Abstract
Members of the epidermal growth factor (EGF)-family bind to ErbB (EGFR)-family receptors which play an important role in the regulation of various fundamental cell processes including cell proliferation and differentiation. The normal rodent kidney has been shown to express at least three members of the ErbB receptor family and is a major site of EGF ligand synthesis. Polycystic kidney disease (PKD) is a group of diseases caused by mutations in single genes and is characterized by enlarged kidneys due to the formation of multiple cysts in both kidneys. Tubule cells proliferate, causing segmental dilation, in association with the abnormal deposition of several proteins. One of the first abnormalities described in cell biological studies of PKD pathogenesis was the abnormal mislocalization of the EGFR in cyst lining epithelial cells. The kidney collecting duct (CD) is predominantly an absorptive epithelium where electrogenic Na+ entry is mediated by the epithelial Na+ channel (ENaC). ENaC-mediated sodium absorption represents an important ion transport pathway in the CD that might be involved in the development of PKD. A role for EGF in the regulation of ENaC-mediated sodium absorption has been proposed. However, several investigations have reported contradictory results indicating opposite effects of EGF and its related factors on ENaC activity and sodium transport. Recent advances in understanding how proteins in the EGF-family regulate the proliferation and sodium transport in normal and PKD epithelial cells are discussed here.
Keywords: Polycystic kidney disease, ADPKD, ARPKD, ENaC, epidermal growth factor, ErbB receptor, ErbB2
1. Introduction
Polycystic kidney disease (PKD) is a common genetic disease that is associated with a high morbidity and mortality [1–6]. Autosomal dominant (ADPKD) and autosomal recessive (ARPKD) polycystic kidney diseases are characterized by the progressive growth and expansion of cysts or dilatation of collecting ducts (CD), respectively, that ultimately destroy the normal renal parenchyma. ADPKD is one of the most common monogenic diseases and in 50% of cases results in end-stage renal disease (ESRD) [4, 7]. ARPKD is a rare genetic disorder and typically presents in utero or during the neonatal period. ADPKD is caused by mutation in PKD1 or PKD2 genes. Mutation in the PKHD1 gene is responsible for ARPKD [4, 8]. At first glance, it seems that the three proteins polycystin-1 (PC-1), polycystin-2 (PC-2) (encoded by PKD1 or PKD2, respectively), and fibrocystin (encoded by PKHD1) are involved in cyst formation and might be regulated by similar mechanisms. There are, however, important differences that suggest that regulation of ADPKD and ARPKD are more complicated than anticipated [9]. In ADPKD, cysts, which can arise from any tubular segment, sprout from the nephron and no longer communicate with the tubule from which they originate. The majority of cysts disconnected from tubular structures still accumulate fluid within the lumen [6, 10]. In contrast, cysts in ARPKD are dilated CDs that remain contiguous with the remaining nephron, allowing urine to continue to flow through the collecting system [6, 11].
The CD is a final site of renal regulation of Na+ and water balance. Within the CD, Na+ diffuses from the lumen into principal cells through the epithelial Na+ channel (ENaC) and is extruded at the basolateral membrane in exchange for uptake of K+ by the Na/K-ATPase. Cellular proliferation and fluid secretion can be accelerated by growth factors such as epidermal growth factor (EGF). EGF is a small mitogenic protein that is thought to be involved in mechanisms such as normal cell growth. ErbB receptors have been recognized as targets in anticancer therapy and are now used in the treatment of breast and colon malignancies. Other than tumor biology, EGF signaling is critically involved in renal electrolyte homeostasis [12]. Moreover, EGF plays an important role in the expansion of renal cysts. Cyst growth is the result of proliferation of incompletely differentiated epithelial cells and accumulation of fluid within the cysts. Epithelial cells from cysts from patients with both ADPKD and ARPKD are unusually susceptible to the proliferative stimulus of EGF [6]. Moreover, cyst fluids from these patients contain mitogenic concentrations of EGF, and this EGF is secreted into the lumens of cysts in amounts that can induce cell proliferation [13]. It was first shown that EGF is an important mediator of the proliferative abnormality seen in cyst formation in human ADPKD and that this involves a mislocalization of the EGFR to the apical epithelial cell membranes [13]. Subsequent studies found this to be a common feature of PKD, being observed not only in human ADPKD and ARPKD, but also in a variety of spontaneously occurring mouse models of ARPKD, as well as mouse models derived by targeted mutations of genes responsible for ADPKD. Here we discuss recent results supporting functional interactions between EGF-family proteins, proliferation and sodium transport in normal and PKD cells, as well as detailing the molecular mechanisms underpinning such interactions and discuss the biological consequences of these regulations. Hence we aim to provide critical insight into the physiological control of cell proliferation and ion channel function by EGF and its related growth factors.
2. ErbB family
The epidermal growth factor receptor (EGFR) family, also known as ErbB receptors, consists of four transmembrane receptors belonging to the receptor tyrosine kinase (RTK) superfamily and includes EGFR (ErbB1/HER-1), ErbB2 (neu/HER-2), ErbB3 (HER-3), and ErbB4 (HER-4) [14]. The gene symbol for EGFRs, ErbB, originates from the name of a viral oncogene to which these receptors are homologous: Erythroblastic Leukemia Viral Oncogene. EGFR was the first discovered RTK [15]. Since then, EGFR (ErbB1/HER-1) is the best characterized and best known ErbB receptor. Most of the principles and paradigms that underlie the action of RTK were first established for the EGFR [14, 16]. In the following discussion, we use the term “ErbB receptors” for the whole ErbB-family and the term “EGFR” in description of experiments where only ErbB1 (EGFR/Her-1) is examined.
EGFR as well as other ErbB receptors is a transmembrane receptor consisting of an extracellular ligand-binding domain with conserved two cysteine-rich domains necessary for ligand binding; a single membrane-spanning domain which has a passive role in signaling and functions as an “anchor” of receptor in plasma membrane [17] and a cytoplasmic protein tyrosine kinase domain, where six tyrosine autophosphorylation sites are located. After ligand binding, ErbB receptors dimerize, which is a required critical step for intrinsic RTK to be activated and specific tyrosine-containing residues become autophosphorylated [18]. Phosphorylated ErbB receptors can recruit different adapter proteins with Src homology domain-2 (SH2) or phosphotyrosine binding domains (PTB) [19]. Signals from dimerized, activated ErbB receptors lead to activation of multiple intracellular signal transduction pathways including cell growth, proliferation, survival/decreased apoptosis, migration etc. Depending on the pairing of ErbB family receptors (homodimers or heterodimers) there can be downstream stimulation of different combinations of signaling pathways [20].
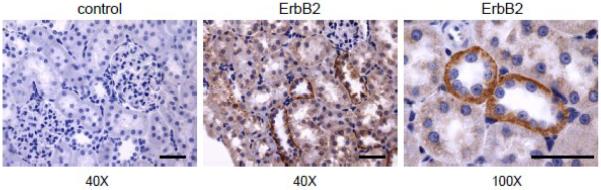
Although EGFR is the predominant ErbB receptor expressed in the normal adult mammalian kidney tubule, ErbB2 and ErbB4 have also been detected in developing ureteric buds [21, 22]. However, major roles for ErbB2, ErbB3 and ErbB4 in neural and cardiac development are demonstrated in null mice by embryonic lethality at embryonic day (E)10 prior to the onset of metanephric development [21]. We have shown recently that all ErbB receptors are expressed at least at low levels in cultured principal mpkCCDc14 cells. However, ErbB2 is the most predominant member of the ErbB family in these cells [23]. Both ErbB2 and ErbB4 have been detected in adult human and rat CDs, respectively [24]. We have recently also detected ErbB2 in adult rat CDs. Fig. 1 shows representative immunohistochemistry staining for ErbB2 at 40× and 100× magnifications in a cortical section of Sprague-Dawley (SD) rat kidneys. Immunohistochemistry analysis was performed as described previously [23, 25]. A negative control (left) (stained with secondary antibodies in the absence of primary antibodies) is also shown. Additional controls (without primary and secondary antibodies) were also negative (data not shown). As clearly seen in Fig. 1, ErbB2 is highly expressed in the CD of SD rat kidney and is localized mostly at the basolateral surface.
Fig. 1. ErbB2 expression in rat kidney tissue.
Representative immunohistochemical staining for ErbB2 detection in kidney cortical sections of Sprague-Dawley rat. Original magnifications are 40× (b) and 100× (c). Scale bars are 50 μm. Negative control (a) (stained with secondary antibodies in the absence of primary antibodies) at 40× is also shown. The kidney was fixed for 24 hours in Zinc Formalin and processed for paraffin embedding as described previously [23, 25]. The kidney sections were cut, dried and deparaffinized for subsequent labeled streptavidin-biotin immunohistochemistry. Tissue sections were incubated for 90 minutes in a 1:250 concentration of anti-ErbB2 (Cat. #ab2428; Abcam Inc., Cambridge, MA).
