mobilized EPC in the circulation are defined by expression of CD34, a common cell surface marker for hematopoietic stem cells and endothelial cells, and the co-expression of the stem cell marker CD133(4, 30). The strong association of increased circulating EPC numbers to neovascularization has led to the measure of circulating CD34+ CD133+ EPC as a sensitive and specific marker of new vessel formation (4, 20, 30). The contribution of EPC to vascular pathologic processes is increasingly recognized as a central component in pathogenesis, hence strategies aimed at modifying EPC are effective in regulating angiogenesis and therapies in acute myocardial infarction (6, 34) and cancer (12). The cyclic physiologic neovascularization in the reproductive organs of women, i.e corpus luteum and endometrium, has also recently been shown to be dependent upon bone marrow derived EPC (3). In this study, we investigated the changes in DLCO over the menstrual cycle by weekly measurements of the subdivisions Vc and Dm together with factors that might alter theta especially hemoglobin. To further investigate angiogenesis as a possible cause of the changes in Vc,, pro-angiogenic factors and circulating pro-angiogenic EPC were determined. To evaluate the concept of hormonal effects on lung microvessel density, murine lung vascularity was quantitated in ovariectomized mice treated with estrogen at levels comparable to those present in women during the luteal phase or with placebo.
Materials and Methods
Healthy, nonsmoking women with regular menstrual cycles were enrolled in the study. Volunteers were seen weekly for physiologic tests, including pulse, oxygen saturation, lung diffusion capacity, and for blood sampling. Healthy, nonsmoking men were enrolled as control subjects. This study was approved by the Institutional Review Board at the Cleveland Clinic and all subjects gave written informed consent.
Lung function and diffusing capacity for carbon monoxide
The forced expiratory volume at 1 second (FEV1) and vital capacity (FVC) were measured using an Eagle™ spirometer (InSpire Health Louisville, Colorado) (27). The single breath carbon monoxide diffusing capacity was performed weekly using Eagle™ equipment (InSpire Health Louisville, Colorado) with volunteers rested and in a seated position (23). On each visit, DLCO was measured at 2 different oxygen concentrations (21% and 42%). DLCO measurements were performed at the same time of the day on each visit to minimize the effect of diurnal variations. The single breath DLCO method was performed in duplicate, ~3 minutes apart using a washout volume of 750 ml and an alveolar volume of 750 ml. The breathhold time was ~10 seconds. DLCO was adjusted for hemoglobin (Hgb) using the Amercian Thoracic Society (ATS) guidelines (23). Exhaled O2 was obtained from the alveolar gas at each measurement of the diffusing capacity. Dm and Vc were calculated from DLCO measurements at inspired O2 concentrations 21% and 42% as described by Roughton and Forster (31). Theta was calculated from the formula described by Cotes (10, 32):
PcO2 represents the capillary partial pressure of oxygen and was determined to be nearly equivalent to the alveolar partial pressure of oxygen (PA O2 ) measured at end expiration (32):
End expiration PAO2 was determined from the direct measure of end expiration oxygen concentration, and the daily barometric pressure. Since measurements of Dm and Vc are best made when Hgb is fully saturated with oxygen, DLCO was measured over a range of inspired O2 concentrations 21%, 42%, 60% and 80% to confirm that 1/DLCO relative to FiO2 was linear (R2 = 0.97). This validated the use of inspired oxygen concentrations of 21% and 42% to determine Dm and Vc in the study.
Prior to DLCO determinations, single-breath on-line measurement of fractional NO concentration in expired breath (FENO) was also measured at each visit using the NIOX (Aerocrine, NY) (1).
Hemoglobin, carboxyhemoglobin, and 2,3-Diphosphoglycerate (2,3-DPG)
The NPT7 series (Radiometer, Copenhagen, Denmark) was used to measure hemoglobin (Hgb) and carboxyhemoglobin (COHgb) levels from whole blood samples collected in a heparin tube, using spectroscopy. 2,3-DPG levels were measured in whole blood samples collected in heparin tubes using a spectrophotometric assay of NADPH oxidation (Roche Diagnostics, Mannheim, Germany). The concentration of 2,3-DPG was expressed in μmol/gm hemoglobin.