ErbB receptors are predominantly located at the basolateral surface of normal adult polarized tubule epithelial cells, but on the apical surfaces of fetal ureteric bud-derived collecting ducts [187, 188,191]. ErbB ligands have autocrine properties, which may explain preferential co-localisation of the ligands and their receptor at the basolateral surface. Development of epithelial cell polarity requires a well orchestrated, domain-specific segregation of lipids, membrane proteins, and cytoskeleton. Intracellular transport to the plasma membrane is regulated in part by intrinsic signals recognized at different steps in polar sorting pathways. A number of polar sorting determinants have now been identified in membrane proteins, and principles for both sequence and structural requirements are beginning to emerge. For example, a 23-amino acid segment located near the cytoplasmic face of the membrane spanning domain (residues Lys-652 to Ala-674) is necessary and sufficient for targeting EGFR from the trans-Golgi network directly to the basolateral plasma membrane [26, 27]. ErbB receptor localization and regulation will be discussed further below.
3. EGF and its related growth factors
Regulation of ErbB receptor function is controlled by their ligands, members of the EGF-related peptide growth factor family [28]. All EGF-family members, like many other growth factors, are derived from membrane-bound precursor proteins [29, 30]. There are over 12 ligands that can bind ErbBs and induce dimerization of distinct functional receptor pairs. EGF, transforming growth factor-α (TGFα), and amphiregulin are specific for EGFR and primarily induce the formation of EGFR/EGFR homodimers and EGFR/ErbB2 heterodimers [31]. Betacellulin, heparin binding EGF (HB-EGF), and epiregulin can bind to either EGFR or ErbB4. Neuregulins-1 and -2 can bind both ErbB3 and ErbB4; neuregulins-3 and -4 are specific only for ErbB4. Currently, no known ligands have been identified for ErbB2 homodimers. However, ErbB2 is the preferred heterodimerization partner of other ErbB family members and ErbB2-containing heterodimers have the strongest signaling output [32, 33]. The sequence of the ErbB3 catalytic domain suggests that this receptor does not have RTK activity [34]. Thus ErbB3 may function as a platform for heterodimerization and subsequent transphosphorylation by other members of the ErbB family. Hence ErbB2 and ErbB3 can be activated through heterodimerization [34, 35]. It was proposed recently that the kinase domain of ErbB3 retains phosphoryl-transferase activity. Kinase activity appears to be ~1,000 fold weaker than for EGFR but may be sufficient for receptor trans-phosphorylation in the context of an ErbB heterodimer [36].
ErbB ligands exist as inactive transmembrane precursors, and need the proteolytic cleavage of their ectodomain to be released as mature soluble ligands. All EGF-family members are synthesized as membrane-anchored precursors that can be processed by specific metalloproteases to release soluble bioactive factors from the cell surface. This cleavage is performed by ADAM (a disintegrin and metalloprotease) family members [37–40] and tightly regulated by different factors. For example, intravenous Angiotensin II (Ang II) infusion induced ADAM17 redistribution to the apical cell membrane, followed by release of TGF-α and activation of the EGFR [41]. This led to the development of glomerulosclerosis, interstitial infiltration of monocytes, fibrosis, and proteinuria. ErbB receptor ligands are cleaved by multiple ADAMs, including ADAM9, 10, 12, 15, 17, and 19. In addition to these ErbB receptor ligands, many ADAMs have multiple substrates, and thereby appear to be involved in various signaling pathways and cellular functions [40]. A role for matrix metalloproteinases (MMPs) in cleaving of ErbB receptor ligands is also proposed [42, 43]. MMPs are a large family of zinc-contained proteolytic enzymes that have the capability to degrade extracellular matrix proteins [44]. It was shown recently that MMP9 protects mesenchymal cells from apoptosis during kidney development and stimulates ureteric bud branching morphogenesis, most likely by releasing the soluble form of stem cell factor, suggesting that normal renal development requires MMP9 [45].
4. Activation of ErbB receptors and physiological functions
Addition of ErbB receptor ligands to tubular cells promotes several biological responses, including cell proliferation. The physiological role of ErbB receptors and particularly of EGFR and ErbB2 is well established, especially in cancer. Activating mutations and overexpression of members of the ErbB-family have been implicated in a variety of cancers, including mammary and squamous carcinomas, glioblastomas, lung cancer as well as other malignant diseases [14, 46, 47]. The role of ErbB-family members in renal development, physiology and pathophysiology is also well recognized [21]. Under physiological conditions, ErbB activation appears to play an important role in the regulation of renal hemodynamics and electrolyte handling by the kidney, while in different pathophysiological states, ErbB activation may mediate either beneficial or detrimental effects to the kidney [21]. ErbB signaling is critically involved in cell signaling, cell growth, proliferation and renal electrolyte homeostasis. Most of the ErbB-family ligands are expressed in the kidney. For example, HB-EGF is synthesized in the proximal tubule, EGF in thick ascending limb of Henle's loop (TAL) and distal convoluted tubule (DCT), and TGFα in the DCT and collecting ducts [48, 49]. However, EGF and its related growth factors are present in the lumen at levels several orders higher than in plasma. As described above, ErbB receptors are expressed at the basolateral surfaces of normal adult kidneys. Thus, ErbB receptors are not accessible to their ligands in the lumen. This may serve as a protective mechanism for the distal nephron. One of such examples is mislocalization of ErbB receptors in PKD which will be discussed below. Another potential mechanism is a disturbance of epithelial cellular integrity. Following tubular injury, tight junctions can be disrupted and thus allow ErbB receptor ligands to traverse to the basolateral surface and consequently activate corresponding receptors. It was shown that EGF via the Raf-1/ERK1/2 pathway regulates the content and distribution of claudins [50, 51], proteins that are the most important components of the tight junctions, where they establish the paracellular barrier that controls the flow of molecules in the intercellular space between epithelial cells [52]. HB-EGF has also been shown to play an important role in the regulation of tight junction proteins and transepithelial resistance [53, 54].
As discussed above, activation of ErbB receptors is accomplished through ligand binding to the extracellular domain, followed by receptor homo- or heterodimerization and tyrosine transphosphorylation. Combinatorial interactions are very important in ErbB receptor polarization and downstream signaling since ErbB homodimers and heterodimers differ in their biological properties [55].
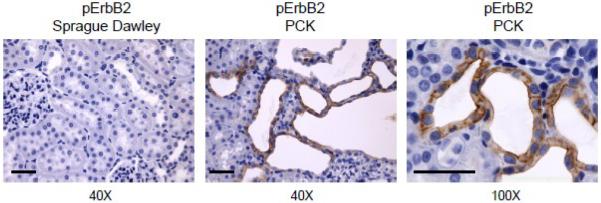
Once activated by site-specific phosphorylation, ErbB receptors serve as molecular integrators through either direct phosphorylation of target molecules or by serving as scaffolds for adaptor proteins. Activation of ErbB receptors leads to initiation of various signaling cascades. The great diversity of ligands and receptor dimer pairs allows for the activation of numerous signaling pathways that coordinately regulate complex processes including developmental growth control and adult homeostasis. Fig. 2 demonstrates one of such examples when ErbB receptor is phosphorylated and misslocalized during pathophysiological conditions. This figure shows immunohistochemistry staining for phosphorylated ErbB2 (pErbB2) at 40× in a cortical section of SD rat kidneys and in PCK rats (Charles River Laboratories International, Inc., Wilmington, MA) at 40× and 100× magnifications. The PCK rat harbours an autosomal recessive Pkhd1 gene mutation and is a model of ARPKD [56]. As clear seen in this figure, pErbB2 is upregulated in cystic kidneys compared to normal rat kidney and is localized at the apical membrane of CDs (Fig. 2).
Fig. 2. pErbB2 expression in normal and cystic rat kidney tissue.
Representative immunohistochemical staining for pErbB2 detection in kidney cortical sections of Sprague Dawley (a) and PCK (b, c) rats. Original magnifications are 40× and 100× (scale bars: 50 μm). Tissue sections were stained with anti-pErbB2 (Phospho-HER2/ErbB2 (Tyr1221/1222); 1:100; Cat. #2243; Cell Signaling Technology Inc., Boston, MA).