Estrogen, VEGF and stem cell factor (SCF)
Estrogen was measured in serum samples by radioimmunoassay (Specialty Lab, CA). VEGF and SCF levels were measured in serum samples using quantikine ELISA kits (R & D system, MN).
Flow cytometry evaluation of EPC numbers and colony forming assay
1×106 mononuclear cells isolated from the peripheral blood were labeled with anti-human CD34-FITC (Becton Dickinson, NJ) and anti-human CD133-PE (Miltenyi Biotec, Auburn, CA) monoclonal antibodies to quantify the CD34+CD133+ progenitor cells. To control for nonspecific antibody binding, isotype matched irrelevant antibodies were used. Following incubation, cell suspensions were washed with PBS/1%BSA/0.02%sodium azide and suspended in FACS flow (Becton Dickinson, NJ). The FACScan flow cytometer (Becton Dickinson, NJ) was used to count 0.5×106 events. Data was analyzed using Cell-Quest 3.3 Software (Becton Dickinson, NJ). Endothelial nature of EPC were confirmed by colony formation as described by Hill et al. (15)
Animal studies
Female FVB/N wild-type mice (4 weeks old) (Jackson Laboratory, Bar Harbor, Maine) were anesthetized by intraperitoneal injection of 150mg ketamine/kg body weight and ovariectomized followed by subcutaneous implantation of 1.7 mg 17β-estradiol pellets (Innovative Research of America) into the back. Control mice received placebo pellets. Circulating 17β-estradiol levels were measured at days 7 and 14 with a commercially available enzyme-linked immunoassay (estradiol ELISA, Cayman Chemical Co, Inc). After 2 weeks, the animals were euthanized. The lungs were insufflated by injecting 10ml saline via the trachea. Then 5ml of 2% paraformaldehyde was injected via the trachea to rapidly fix the lungs. The pulmonary arteries and veins were banded and the lungs were harvested and placed in 2% paraformaldehyde. Subsequently, the lungs were placed into 10% buffered formalin for 48 hours. All procedures were performed in accordance with St Elizabeth’s Medical Center Animal Care and Use Committee.
To analyze the lung microvessel density, 5μm sections of paraffin embedded tissues were stained with polyclonal rabbit anti-human Von Willebrand Factor, which cross-reacts with the mouse antigen (Dako Cytomation, Glostrup, Denmark). Samples were pre-treated with proteinase K for 4 min and staining was visualized by using diaminobenzidine and hydrogen peroxidase. All samples were stained by using an automated biotin-avidin peroxidase system (Ventana-320-ES; Ventana Medical Systems, Tucson AZ). Secondary antibody alone was used as negative control. Sections were counterstained with hematoxylin. To quantify the microvessel density, pictures were taken from 5 random fields at a final magnification of 400x, using a Leica DMR light microscope, equipped with a Retiga EX digital camera and by using Q imaging software. The number of blood vessels was counted on each picture and the sum of 5 fields was calculated for each mouse. Quantification of the lung blood vessel density was performed in blinded fashion and the results were confirmed in a second blinded analysis. Alveolus areas were calculated using Image-Pro Plus v. 5.1 software in each image. The software calibrated each image for the specific microscope and objective used. The alveolar radius was derived from the area using the formula: radius (r) = √ (area/Π). And the volume of the alveolus was calculated with the formula:
Statistical Analysis
The study data consisted of multiple observations for each subject, and it was assumed that observations of a variable within a subject might be positively correlated. Therefore, a method accounting for this correlation was used to provide estimates of mean values for study variables, and to be the basis for measures of association within subjects and for comparisons of weeks or phases. Linear mixed models with random subject intercepts satisfied these requirements. Associations between subjects were studied by first obtaining averages of variables over each patient’s set of observations, resulting in only a single value of each variable per patient. Then Spearman correlations were used to estimate the correlations of the average patient values. Spearman correlations were also estimated based on the entire set of observations in the data, though the associations estimated using the data in this manner represent a combination of between- and within-subject relationships. The linear mixed model analyses and computation of associated estimates and P-values were performed using SAS PROC MIXED in version 9.1 (SAS Institute, Cary, NC). Spearman correlations and associated P-values were calculated using R version 1.9 (www.R-project.org). All data are presented as means ± SEM. For the mice data, the Wilcoxon test was used to calculate the P-values using JMP 5.0.1 (SAS Institute, Cary, NC).