5. PKD
Polycystic kidney diseases (PKD) are a group of inherited disorders characterized by development of renal cysts. Of these, the most common lethal monogenic forms are ADPKD, characterized by progressive development of fluid-filled cysts; and the mainly infantile, ARPKD [4, 6, 11, 57]. ADPKD is inherited as a dominant trait, initiated in utero but usually leading to onset of renal failure in mid-life. It is characterized by early separation of focal spherical cysts from all segments of the nephron and progressive expansion by cell proliferation and fluid secretion throughout life. In contrast, ARPKD is inherited as a recessive trait, often lethal in the perinatal period, and is characterized by ecstatic distension of CDs to form numerous spindle-shaped renal cysts [4–6, 11, 58]. Because of these differences, ADPKD and ARPKD are treated as two distinct diseases in clinical practice [59].
5.1. ADPKD
ADPKD is one of the most common mono-genetic diseases affecting humans [60] and occurs approximately in 1 in 800 live births. Recent advances in ADPKD are thoroughly reviewed by Torres and Harris [5]. The primary phenotype of ADPKD is the progressive development in both kidneys of multiple fluid filled cysts, which eventually result in ESRD [6]. Two genes have been shown definitively to result in ADPKD when mutated: PKD1, which accounts for 85%, and PKD2, which is responsible for 15% [3]. The PKD1- encoded protein, polycystin-1 (PC-1) is a large (~460 kDa) membrane protein with a long, modular extracellular N-terminal component capable of protein-protein interactions, 11 transmembrane domains, and a short (~200 amino acid) intracellular C-terminal domain [61] which is capable of physical interactions with the PKD2-encoded polycystin-2 (PC-2), proteins of focal adhesion and cell-cell adherent junctional complexes as well as the α1-subunit of NaK-ATPase [62, 63]. mRNA and protein expression studies have shown that PC-1 is developmentally regulated and highly expressed in fetal kidneys in ureteric bud-derived CD [64–66]. On the basis of its predicted structure and functional features, PC-1 is postulated to be a plasma membrane receptor involved in cell–cell interaction and the regulation of several signaling pathways linked to cell proliferation [6, 67–69]. The PC-1 C-terminal domain also contains specific tyrosine (Y) and serine (S) sites for phosphorylation. PC-1 and PC-2 interact physically [62, 65, 70–73] and form a Ca2+-permeable non-selective cation channel when expressed heterologously [63, 74, 75]. Furthermore, it was recently shown that PC-1/PC-2 complex regulates pressure sensing [76]. A newly identified coiled-coil domain located in the C-terminus of PC-2 has been shown to mediate dimerization of the C-terminus of PC-2 and recognition of PC-1 [77] and is proposed to be essential for assembly and function of heteromeric polycystin complexes [78].
PC-2, also called TRPP2, belongs to the transient receptor potential (TRP) superfamily of ion channels [79–81]. PC-2 has six transmembrane domains, a pore forming region, and cytoplasmic N- and C-terminal tails [81–83]. In addition to forming a heteromeric complex with PC-1, PC2 has also been proposed to form heteromeric channels with TRPV4 and TRPC1 [84–86]. It was shown that PC-2 and TRPV4 form a mechanical and thermal sensor in cilia and suggested that TRPV4 is the mechanically sensitive component of the complex [84]. The PC-2/TRPC1 heteromeric channel is activated in response to GPCR activation and shows a pattern of single-channel conductance, amiloride sensitivity and ion permeability distinct from that of PC-2 or TRPC1 alone [85]. Kobori et al. using atomic force microscopy recently proposed that PC-2 and TRPC1 form a hetero-tetramer with a 2:2 stoichiometry and an alternating subunit arrangement [86]. Several protein-protein interactions have been reported previously between TRP channels, most of them are known to occur between members of the same group. Similarly to PC-2/TRPC1 and PC2/TRPV4 complexes, we have shown recently that members of other subfamilies of TRP channels, TRPA1 and TRPV1, can assemble into a complex on the plasma membrane and that TRPV1 influences intrinsic characteristics of the TRPA1 channel [87].
5.2. ARPKD
ARPKD is a hereditary renal cystic disease in infants and children affecting the kidneys and liver and occurs in approximately 1 in 20,000 live births. Renal failure and hepatic fibrosis develop in most babies who survive the perinatal period. The disease is characterized by the expansion and elongation of collecting tubules into multiple small cysts [6]. ARPKD is caused by mutations in the PKHD1 (polycystic kidney and hepatic disease 1) gene [88–90]. The PKHD1-encoded protein, fibrocystin-1 (polyductin) spans the cell membrane of kidney cells and is developmentally regulated. Based on fibrocystin domain structure, intracellular phosphorylation sites and capability to physically interact with PC-2, it is thought to act as a mechano-receptor, receiving external signals and initiating various intracellular signaling processes. This protein also may be involved in adhesion and proliferation. However, exact role of fibrocystin is unknown. Although the human genome contains a PKHD1 homolog, PKHDL1, this is not ARPKD-associated but is thought to be an ancestral variant of PKHD1 [91]. Fibrocystin-1 has been shown to be associated with the basal bodies/primary cilia of epithelial cells [92–94] and co-localized with PC2 in vitro and in vivo [95–97]. We have shown recently that PKHD1 mutation in human ARPKD is associated with alterations in cell spreading, matrix adhesion, and migration due to abnormalities of focal adhesion protein (FAK, c-Src, paxillin) content, distribution and activation by site-specific phosphorylation [98].
The overexpression and abnormally located ErbB receptors on the apical surface of cyst-lining epithelia in human and murine models of ARPKD have been shown specifically to bind with high affinity EGF/TGF-α. Overexpressed ErfB receptors autophosphorylate and generate mitogenic signals in response to growth factors [99–102]. However, surprisingly, neither overexpression nor mislocalization of EGFR was seen in PCK rats, the only current genetically orthologous rodent model of ARPKD. Moreover, the specific inhibitors of EGFR(ErbB1): EKI-785 and EKB-569, had no effect on renal cystic progression or fibrocystic liver disease [103]. Subsequently, high expression levels of ErbB2 were observed in the renal cystic epithelia in PCK rats [104]. More recently we have not only observed that ErbB2 is predominantly localized on the basolateral surface in the PCK rats (data not shown) but also shown that phosphorylated pErbB2 is abnormally expressed and mislocalized on the apical surface of cystic epithelia (Fig. 2) compared to normal rat CDs.
6. EGF-mediated proliferation
EGF-ErbB receptor-mediated interactions are key elements in renal tubular cell proliferation, not only in normal kidneys but also in cyst formation and enlargement. Activation of the intrinsic kinase domain results in phosphorylation of specific tyrosine residues within the cytoplasmic tail [105] that serve as docking sites for a range of proteins, the recruitment of which leads to the activation of intracellular signaling pathways [105, 106]. The duration and strength of intracellular signaling of ErbB receptors depend on speed of down-regulation by internalization, as well as the ratio of receptors that are targeted for recycling or degradation. Homodimers of EGFR have a signal which does not last long, in part due to receptor downregulation and degradation after ligand-dependent activation [107]. EGF is a well-known mitogen for normal renal epithelia, and has been shown to hyper-stimulate proliferation in ADPKD and ARPKD cystic epithelia [13, 108]. As mentioned above, EGF and EGF-reactive peptide species are secreted into the apical medium of cultured ADPKD epithelia, and high, potentially mitogenic concentrations have been measured in cyst fluids collected from ADPKD patients [6, 13]. Increased expression of TGFα has been observed in PKD cells compared with normal kidney cells [109] and transgenic mice overexpressing TGFα develop cystic kidneys [110]. Abnormal expression of amphiregulin and heparin-binding EGF (HB-EGF) has also been reported in both autosomal dominant and autosomal recessive PKD [13, 108–110]. In non-orthologous murine models of ARPKD HB-EGF showed the most marked activation of EGFR and was seen on apical membranes of cystic epithelia, while amphiregulin was ineffective and not on apical membranes. These studies suggested that the relative potencies of growth factors in the context of cyst expansion are a function of membrane ligand bound/receptor trafficking, as well as ligand availability [111].
Ligand-induced activation of ErbB receptors can stimulate a varety of intracellular pathways including PKC/AKT, PLCγ, MEK/Erk or c-Src. Phosphorylation of EGFR at Y845 in the kinase domain is implicated in stabilizing the activation loop, maintaining the active state of the enzyme and providing a binding surface for substrate proteins. c-Src binds to and phosphorylates EGFR at Y845 and is required for ErbB family-mediated signaling and proliferation in normal cells as well as in cystic renal epithelia [104, 112, 113]. It was also shown that increased pY418-Src activity correlated with cystic expansion in BPK mice and PCK rats and that inhibition of Src activity could attenuate of renal cystic disease in these respective phenotypic and genotypic rodent models of ARPKD [104].