Results
Ten healthy, non smoking women (age 31 ± 1) were enrolled in the study. They were on no medications except four of the ten were on hormonal contraceptives. Hormonal contraceptives were permitted, as previous studies have shown that changes in DLCO were detectable in menstruating women, whether or not on hormonal contraceptives (33). Four healthy, non smoking men (age 45 ± 2) with no medical history, on no medications were also enrolled as gender discordant controls. All volunteers were longitudinally assessed over 4 weeks with one visit each week. Four women were followed for 7 weeks with duplicate measurements. The menstrual cycle was divided into weeks. Week 1 was defined by the first day of menstruation. Menstrual cycles varied from 28 to 31 days in length.
Lung Function and Physiology in Men and Women
DLCO, alveolar volume (VA) and Hgb were lower in women than men (Table 1). However, O2 saturation was higher in women compared to men (p=0.0006). Table 1 summarizes the mean values of men and women.
Table 1.
Population of Men and Women
| Variable | Women N = 10 |
Men N = 4 |
p-value |
|---|---|---|---|
| Age | 31 ± 1 | 45 ± 2 | 0.002 |
| Weight (kg) | 65 ± 1 | 87 ± 3 | <0.0001 |
| O2 saturation (%) | 98.7 ± 0.1 | 97.6 ± 0.3 | 0.0006 |
| Respiratory rate (breath/min) |
11.7 ± 0.2 | 12.9 ±0.4 | 0.008 |
| DLCO (ml/min/mmHg) | 24.0 ± 0.4 | 30.4 ± 0.8 | <0.0001 |
| VA (L) | 5.5 ± 0.1 | 6.6 ± 0.1 | <0.0001 |
| Dm (ml/min/mmHg) | 62 ± 6 | 58 ± 4 | 0.6 |
| Vc (ml) | 55 ± 3 | 71 ± 6 | 0.01 |
| FVC (L) | 3.97 ± 0.06 | 4.58 ± 0.08 | <0.0001 |
| FEV1 (L) | 3.28 ± 0.05 | 3.75 ± 0.06 | <0.0001 |
| NO (ppb) | 14.7 ±1.1 | 17.0 ± 1.9 | 0.3 |
| Hgb (gm/dl) | 13.5 ± 0.2 | 15.7 ± 0.3 | <0.0001 |
Lung Diffusing Capacity over the Menstrual Cycle
DLCO in women decreased by 10% between week 1 and week 2 of the menstrual cycle (p = 0.013). The values subsequently increased over weeks 3 – 4 (Figure 1). Men had no significant variation in DLCO among consecutive weeks (p = 0.72). For control of reproducibility of measures, the within day variability was determined in subjects. As per ATS recommendations, DLCO measurements were made during the same time of day to eliminate diurnal variation. The same day variability per subject was less than 5% in all individuals. Repetitive measurements were performed over 6 different days in one volunteer. The maximal within day variability over 6 days was 5.4%. Duplicate measures performed in 4 women over 7 weeks also provided a measure of variability within week of the menstrual cycle. The maximal mean variability of the duplicate measurements of the 4 women followed over 7 weeks was 4.3% [% variability of DLCO: week 1, 0.5 ± 0.4; week 2, 4.3 ± 1.5; week 3, 0.5 ± 0.3; week 4, 2.3 ± 2.2].
Figure 1.
Coordinate changes in gas transfer (DLCO), pulmonary vascular capillary bed (Vc), endothelial progenitor cells (EPC)(CD34+CD133+) and stem cells factor (SCF) over the menstrual cycle. No changes occur in men over the same time period (not shown).
Hgb concentration varied slightly over the menstrual period, increasing from week 1 to week 4 by 2.5% (p=0.032) [Hgb (gm/dl): follicular phase 13.3 ± 0.2; luteal phase 13.5 ± 0.2; p=0.03. Week 1, 13.2 ± 0.3; week 2, 13.4 ± 0.3; week 3, 13.6 ± 0.3; week 4, 13.6 ± 0.3; p=0.18]. Change in DLCO was not associated with the change in Hgb, e.g. there was no change in Hgb from week 1 to week 2 (p=0.8), the time period when DLCO was decreasing. Similarly, COHgb, which could affect DLCO measurement, did not change significantly between week 1 and week 2 (p=0.37). In addition, VA was unchanged over the menstrual cycle in women. The affinity of carbon monoxide towards Hgb is high and its plasma solubility is low, hence cardiac output effect on DLCO is trivial and not taken into consideration as a significant determinant (10). Nevertheless, pulse rate was measured as an indicator of cardiac effects, and as in previous studies (9, 22), showed no significant change over the menstrual cycle (p=0.88).
In parallel to the change in DLCO, Vc decreased by 25% between week 1 and week 2 (p=0.046) (Figure 1). In contrast, Dm did not change over the menstrual cycle (p=0.87). Similarly, θ did not change overall (p=0.79). There was no change in 2,3 DPG (mean 16 ± 1 μmol/gm hemoglobin; p=0.25) or exhaled NO over the menstrual cycle (p = 0.62). To evaluate a potential effect of contraceptives, the subgroup of women on oral contraceptives was compared to those not on contraceptives. Estrogen varied as expected over the menstrual cycle with the lowest value at week 1 (200 pg/ml), and was lower overall in the follicular phase compared to the luteal phase [Estrogen (pg/ml): follicular phase 266 ± 32; luteal phase 361 ± 33; p=0.037] Estrogen was lower in women on birth control during week 4 (estrogen pg/ml: 185 ± 170 vs 455 ± 129; p = 0.006 ). Nevertheless, DLCO of women on birth control behaved similarly to those not on birth control (all comparisons p> 0.5), while Vc in week 2 of those women not on birth control tended to be lower than those on birth control (44 ± 4 vs 59 ± 5; p = 0.04).
Circulating EPC over the Menstrual Cycle
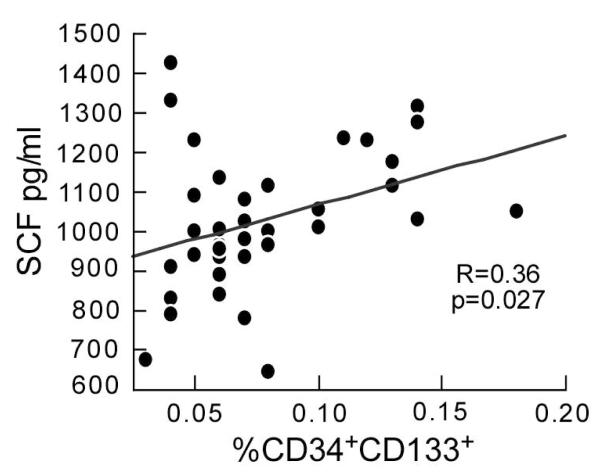
The CD34+CD133+ EPC in the circulation varied over the menstrual cycle, also decreasing by ~24% over the time of menses from week 1 to week 2 (p=0.021) in parallel with the drop in Vc and DLCO (Figure 1). Unlike previous study (2, 24), the changes in VEGF in this study did not achieve significance at the times studied over the menstrual cycle; however, SCF showed variability (p=0.041) with the lowest values in weeks 2 and 3 (figure 1). The fact that VEGF did not reach statistical significance for variation during the menstrual cycle in this study may be secondary to the timing and definition of the weeks of the cycle. In previous studies (2, 24), the phases were defined by the luteinizing hormone surge at midcycle, and by serial vaginal ultrasounds to evaluate changes in vascularity and track dominant follicle growth. Consistent with a coordinate role in regulating endothelial precursors, SCF correlated with CD34+CD133+ cells, with greater numbers of CD34+CD133+ cells associated with higher levels of SCF within volunteers (p=0.038) and across all volunteers (R=0.36, p=0.026) (Figure 2).
Figure 2.
Association of stem cell factor (SCF) to circulating CD34+CD133+ EPC. The points represent all measurements obtained for women over the study period.