Over-expression, constitutive activation and abnormal location of EGFR and ErbB2 receptors on the apical (luminal) surface of cyst-lining epithelia, together with secretion of soluble ligands by PKD cyst lining epithelia, creates a sustained cycle of autocrine–paracrine stimulation of proliferation in cysts [13, 100, 102, 114]. A precisely controlled balance between cellular proliferation and apoptosis is essential for normal growth and differentiation of the kidney and maintenance of normal renal structure. These fundamental processes are disturbed in cystic kidneys [6, 115]. Moreover, it was shown recently that EGFR membrane polarity is regulated by multiple hierarchical sorting pathways in renal epithelial cells and some of these pathways are perturbed in the BPK ARPKD mouse model [116].
Another common feature in PKD kidneys is a failure to switch off fetal genes and proteins, such as ErbB2, after completion of the developmental period [187] ErbB2 receptor which is highly expressed in several human cancers, including renal carcinomas, as well as in PKD, can potentiate EGFR signaling by slowing down the internalization and degradation of EGFR [117]. Although ErbB2 requires heterodimerization with another ligand-bound ErbB family member, such as EGFR, to be activated by autophosphorylation at Y1248 and Y1221/1222 [118], heterodimers are not only the favored complexes. As noted above, activation of ErbB2 is known to produce the strongest ErbB-related intracellular signaling responses. The importance of the ErbB2-receptor variant is further demonstrated both in cultured cells, where overexpression and activation of ErbB2 enhances proliferation and migration [119–122] and in transgenic mice where overexpression of ErbB2 induces renal cyst formation [108, 123].
7. EGF-mediated regulation of sodium transport in normal and PKD cells
The epithelial Na+ channel (ENaC) is a member of the ENaC/Deg channel superfamily [124–128]. ENaC activity is the rate limiting step for Na+ (re)absorption across many epithelia, including those in the distal nephron, lungs and colon. Dysfunction and aberrant regulation of this channel leads to a spectrum of diseases ranging from hyper- and hypotension associated with improper renal Na+ conservation and wasting, respectively, to respiratory syndromes linked to cystic fibrosis [129, 130]. Moreover, several forms of congenital hypo- and hypertension, such as pseudohypoaldosteronism and Liddle's syndrome, respectively, result from ENaC dysfunction with the prior being caused by loss of function mutations in the channel and the latter by gain of function mutations [131].
EGF-family ligands regulate various epithelial channels, including ENaC, TRPC5, TRPM6 and PC-2. As we discussed above, EGF and it related growth factors have direct actions on the primary route of Na+ reabsorption in the distal nephron. This site of the nephron gives rise to many of the cystic epithelial cells in PKD particularly in ARPKD which appears to be limited to distal tubular cystic development [6, 10, 132–134]. Activity of ENaC is the rate limiting step in Na+ reabsorption at this site [125, 126, 128]. Thus, ENaC dysfunction has been linked to a broad spectrum of human diseases including PKD where it may have an important pathophysiological role at the very earliest stages of the disease. Here we will summarize recent advances in the understanding of regulation of epithelial ion channels by EGF-family ligands in the normal and cystic kidney. This review will certainly not be complete, in part due to the considerable bulk of data and to the recent burst of new findings on this topic, and we apologize in advance to the authors of any relevant studies that we fail to mention
7.1. Role of EGF in regulation of ion channels in the kidney
EGF and its related growth factors are involved in regulation of various epithelial ion channels in the kidney. For instance, EGF stimulates store-operated Ca2+ channels in human mesangial cells through an intracellular signaling mechanism involving tyrosine kinase and PKC [135]. Later it was shown that EGF activates store-operated Ca2+ channels by a PLC-dependent but IP3 receptor independent pathway [136].
The TRP superfamily of signal-transduction-gated ion channels is widely expressed in the kidney [79, 137–139]. Bezzerides et al. have shown that EGF stimulation rapidly induces TRPC5 channel translocation to the plasma membrane. The small G protein Rac1 mediated the rapid insertion of TRPC5 into the plasma membrane through stimulation of PI(4)P 5-kinase, and increased channel availability. The authors proposed that rapid insertion of TRP channels may be a general process of cells in response to stimuli that affect cell morphology and perhaps, direction of movement [140]. We have also shown that RhoA and Rac1, small G proteins in the Rho family affect ENaC trafficking [25, 141–144]. However, a role of growth factors in Rho-mediated increase of ENaC activity is not clear yet.
A role for ErbB receptor activation in the regulation of Mg2+ channels in the kidney has also recently been proposed. The regulation of magnesium in the body principally occurs within the kidney. Genetic analysis has revealed that the TRPM6 channel is mutated in patients with primary hypomagnesemia and secondary hypocalcemia [145, 146]. Moreover, Groenestege et al., described a mutation in the EGF gene, encoding pro-EGF, which is responsible for a rare form of renal Mg2+ wasting, isolated recessive hypomagnesemia [147]. TRPM6 is a magnesium channel that is expressed in the intestine and renal distal tubules. Two different laboratories studied the effects of EGF on TRPM6 channels. Miwa and colleagues have shown that EGF increased the phosphorylation of ERK1/2 and TRPM6 expression in renal epithelial NRK-52E cells. Furthermore, EGF enhanced the influx of magnesium. Thus it was proposed that the phosphorylation of ERK1/2 may up-regulate TRPM6 expression and magnesium influx, resulting in an increase in cell proliferation with a shift from G1 to S phase [148]. Recently they have shown that the ERK/AP-1-dependent pathway is involved in the transcriptional regulation of TRPM6 expression [149]. Bindels and colleagues reported that EGF-mediated stimulation of TRPM6 occurs via signaling through Src kinases and the small GTPase Rac1, thereby redistributing endomembrane TRPM6 to the plasma membrane [150]. It is interesting that this effect was specific since stimulation of the EGFR increased current through TRPM6 but not TRPM7 [150]. Recent study showed that EGFR inhibitor erlotinib is capable of affecting TRPM6 regulation and thereby altering Mg2+ handling in vivo. Erlotinib can influence Mg2+ handling but its effect on the systemic Mg2+ concentration seems less potent than that observed with antibody-based EGFR inhibitors. Thus these data suggest that typical human dosages of erlotinib are unlikely to severely affect serum Mg2+ concentrations [151].
7.2. EGF-mediated regulation of polycystins
PC-2 can be activated in response to EGF in the kidney epithelial cell line LLC-PK1 [152]. PC-2 overexpression increases EGF-induced inward currents in LLC-PK1 cells, while the knockdown of endogenous PC-2 by sRNAi or the expression of a pathogenic missense variant, PKD2-D511V, blunts the EGF-induced response [152]. The physiological relevance of EGF-induced activation of TRPP2 in LLC-PK1 cells was supported by whole animal studies in which homozygous deletion of the EGFR gene resulted in cystic dilatation of collecting ducts [70, 153]. EGF-induced activation of PC-2 occurred independently of store depletion but required the activity of phospholipase C (PLC) and phosphatidylinositol 3-kinase (PI3-kinase). Interestingly, PC-2 interacted with PLC-γ2 and colocalized in the primary cilium with EGFR and PIP2 [152]. The authors proposed that PC-2 is under negative regulation by PIP2, which can be reversed by EGFR activation.
In addition, yeast two-hybrid screen analysis identified mDia1 (mammalian Diaphanous-related formin 1 protein) as a PC-2-interacting protein [154]. mDia1 is a member of the small G protein RhoA-binding-formin protein family that participates in cytoskeletal organization, cytokinesis, and signal transduction. mDia1 functioned as an intracellular, voltage-dependent regulator of PC-2. At resting potentials, autoinhibited mDia1 bound to and blocked PC-2, whereas at positive potentials activated mDia1 released the block on PC-2 leading to channel activation. EGF or membrane depolarization activated PC-2 through the sequential activation of RhoA and mDia1. The voltage-dependent block of PC-2 by mDia1 served two physiologically relevant roles, to underlie the activation mechanism of PC-2 by EGF and to set the resting membrane potential of kidney epithelial cells to its resting value by preventing constitutive activation of PC-2 [155].
7.3. EGF and its related growth factor-dependent regulation of ENaC-mediated sodium transport in principal cells
EGF has been known for almost two decades to regulate sodium transport. However, several investigations have reported contradictory results indicating opposite effects of EGF on ENaC activity and sodium transport. EGF appears to affect sodium transport and ENaC in a context-dependent manner: it increases sodium absorption in the airways [156] and intestine [157], but decreases sodium transport in the isolated and perfused rabbit CD [158–161], immortalized murine CD mCT1 cells [162, 163], in cystic fibrosis JME/CF15 cells [164], in Chinese hamster ovary (CHO) cells overexpressing ENaC subunits and EGFR [165] and in Madin-Darby canine kidney cells [166, 167]. Markadieu et al. demonstrated that EGF transiently increases sodium transport in Xenopus laevis kidney A6 cells in a phosphatidylinositol 3-kinase (PI 3-kinase)-dependent manner, and that this stimulation is increased by the inhibition of the MAPK pathway [168].
Recently Liu et al. have proposed that ENaC shows a biphasic response to EGF and TGF-α in A6 cells [169]. These authors also showed that acute treatment with EGF and TGF-α enhanced ENaC activity by increasing Po and that PI 3-kinase was involved in the activation of sodium transport [169]. Similarly, EGF via the small G protein Rac1, acutely activates ENaC in alveolar epithelial cells [170]. However, chronic treatment with EGF and TGF-α inhibited ENaC activity by decreasing the number of channels (N) in the plasma membrane through MAPK1/2 pathways [169]. Short-term blockade of the MAPK1/2 has also been shown to down regulate both the basal Na+ current and the aldosterone and vasopressin stimulated Na+ current in mpkCCDc14 principal cells. The authors proposed that the activity of Na, K-ATPase but not that of ENaC was inhibited by MEK1/2 inhibitors in both unstimulated and aldosterone- or vasopressin-stimulated CCDs [171]. Similarly, inhibitors of the MAPK1/2 cascade, protected Na+ reabsorption from PKC, demonstrating that the MAPK1/2 cascade, in some instances, plays a central role in down-regulation of ENaC activity [172]. Furthermore, we have shown that the small G protein K-Ras activates ENaC activity via PI 3-kinase in both heterologous and native principal cells [173–175]. K-Ras is an aldosterone-induced protein and most likely its effect on ENaC is mediated by this hormone [176–179]. However, K-Ras expression and activity could also be regulated by growth factors such as EGF [180, 181]. Furthermore, it was proposed that EGFR is transactivated by aldosterone [182]. For example, it was shown recently that aldosterone-induced mesangial cell proliferation is mediated by EGFR transactivation which was dependent on the K-Ras/c-Raf/MEK/ERK and PI 3-kinase/Akt/mTOR/p70S6K1 signaling pathways [183]. However it is still remains to be shown whether K-Ras or other small G proteins are involved in EGF-mediated regulation of ENaC activity.
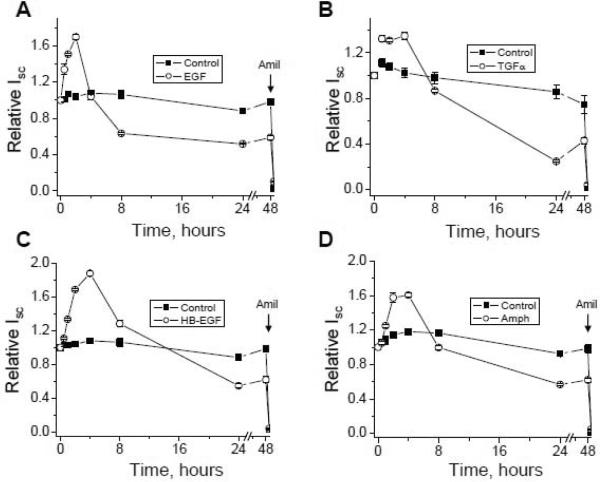
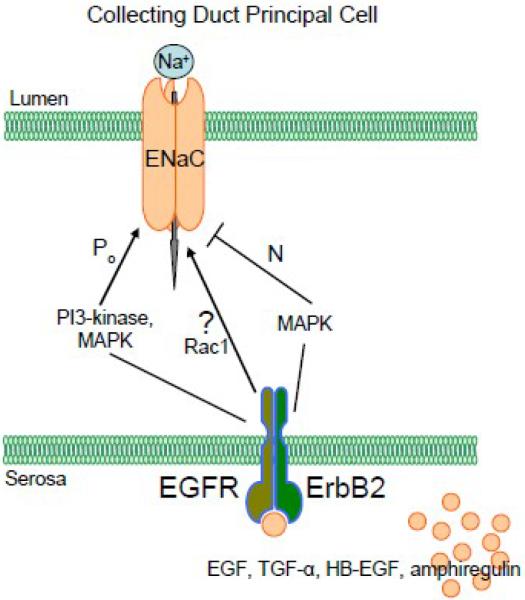
Our recent data are consistent with the idea that EGF and its related growth factors (TGF-α, HB-EGF and amphiregulin) have a biphasic effect on sodium absorption in the mammalian kidney as represented by mpkCCDc14 cells and that inhibition of MEK1/2 stimulates acute increase and abolishes chronic decrease of sodium transport in response to EGF action [23]. As shown in Fig. 3, addition of all four studied EGF family growth factors to polarized mpkCCDc14 principal cells with steady-state basal transport rates significantly increased Na+ reabsorption above basal levels in a time-dependent manner. Significant increases were detectable after 30 minutes, the earliest time point measured, with a maximum reached by 2 hours. After 4 hours of stimulation, a slow and continuous decrease of Isc (short circuit current) was observed. Chronic treatment of monolayers with EGF and its related growth factors (added basolaterally) leads to significant inhibition of Isc. As illustrated in Fig. 3, addition of the Na+ channel inhibitor amiloride (10 μM) at the end of experiments to the apical bathing solution caused a rapid decrease in Isc. Nearly all of the basal Isc was inhibited by amiloride. Thus, the addition of EGF caused a transient increase in Isc followed by a chronic decline in Isc and these effects were mediated by receptors located at the basolateral membrane. The acute effect of EGF was mediated via PI3-kinase and both phases were mediated by MEK1/2. Acute effect is most likely mediated by changes in the channel's open probability and long term effect occurred due to the decrease of the number of active channels at the plasma membrane [23, 169]. Furthermore, our study underlined the critical role of ErbB2 in the regulation of sodium transport in these collecting duct cells and suggested that EGF influences ENaC and sodium absorption via the ErbB2 receptor, most probably by forming ErbB2/EGFR heterodimers [23]. Fig. 4 shows our favored possible model for regulation of ENaC in normal CD monolayers by EGF-family ligands. As demonstrated in this figure, there are at least two independent pathways involved in the regulation of ENaC. However, it is very likely that other proteins are involved in regulation of ENaC. As we discussed previously, Bezzerides et al. demonstrated that EGF stimulation rapidly induces TRPC5 channel translocation to the plasma membrane and Rac1 mediates this insertion[140]. Bindels and colleagues also reported that EGF-mediated stimulation of TRPM6 occurs via signaling through Src kinases and Rac1 [150]. Importantly, Fujita and colleagues have established that signaling cross-talk between Rac1 and the mineralocorticoid receptor modulates receptor activity and identified Rac1 as a therapeutic target for chronic kidney disease[184, 185]. Garvin's and Helms' laboratories also recently identified an important role for Rac1 in NaCl-induced superoxide generation in the medullary thick ascending limb[186] and in the alveolar epithelium[170]. Thus, small GTPase Rac1 is a probable effector of EGF and its related growth factors with respect to ENaC activity. However this hypothesis requires further studies.
Fig. 3. Time course of biphasic effect of ErbB receptor ligands on ENaC-mediated sodium transport in mpkCCDc14 principal cells.
Summary graph of relative Isc in mpkCCDc14 cells in response to EGF (10 ng/ml; a), TGF-α (10 ng/ml; b), HB-EGF (50 ng/ml; c) and amphiregulin (100 ng/ml; d). MpkCCDc14 cells were serum-starved overnight. EGF-family ligands and vehicle (control) were added basolaterally at time 0 and current was normalized to the starting level. Amiloride (10 μM; arrows) was added to the apical membrane at the end of experiment. If error bars are not visible, they are smaller than the size of symbols. Values are means ± SE from at least 6 experiments. Some results represented here were previously published in a different format [23].
Fig. 4.
A schematic representation of EGF and its related growth factor-mediated regulation of ENaC activity in collecting duct principal cells.