Correlation of Lung Gas Transfer and EPC
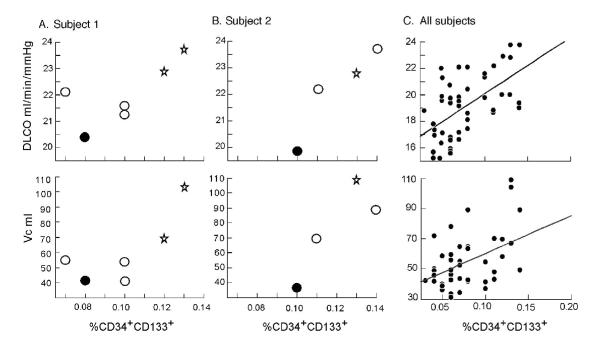
DLCO at 21% O2 or at 42% O2 was related to CD34+CD133+ cells in peripheral blood when all measurements were taken into account (R=0.47, p=0.001 and R=0.56, p<0.001 respectively) (Figure 3). This correlation held within volunteers for DLCO measured at 42% O2 (p=0.023) (Figure 3) but did not reach statistical significance for DLCO at 21% O2 (p=0.24). The membrane diffusing capacity Dm did not correlate with CD34+CD133+ cells (p=0.91); however, Vc was directly related to CD34+CD133+ cell numbers for all measurements (R=0.36, p=0.01) and within volunteers (p=0.013) (Figure 3).
Figure 3.
Relation of DLCO measured at 42% O2 and Vc to circulating CD34+CD133+ EPC. Values from two representative women over the study period are shown [A studied over 6 weeks and B over 4 weeks]. Each point was measured at a different week of the menstrual cycle, with the first week starting at the first day of menses. Stars represent menses, black circles the follicular phase and white ones the luteal phase. [C] Relation of DLCO measured at 42% and Vc to CD34+CD133+ EPC for all women over the menstrual cycle.
Vasculogenesis in the Lungs of Mice with Inductive Ovulation
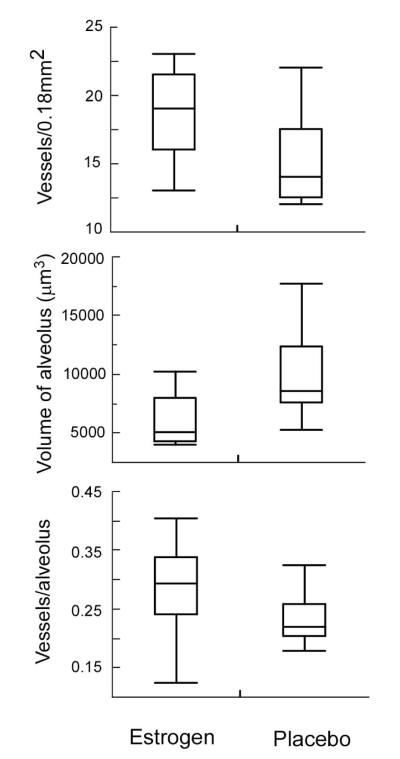
To directly investigate changes in lung vasculature under the influence of estrogen in vivo, ovariectomized mice were implanted with either estrogen or placebo pellets. The estradiol pellets ensured continuous release of estrogen at a dose comparable to the upper physiologic range found in non-pregnant pre-menopausal humans during the midcycle to the luteal phase of the normal menstrual cycle, but levels which are ~3-fold higher than those in the non-pregnant mouse (19, 41). The number of microvessels per lung field was greater in the estrogen-treated mice than control ovariectomized mice after 2 weeks of estrogen [number of microvessels/lung field (0.18 mm2): estrogen-treated 18 ± 1; placebo group 15 ± 1; p=0.037]. Interestingly, alveolar size and volume were smaller in the estrogen-treated mice as compared to the placebo control [average area of alveolus (μm2): estrogen-treated group 394 ± 32; placebo group 554 ± 45; p=0.015; average volume of alveolus (μm3): estrogen-treated group 6000 ± 748; placebo group 9990 ± 1244; p=0.015] (Figures 4, 5). Thus, mice receiving estrogen had greater numbers of vessels and smaller alveoli, suggesting a potential better matching of ventilation to perfusion (26). Furthermore, smaller size of alveoli with associated rich networks of capillaries (Figure 4B) predicts that surface area available for gas transfer is greater in estrogen exposed mice. In fact, the ratio of number of microvessels to number of alveoli per high power field trended to be greater in the estrogen-exposed mice as compared to the control mice [number of vessels/alveolus: estrogen-treated group 0.28 ± 0.08; placebo group 0.23 ± 0.04; p=0.047] (Figure 5).
Figure 4.