7.4. Regulation of sodium transport in PKD cells
Since EGF and its related growth factors regulate ENaC activity, it is possible that ENaC inhibition facilitates cyst formation in PKD [187]. Considerable experimental data support the idea that there is coordinated interaction between ENaC and the cystic fibrosis transmembrane conductance regulator Cl− channel (CFTR) suggesting the possibility that ENaC may play some role in cystic fibrosis and other disease processes associated with dysfunctional CFTR [188–192]. Increased airway Na+ absorption in vivo caused airway surface liquid volume depletion, increased mucus concentration, delayed mucus transport and mucus adhesion to airway surfaces. Defective mucus transport caused a severe spontaneous lung disease sharing features with cystic fibrosis [193]. The airway surface liquid volume modulates the activity of ENaC by modification of the serine protease-protease inhibitor balance and alterations in this balance contribute to excessive Na+ absorption in cystic fibrosis [194]. It was shown that the CFTR Cl− channel exists in apical membranes of human ADPKD cells and may play an important role in cyst formation or enlargement [195]. Cl− currents in cultured ADPKD cells were activated by forskolin and cAMP and had properties identical to those that characterized CFTR [195]. Later Magenheimer et al. have shown that cAMP-stimulated fluid secretion occurs early in embryonic renal tubule development in wild-type and PKD kidneys at the time when renal cysts first appear in ADPKD, suggesting that a cAMP-driven mechanism may be involved in the initial stages of cyst formation in ADPKD [196]. Moreover, CFTR inhibitors partially inhibited cyst growth and preserved renal function in a mouse model of ADPKD [197]. However, renal cyst formation in a murine model of ARPKD did not require functional activity of CFTR [198].
In ADPKD, all the segments of the nephron are involved in forming the fluid-filled cysts. In ARPKD, dilated tubules rather than isolated cysts are predominantly formed from the CD, which is the nephron segment where ENaC plays a major role in the regulation of sodium transport [199, 200]. ENaC dysfunction associated with improper handling of NaCl has been demonstrated in renal epithelial cells from animal models and human ARPKD cell lines [201–204].
Measurements of transepithelial 22Na transport performed on monolayers of ARPKD and age-matched human fetal collecting tubule (HFCT) cells revealed net Na+ absorption in both models. However, ARPKD cells absorbed Na+ at a rate approximately 50% greater than that of HFCT. Furthermore, northern blot analyses of ARPKD whole kidney and western immunoblot of ARPKD cells showed approximately two fold greater expression of the α-ENaC. These results suggest that ARPKD cyst-lining cells absorb Na+ by a pathway that involved ENaC activity [205]. Later it was shown that ENaC-mediated Na+ absorption is upregulated in a murine ARPKD model epithelium lacking apical monocilia [202]. Three- to fourfold elevated Isc was measured in mutant orpk (Oak Ridge PKD) monolayers versus rescued controls. Examination of this enhanced end point in mutant monolayers revealed that that this effect was mediated by ENaC. These data suggested that ENaC expression and/or function are upregulated in the apical membrane of mutant orpk CD principal cell monolayers [202]. However, Veizis et al. have shown that amiloride sensitive Na+ absorption is decreased in CD cells from the non-orthologous BPK mouse model of ARPKD [203]. Moreover, they present evidence that addition of EGF to the apical bathing solution (24 hrs) led to inhibition of Na+ transport and decreased ENaC expression in cystic cells and that this effect was mediated through ERK kinase [204]. Thus the authors suggest that the mislocalized apical ErbB receptors are functionally coupled to the ERK pathway and that abnormal EGF-dependent regulation of ENaC function and expression may contribute to PKD pathophysiology [204].
Thus, role of ENaC-mediated sodium absorption and its regulation by EGF in PKD is obvious. However, several investigations have reported contradictory results. Thus, the exact mechanism by which ENaC-mediated sodium transport involved in development and progression of PKD, and especially ARPKD, is not completely clear. As indicated by Guay-Woodford [134], although identified abnormalities in the tubular epithelia diverse and not necessarily reproducible from one model to another, these abnormalities provide the framework for evaluating interventions that target specific processes and pathways involved in PKD pathogenesis. Hence investigation of the modulation of sodium transport by growth factors in PKD models would facilitate a more complete understanding of the pathologic development of PKD. Additional studies using cells with endogenous genotypic and phenotypic effects of PC-1, PC-2 and Fibrocystin-1 mutations will be required to understand the role of ENaC in PKD and EGF-mediated regulation of sodium transport in PKD. Our preliminary immunohistochemistry analysis revealed that ENaC subunits are expressed on the apical surface of CDs in PCK rats, the genotypic model of ARPKD. Moreover, we were able to record ENaC activity with patch clamp electrophysiology (unpublished observations) in freshly isolated opened cysts from the PCK rats. However, these observations require further investigations.
8. Clinical Relevance
ErbB family members are implicated in the development of cardiac and renal diseases such as cancer, hypertension and ESRD. Therefore, the therapeutic potential of targeting of ErbB receptors and EGF-family signaling pathways may play key roles in the kidney. A number of studies have investigated the potential role of EGF and its related growth factors in acute renal injury and both ADPKD and ARPKD [4, 6, 21, 58]. Addition of ErbB receptor ligands to epithelial cells promotes cell proliferation, migration, and epithelial-mesenchymal cell transdifferentiation. Chronic ErbB activation and mislocalization are thought to be important in the evolution of PKD. The cyst fluid of PKD patients contains mitogenic concentrations of ErbB receptor ligands [109, 206–208]. Thus this might represent a protective mechanism of diseased kidney in PKD.
ADPKD and ARPKD have overlapping but distinct pathogeneses. Identification of the causative mutated genes and elucidation of the function of their encoded proteins and signaling mechanisms mediating their regulation will shed new light on these diseases. Improved understanding of pathogenesis and mechanisms involved in both ADPKD and ARPKD provide an excellent opportunity for the development of pathophysiology-based therapies. Some of these have proven effective in preclinical studies, and clinical trials have begun [59, 209]. Our enhanced understanding of the PKD phenotype at a cellular level will facilitate identification of other interventional targets.
9. Conclusion
The study of regulation of ion channels and proliferation by ErbB receptor ligands has expanded considerably in recent years. In vitro and in vivo studies have provided direct evidence of the role of ErbB signaling in the kidney. Collectively, these results suggest that ErbB receptors could be major determinants in the development of renal lesions, possibly through enhanced cell proliferation and regulation of sodium reabsorption. However it is not exactly clear yet how ErbB receptors ligands affect proliferation and sodium reabsorption in PKD.
Inappropriate expression, activation and downstream signaling of ErbB receptors, and particularly ErbB2, contributes to the pathological phenotype of PKD and suggest that inhibition of this pathway represents a promising therapeutic target in this disease. Undoubtedly, many questions remain regarding regulation of sodium reabsorption and proliferation in normal and PKD cells by ErbB receptors and their ligands. For example, our data demonstrate that the ErbB2 receptor is involved in ENaC-mediated sodium transport in mpkCCDc14 principal cells. It is most likely that ErbB2 forms a heterodimer with EGFR, although a role for ErbB3 and ErbB4 cannot be excluded. Neuregulins-1 through -4, which can bind either both ErbB3 and ErbB4 or only ErbB4 could be utilized in future experiments to define any potential involvement of ErbB3 and ErbB4 in sodium transport. Furthermore, ErbB receptors after changing their polarity in PKD cells come into close proximity with ENaC and could thus potentially either form a complex with the channel or change its mechanisms of regulation. Future directions for study will require direct measurements of ENaC channel activity in response to ErbB-family ligands in genotypic ADPKD and ARPKD cell models.
Acknowledgements
The authors apologize to the investigators of ErbB receptors, PKD and sodium transport whose work was not directly discussed due to space limitations. Glen Slocum and Christine Duris are recognized for excellent technical assistance with immunohistochemistry experiments. This work was supported by the American Heart Association SDG 0730111N, Carl W. Gottschalk Research Scholar Grant from the American Society of Nephrology and American Society of Physiology S&R Foundation Ryuji Ueno Award (to A. Staruschenko) and NIH DK62345 (to P.D. Wilson).
Abbreviations
- ADAM
a disintegrin and metalloprotease
- Ang II
Angiotensin II
- ADPKD
autosomal dominant polycystic kidney disease
- ARPKD
autosomal recessive polycystic kidney disease
- CCD
cortical collecting duct
- CD
collecting duct
- DCT
distal convoluted tubule
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ENaC
epithelial Na+ channel
- ESRD
end-stage renal disease
- GPCR
G-protein coupled receptor
- HB-EGF
heparin-binding EGF
- HFCT
human fetal collecting tubule
- Isc
short-circuit current
- mDia1
mammalian Diaphanous-related formin 1 protein
- MMPs
matrix metalloproteinases
- PC-1
polycystin-1
- PC-2
polycystin-2
- PI 3-kinase
phosphatidylinositol 3-kinase
- PKD
polycystic kidney disease
- PLC
phospholipase C
- PTB
phosphotyrosine binding domain
- RTK
receptor tyrosine kinase
- SD
Sprague-Dawley
- SH2
Src homology domain 2
- TAL
thick ascending limb
- TGF-α
transforming growth factor alpha
- TRP
transient receptor potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr., Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP. Volume progression in polycystic kidney disease. N. Engl. J. Med. 2006;354:2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- [2].Patel V, Chowdhury R, Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr. Opin. Nephrol Hypertens. 2009;18:99–106. doi: 10.1097/MNH.0b013e3283262ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Boletta A, Germino GG. Role of polycystins in renal tubulogenesis. Trends Cell Biol. 2003;13:484–492. doi: 10.1016/s0962-8924(03)00169-7. [DOI] [PubMed] [Google Scholar]
- [4].Harris PC, Torres VE. Polycystic kidney disease. Annu. Rev. Med. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149–168. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wilson PD. Polycystic kidney disease. N. Engl. J. Med. 2004;350:151–164. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- [7].Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- [8].Wilson PD. Mouse models of polycystic kidney disease. Curr. Top. Dev. Biol. 2008;84:311–350. doi: 10.1016/S0070-2153(08)00606-6. [DOI] [PubMed] [Google Scholar]
- [9].Kaimori JY, Germino GG. ARPKD and ADPKD: first cousins or more distant relatives? J. Am. Soc. Nephrol. 2008;19:416–418. doi: 10.1681/ASN.2008010033. [DOI] [PubMed] [Google Scholar]
- [10].Sullivan LP, Wallace DP, Grantham JJ. Epithelial transport in polycystic kidney disease. Physiol Rev. 1998;78:1165–1191. doi: 10.1152/physrev.1998.78.4.1165. [DOI] [PubMed] [Google Scholar]
- [11].Wilson PD, Goilav B. Cystic disease of the kidney. Annu. Rev. Pathol. 2007;2:341–368. doi: 10.1146/annurev.pathol.2.010506.091850. [DOI] [PubMed] [Google Scholar]
- [12].Melenhorst WB, Mulder GM, Xi Q, Hoenderop JG, Kimura K, Eguchi S, van GH. Epidermal growth factor receptor signaling in the kidney: key roles in physiology and disease. Hypertension. 2008;52:987–993. doi: 10.1161/HYPERTENSIONAHA.108.113860. [DOI] [PubMed] [Google Scholar]
- [13].Du J, Wilson PD. Abnormal polarization of EGF receptors and autocrine stimulation of cyst epithelial growth in human ADPKD. Am. J. Physiol. 1995;269:C487–C495. doi: 10.1152/ajpcell.1995.269.2.C487. [DOI] [PubMed] [Google Scholar]
- [14].Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- [15].Carpenter G, King L, Jr., Cohen S. Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro. Nature. 1978;276:409–410. doi: 10.1038/276409a0. [DOI] [PubMed] [Google Scholar]
- [16].Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- [17].Kashles O, Szapary D, Bellot F, Ullrich A, Schlessinger J, Schmidt A. Ligand-induced stimulation of epidermal growth factor receptor mutants with altered transmembrane regions. Proc. Natl. Acad. Sci. U. S. A. 1988;85:9567–9571. doi: 10.1073/pnas.85.24.9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Carpenter G, Cohen S. Epidermal growth factor. J Biol. Chem. 1990;265:7709–7712. [PubMed] [Google Scholar]
- [19].Blaikie P, Immanuel D, Wu J, Li N, Yajnik V, Margolis B. A region in Shc distinct from the SH2 domain can bind tyrosine-phosphorylated growth factor receptors. J. Biol. Chem. 1994;269:32031–32034. [PubMed] [Google Scholar]
- [20].Holbro T, Hynes NE. ErbB receptors: directing key signaling networks throughout life. Annu. Rev. Pharmacol. Toxicol. 2004;44:195–217. doi: 10.1146/annurev.pharmtox.44.101802.121440. [DOI] [PubMed] [Google Scholar]
- [21].Zeng F, Singh AB, Harris RC. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp. Cell Res. 2009;315:602–610. doi: 10.1016/j.yexcr.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zeng F, Zhang MZ, Singh AB, Zent R, Harris RC. ErbB4 isoforms selectively regulate growth factor induced Madin-Darby canine kidney cell tubulogenesis. Mol Biol. Cell. 2007;18:4446–4456. doi: 10.1091/mbc.E07-03-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Levchenko V, Zheleznova NN, Pavlov TS, Vandewalle A, Wilson PD, Staruschenko A. EGF and its related growth factors mediate sodium transport in mpkCCD(c14) cells via ErbB2 (neu/HER-2) receptor. J. Cell. Physiol. 2010;223:252–259. doi: 10.1002/jcp.22033. [DOI] [PubMed] [Google Scholar]
- [24].Press MF, Cordon-Cardo C, Slamon DJ. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene. 1990;5:953–962. [PubMed] [Google Scholar]
- [25].Pavlov TS, Chahdi A, Ilatovskaya DV, Levchenko V, Vandewalle A, Pochynyuk O, Sorokin A, Staruschenko A. Endothelin-1 inhibits the epithelial Na+ channel through betaPix/14-3-3/Nedd4-2. J. Am. Soc. Nephrol. 2010;21:833–843. doi: 10.1681/ASN.2009080885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hobert ME, Kil SJ, Medof ME, Carlin CR. The cytoplasmic juxtamembrane domain of the epidermal growth factor receptor contains a novel autonomous basolateral sorting determinant. J. Biol. Chem. 1997;272:32901–32909. doi: 10.1074/jbc.272.52.32901. [DOI] [PubMed] [Google Scholar]
- [27].Hobert ME, Friend LA, Carlin CR. Regulation of EGF signaling by cell polarity in MDCK kidney epithelial cells. J. Cell. Physiol. 1999;181:330–341. doi: 10.1002/(SICI)1097-4652(199911)181:2<330::AID-JCP15>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- [28].Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp. Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- [29].Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cellular Signalling. 2005;17:1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- [30].Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology (Bethesda.) 2010;25:85–101. doi: 10.1152/physiol.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Feigin ME, Muthuswamy SK. ErbB receptors and cell polarity: new pathways and paradigms for understanding cell migration and invasion. Exp. Cell Res. 2009;315:707–716. doi: 10.1016/j.yexcr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- [32].Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin BJ, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol. Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp. Cell Res. 2003;284:54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- [35].Berger MB, Mendrola JM, Lemmon MA. ErbB3/HER3 does not homodimerize upon neuregulin binding at the cell surface. FEBS Lett. 2004;569:332–336. doi: 10.1016/j.febslet.2004.06.014. [DOI] [PubMed] [Google Scholar]
- [36].Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7692–7697. doi: 10.1073/pnas.1002753107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Melenhorst WB, Visser L, Timmer A, van den Heuvel MC, Stegeman CA, van Goor H. ADAM17 upregulation in human renal disease: a role in modulating TGF-{alpha} availability? Am. J. Physiol. Renal Physiol. 2009;297:F781–F790. doi: 10.1152/ajprenal.90610.2008. [DOI] [PubMed] [Google Scholar]
- [38].Blobel CP, Carpenter G, Freeman M. The role of protease activity in ErbB biology. Exp. Cell Res. 2009;315:671–682. doi: 10.1016/j.yexcr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sanderson MP, Dempsey PJ, Dunbar AJ. Control of ErbB signaling through metalloprotease mediated ectodomain shedding of EGF-like factors. Growth Factors. 2006;24:121–136. doi: 10.1080/08977190600634373. [DOI] [PubMed] [Google Scholar]
- [40].Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am. J. Physiol. Cell Physiol. 2006;291:C1–10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- [41].Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat. Med. 2005;11:867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- [42].Kramarenko II, Bunni MA, Raymond JR, Garnovskaya MN. Bradykinin B2 Receptor Interacts with Integrin +−5+−1 to Transactivate Epidermal Growth Factor Receptor in Kidney Cells. Mol. Pharmacol. 2010 doi: 10.1124/mol.110.064840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Suzuki M, Raab G, Moses MA, Fernandez CA, Klagsbrun M. Matrix Metalloproteinase-3 Releases Active Heparin-binding EGF-like Growth Factor by Cleavage at a Specific Juxtamembrane Site. J. Biol. Chem. 1997;272:31730–31737. doi: 10.1074/jbc.272.50.31730. [DOI] [PubMed] [Google Scholar]