Lung tissues from ovariectomized female mice exposed to estrogen [B, D, E] or placebo [A, C] for 2 weeks. [A, B] Lungs of estrogen-exposed mice have greater numbers of vessels as identified by endothelial cells that are positive (brown-staining) for vonWillebrand factor, but smaller alveoli than control ovariectomized female mice. Arrows identify microvessels. Magnification 400x.
[C, D] The alveolar capillary unit from the lungs of ovariectomized mice exposed to estrogen compared to placebo. Immunopositivity for vonWillebrand factor identifies endothelial cells. The “a” identifies alveolar spaces, open arrowheads for capillary lining and black arrowheads for red blood cells. Magnification 1000x.
[E] Capillaries containing erythrocytes are identified as they traverse through alveolar walls. Magnification 1500x.
Figure 5.
Lung histology quantification of vessels and alveoli in mice by treatment groups. Estrogen exposed mice have greater numbers of microvessels and smaller alveoli than mice exposed to placebo. There is a trend towards more vessels per alveolus in estrogen treated mice.
Discussion
This study details the unique characteristics of gas transfer in women, and provides evidence that gas transfer varies over the menstrual cycle due to cyclic changes in the pulmonary vascular capillary bed that are linked to circulating pro-angiogenic endothelial progenitor cells.
Here, the changes in gas transfer over the menstrual cycle are similar to previous findings (33). Analysis of the components of gas transfer reveals that the change in gas transfer is due to change in the pulmonary capillary vascular bed, and not due to alterations in membrane diffusion or hemoglobin affinity. Seaton also identified an increase in gas transfer in the luteal phase, which was attributed to changes in the pulmonary vascular bed, but not blood volume (35). An increase in blood and/or plasma volume is also unlikely to have caused the observed changes in the present study, given minimal change in hemoglobin. Furthermore, estrogen causes plasma volume expansion during the pre-ovulatory phase of the menstrual cycle (37). Thus, the pulmonary vascular bed would have been expected to increase from week 1 to week 2, rather than decrease. Another possible explanation for the change in vascular bed could be pulmonary vasodilatation under the influence of NO. However unlike previous findings (21), exhaled NO did not vary over the menstrual cycle in this study. Even so, the changes in exhaled NO observed in a previous study predicts NO-related vasodilation at week 2 as compared to week 1, which is in opposition to our findings.
The observed change in the physiologic pulmonary vascular capillary bed in women over the menstrual cycle may be related to new vessel formation in the lungs based upon two major findings. First, a greater number of pulmonary microvessels were present in the lungs of mice after receiving two weeks of estrogen at levels comparable to those present during the luteal phase of menstruating women. This provides support for the hypothesis that estrogen can modify vessel density in the lung and that vascular changes can occur over a period of time that is relevant to the female human menstrual cycle. In fact, a recent study showed that EPC migration and proliferation are regulated by estrogen through estrogen receptors and phosphatidylinositol 3-kinase pathways (41). Unexpectedly, a smaller size of alveoli was also found in the estrogen-treated mice. This situation predicts a more efficient matching of ventilation to blood perfusion (26, 39), with a greater total surface area available for gas transfer per unit alveolar volume in the mice exposed to estrogen. Second, changes in circulating EPC numbers and angiogenic factor SCF, and their strong association to gas transfer and the physiologic pulmonary capillary vascular bed capacity, provide support for angiogenesis in the lung over the menstrual cycle. Current concepts suggest that neovascularization in the adult involves bone marrow derived stem cells and local pulmonary stem cells. Whether or not bone marrow derived endothelial progenitors directly incorporate into vessels or if they provide factors to support expansion of tissue-resident endothelial cells for angiogenesis is still not resolved. Furthermore, while endothelial cells and neovascularization may be derived from resident endothelial stem cells, recent study suggests that epithelial to mesenchymal cell transformation may also give rise to endothelial cells for new vessels formation (36). Regardless of the source of the stem cells giving rise to the new vessels, circulating EPC are clearly related to new blood vessel formation in tissues (3, 4, 6, 12, 20, 30, 34). Hence, the direct correlation of circulating EPC to the physiologic pulmonary vascular capillary bed capacity is also supportive of cyclic neovascularization in the female lung, perhaps through release of pro-angiogenic factors by EPC (18, 40).
Abundant evidence supports that new pulmonary vessels can form and regress rapidly, and well within the time frame of the menstrual cycle changes observed in this study, under the direction of pro-angiogenic factors, such as VEGF and SCF, and that EPC are associated with the process (11, 25). For example, a model for the formation and regression of vessels in the airways over 28 days has been studied by Baluk et al. in lungs of mice (7). New blood vessels in mouse trachea form by sprouting angiogenesis under the influence of VEGF expression in the airway. Endothelial sprouts are seen as soon as one day after VEGF expression and maximum vessel density is reached within 7 days and are maintained as long as VEGF is expressed. Upon VEGF withdrawal, vessels regress within 3 days. In a recent study, expression of new capillary vessels in the lung microvasculature occurred within 12 – 24 hours of mobilization of EPC from the bone marrow into the blood, which predicts that new capillary vessels in the lung, and hence Vc and DLCO, should be temporally closely associated to EPC numbers in the blood (5). In multiple model systems, microscopic imaging techniques reveal that basement membrane sleeves and pericytes are left behind after endothelial cell degeneration and provide the scaffold for microvascular regrowth that occurs as rapidly as one week after VEGF is again made available (11, 25). Empty basement membrane sleeves remain for as long as 21 days after vessel regression (25), and so would be available to facilitate vascular restoration over the time period of the menstrual cycle within which changes in estrogen and pro-angiogenic factors occur. The plasticity of tumor vasculature by these processes is evident (13, 14), and a similar process may subserve a physiologic role in the lung for the preparation of women for the greater metabolic needs of childbearing. It is important to note that although a significant association among DLCO, Vc, estrogen and CD34+CD133+ progenitor cells was found in this study, this does not necessarily imply causality. In particular other possible mechanisms that may account for the changes in gas transfer, such as recruitment, vasodilation and/or vasoconstriction of existing pulmonary vessels, were not excluded. Further study of the physiologic processes that allow cyclic peaks of gas transfer in women is warranted, given the potential for application to regenerative strategies to augment oxygen uptake in patients with advanced lung diseases.
Acknowledgements
We thank J. Hammel for his help in the statistical analysis and J.Sharp and M.Baaklini for study coordination, and Edmond Chu Ph.D, Norman Thomas, Carl Keefer and Larry Murdock R.R.T of Inspire Health for their technical assistance.
Grants The study was supported by NIH grants HL60917 and HL081064.
Footnotes
Disclosures All authors participated in the study and have seen and approved the final version. None of the authors have any conflict of interest to declare.
References
- 1.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal R, Conway GS, Sladkevicius P, Payne NN, Bekir J, Campbell S, Tan SL, Jacobs HS. Serum vascular endothelial growth factor (VEGF) in the normal menstrual cycle: association with changes in ovarian and uterine Doppler blood flow. Clin Endocrinol (Oxf) 1999;50:101–106. doi: 10.1046/j.1365-2265.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 5.Asosingh K, Swaidani S, Aronica M, Erzurum SC. Th1- and Th2-dependent endothelial progenitor cell recruitment and angiogenic switch in asthma. J Immunol. 2007;178:6482–6494. doi: 10.4049/jimmunol.178.10.6482. [DOI] [PubMed] [Google Scholar]
- 6.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 7.Baluk P, Lee CG, Link H, Ator E, Haskell A, Elias JA, McDonald DM. Regulated angiogenesis and vascular regression in mice overexpressing vascular endothelial growth factor in airways. Am J Pathol. 2004;165:1071–1085. doi: 10.1016/S0002-9440(10)63369-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butte NF, King JC. Energy requirements during pregnancy and lactation. Public Health Nutr. 2005;8:1010–1027. doi: 10.1079/phn2005793. [DOI] [PubMed] [Google Scholar]
- 9.Chapman AB, Zamudio S, Woodmansee W, Merouani A, Osorio F, Johnson A, Moore LG, Dahms T, Coffin C, Abraham WT, Schrier RW. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J Physiol. 1997;273:F777–782. doi: 10.1152/ajprenal.1997.273.5.F777. [DOI] [PubMed] [Google Scholar]
- 10.Cotes J. Lung function. Blackwell Scientific Publications; Oxford: 1993. [Google Scholar]
- 11.Ezaki T, Baluk P, Thurston G, La Barbara A, Woo C, McDonald DM. Time course of endothelial cell proliferation and microvascular remodeling in chronic inflammation. Am J Pathol. 2001;158:2043–2055. doi: 10.1016/S0002-9440(10)64676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 14.Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–651. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- 15.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 16.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: isolation and characterization. Trends Cardiovasc Med. 2003;13:201–206. doi: 10.1016/s1050-1738(03)00077-x. [DOI] [PubMed] [Google Scholar]
- 17.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–1189. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 18.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 19.Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108:3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 20.Iwami Y, Masuda H, Asahara T. Endothelial progenitor cells: past, state of the art, and future. J Cell Mol Med. 2004;8:488–497. doi: 10.1111/j.1582-4934.2004.tb00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kharitonov SA, Logan-Sinclair RB, Busset CM, Shinebourne EA. Peak expiratory nitric oxide differences in men and women: relation to the menstrual cycle. Br Heart J. 1994;72:243–245. doi: 10.1136/hrt.72.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Littler WA, Bojorges-Bueno R, Banks J. Cardiovascular dynamics in women during the menstrual cycle and oral contraceptive therapy. Thorax. 1974;29:567–570. doi: 10.1136/thx.29.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 24.Malamitsi-Puchner A, Sarandakou A, Tziotis J, Stavreus-Evers A, Tzonou A, Landgren BM. Circulating angiogenic factors during periovulation and the luteal phase of normal menstrual cycles. Fertil Steril. 2004;81:1322–1327. doi: 10.1016/j.fertnstert.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 25.Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, Shalinsky DR, Hu-Lowe DD, McDonald DM. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massaro GD, Mortola JP, Massaro D. Sexual dimorphism in the architecture of the lung’s gas-exchange region. Proc Natl Acad Sci U S A. 1995;92:1105–1107. doi: 10.1073/pnas.92.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 28.Murasawa S, Asahara T. Endothelial progenitor cells for vasculogenesis. Physiology (Bethesda) 2005;20:36–42. doi: 10.1152/physiol.00033.2004. [DOI] [PubMed] [Google Scholar]
- 29.Nardo LG. Vascular endothelial growth factor expression in the endometrium during the menstrual cycle, implantation window and early pregnancy. Curr Opin Obstet Gynecol. 2005;17:419–423. doi: 10.1097/01.gco.0000175362.12470.e0. [DOI] [PubMed] [Google Scholar]
- 30.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 31.Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 1957;11:290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- 32.Roughton FJ, Forster RE, Cander L. Rate at which carbon monoxide replaces oxygen from combination with human hemoglobin in solution and in the red cell. J Appl Physiol. 1957;11:269–276. doi: 10.1152/jappl.1957.11.2.269. [DOI] [PubMed] [Google Scholar]
- 33.Sansores RH, Abboud RT, Kennell C, Haynes N. The effect of menstruation on the pulmonary carbon monoxide diffusing capacity. Am J Respir Crit Care Med. 1995;152:381–384. doi: 10.1164/ajrccm.152.1.7599851. [DOI] [PubMed] [Google Scholar]
- 34.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 35.Seaton A. Pulmonary capillary blood volume in women: normal values and the effect of oral contraceptives. Thorax. 1972;27:75–79. doi: 10.1136/thx.27.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 37.Stachenfeld NS, Taylor HS. Effects of estrogen and progesterone administration on extracellular fluid. J Appl Physiol. 2004;96:1011–1018. doi: 10.1152/japplphysiol.01032.2003. [DOI] [PubMed] [Google Scholar]
- 38.Thurlbeck WM. Postnatal growth and development of the lung. Am Rev Respir Dis. 1975;111:803–844. doi: 10.1164/arrd.1975.111.6.803. [DOI] [PubMed] [Google Scholar]
- 39.Torbati D, Ramirez J, Hon E, Camacho MT, Sussmane JB, Raszynski A, Wolfsdorf J. Experimental critical care in rats: gender differences in anesthesia, ventilation, and gas exchange. Crit Care Med. 1999;27:1878–1884. doi: 10.1097/00003246-199909000-00028. [DOI] [PubMed] [Google Scholar]
- 40.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Zhao X, Huang L, Yin Y, Fang Y, Zhao J, Chen J. Estrogen induces endothelial progenitor cells proliferation and migration by estrogen receptors and PI3K-dependent pathways. Microvasc Res. 2007 doi: 10.1016/j.mvr.2007.02.009. [DOI] [PubMed] [Google Scholar]