- [44].Visse R, Nagase H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases: Structure, Function, and Biochemistry. Circ. Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- [45].Arnould C, Lelievre-Pegorier M, Ronco P, Lelongt B. MMP9 Limits Apoptosis and Stimulates Branching Morphogenesis During Kidney Development. J. Am. Soc. Nephrol. 2009;20:2171–2180. doi: 10.1681/ASN.2009030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010;277:301–308. doi: 10.1111/j.1742-4658.2009.07448.x. [DOI] [PubMed] [Google Scholar]
- [47].Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- [48].Gesualdo L, Di PS, Calabro A, Milani S, Maiorano E, Ranieri E, Pannarale G, Schena FP. Expression of epidermal growth factor and its receptor in normal and diseased human kidney: an immunohistochemical and in situ hybridization study. Kidney Int. 1996;49:656–665. doi: 10.1038/ki.1996.94. [DOI] [PubMed] [Google Scholar]
- [49].Wilson PD. A plethora of epidermal growth factor-like proteins in polycystic kidneys. Kidney Int. 2004;65:2441–2442. doi: 10.1111/j.1523-1755.2004.00667.x. [DOI] [PubMed] [Google Scholar]
- [50].Flores-Benitez D, Ruiz-Cabrera A, Flores-Maldonado C, Shoshani L, Cereijido M, Contreras RG. Control of tight junctional sealing: role of epidermal growth factor. Am. J. Physiol. Renal Physiol. 2007;292:F828–F836. doi: 10.1152/ajprenal.00369.2006. [DOI] [PubMed] [Google Scholar]
- [51].Flores-Benitez D, Rincon-Heredia R, Razgado LF, Larre I, Cereijido M, Contreras RG. Control of tight junctional sealing: roles of epidermal growth factor and prostaglandin E2. Am. J. Physiol. Cell Physiol. 2009;297:C611–C620. doi: 10.1152/ajpcell.00622.2008. [DOI] [PubMed] [Google Scholar]
- [52].Amasheh S, Milatz S, Krug SM, Markov AG, Gunzel D, Amasheh M, Fromm M. Tight junction proteins as channel formers and barrier builders. Ann. N. Y. Acad. Sci. 2009;1165:211–219. doi: 10.1111/j.1749-6632.2009.04439.x. [DOI] [PubMed] [Google Scholar]
- [53].Singh AB, Sugimoto K, Dhawan P, Harris RC. Juxtacrine activation of EGFR regulates claudin expression and increases transepithelial resistance. Am. J. Physiol. Cell Physiol. 2007;293:C1660–C1668. doi: 10.1152/ajpcell.00274.2007. [DOI] [PubMed] [Google Scholar]
- [54].Smith JP, Pozzi A, Dhawan P, Singh AB, Harris RC. Soluble HB-EGF induces epithelial-to-mesenchymal transition in inner medullary collecting duct cells by upregulating Snail-2. Am. J. Physiol. Renal Physiol. 2009;296:F957–F965. doi: 10.1152/ajprenal.90490.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Muthuswamy SK, Gilman M, Brugge JS. Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers. Mol. Cell Biol. 1999;19:6845–6857. doi: 10.1128/mcb.19.10.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Katsuyama M, Masuyama T, Komura I, Hibino T, Takahashi H. Characterization of a novel polycystic kidney rat model with accompanying polycystic liver. Exp. Anim. 2000;49:51–55. doi: 10.1538/expanim.49.51. [DOI] [PubMed] [Google Scholar]
- [57].Harris PC. 2008 Homer W. Smith Award: insights into the pathogenesis of polycystic kidney disease from gene discovery. J. Am. Soc. Nephrol. 2009;20:1188–1198. doi: 10.1681/ASN.2009010014. [DOI] [PubMed] [Google Scholar]
- [58].Wilson PD. Polycystic kidney disease: new understanding in the pathogenesis. Int. J Biochem. Cell Biol. 2004;36:1868–1873. doi: 10.1016/j.biocel.2004.03.012. [DOI] [PubMed] [Google Scholar]
- [59].Torres VE, Harris PC. Mechanisms of Disease: autosomal dominant and recessive polycystic kidney diseases. Nat. Clin. Pract. Nephrol. 2006;2:40–55. doi: 10.1038/ncpneph0070. [DOI] [PubMed] [Google Scholar]
- [60].Gabow PA. Autosomal dominant polycystic kidney disease. N. Engl. J Med. 1993;329:332–342. doi: 10.1056/NEJM199307293290508. [DOI] [PubMed] [Google Scholar]
- [61].Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, Gamble V, Harris PC. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat. Genet. 1995;10:151–160. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- [62].Newby LJ, Streets AJ, Zhao Y, Harris PC, Ward CJ, Ong AC. Identification, characterization, and localization of a novel kidney polycystin-1-polycystin-2 complex. J. Biol. Chem. 2002;277:20763–20773. doi: 10.1074/jbc.M107788200. [DOI] [PubMed] [Google Scholar]
- [63].Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- [64].Ibraghimov-Beskrovnaya O, Bukanov NO, Donohue LC, Dackowski WR, Klinger KW, Landes GM. Strong homophilic interactions of the Ig-like domains of polycystin-1, the protein product of an autosomal dominant polycystic kidney disease gene, PKD1. Hum. Mol. Genet. 2000;9:1641–1649. doi: 10.1093/hmg/9.11.1641. [DOI] [PubMed] [Google Scholar]
- [65].Geng L, Burrow CR, Li HP, Wilson PD. Modification of the composition of polycystin-1 multiprotein complexes by calcium and tyrosine phosphorylation. Biochim. Biophys. Acta. 2000;1535:21–35. doi: 10.1016/s0925-4439(00)00079-x. [DOI] [PubMed] [Google Scholar]
- [66].Ward CJ, Turley H, Ong AC, Comley M, Biddolph S, Chetty R, Ratcliffe PJ, Gattner K, Harris PC. Polycystin, the polycystic kidney disease 1 protein, is expressed by epithelial cells in fetal, adult, and polycystic kidney. Proc. Natl. Acad. Sci. U. S. A. 1996;93:1524–1528. doi: 10.1073/pnas.93.4.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP. Calcium Restores a Normal Proliferation Phenotype in Human Polycystic Kidney Disease Epithelial Cells. J. Am. Soc. Nephrol. 2006;17:178–187. doi: 10.1681/ASN.2005060645. [DOI] [PubMed] [Google Scholar]
- [68].Li X, Magenheimer BS, Xia S, Johnson T, Wallace DP, Calvet JP, Li R. A tumor necrosis factor-alpha-mediated pathway promoting autosomal dominant polycystic kidney disease. Nat. Med. 2008;14:863–868. doi: 10.1038/nm1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- [70].Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc. Natl. Acad. Sci. U. S. A. 1997;94:6965–6970. doi: 10.1073/pnas.94.13.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, Sukhatme VP. Specific association of the gene product of PKD2 with the TRPC1 channel. Proc. Natl. Acad. Sci. U. S. A. 1999;96:3934–3939. doi: 10.1073/pnas.96.7.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- [73].Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat. Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- [74].Babich V, Zeng WZ, Yeh BI, Ibraghimov-Beskrovnaya O, Cai Y, Somlo S, Huang CL. The N-terminal extracellular domain is required for polycystin-1-dependent channel activity. J Biol. Chem. 2004;279:25582–25589. doi: 10.1074/jbc.M402829200. [DOI] [PubMed] [Google Scholar]
- [75].Delmas P, Nauli SM, Li X, Coste B, Osorio N, Crest M, Brown DA, Zhou J. Gating of the polycystin ion channel signaling complex in neurons and kidney cells. FASEB J. 2004;18:740–742. doi: 10.1096/fj.03-0319fje. [DOI] [PubMed] [Google Scholar]
- [76].Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, Retailleau K, Loufrani L, Patel A, Sachs F, Delmas P, Peters DJ, Honore E. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- [77].Giamarchi A, Feng S, Rodat-Despoix L, Xu Y, Bubenshchikova E, Newby LJ, Hao J, Gaudioso C, Crest M, Lupas AN, Honore E, Williamson MP, Obara T, Ong AC, Delmas P. A polycystin-2 (TRPP2) dimerization domain essential for the function of heteromeric polycystin complexes. EMBO J. 2010;29:1176–1191. doi: 10.1038/emboj.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Yu Y, Ulbrich MH, Li MH, Buraei Z, Chen XZ, Ong AC, Tong L, Isacoff EY, Yang J. Structural and molecular basis of the assembly of the TRPP2/PKD1 complex. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11558–11563. doi: 10.1073/pnas.0903684106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Woudenberg-Vrenken TE, Bindels RJ, Hoenderop JG. The role of transient receptor potential channels in kidney disease. Nat. Rev. Nephrol. 2009;5:441–449. doi: 10.1038/nrneph.2009.100. [DOI] [PubMed] [Google Scholar]
- [80].Kiselyov K, Soyombo A, Muallem S. TRPpathies. J Physiol. 2007;578:641–653. doi: 10.1113/jphysiol.2006.119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Zhou J. Polycystins and Primary Cilia: Primers for Cell Cycle Progression. An. Rev. Physiol. 2009;71:83–113. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
- [82].Tsiokas L. Function and regulation of TRPP2 at the plasma membrane. Am. J. Physiol. Renal Physiol. 2009;297:F1–F9. doi: 10.1152/ajprenal.90277.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- [84].Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J. Cell Biol. 2008;182:437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep. 2008;9:472–479. doi: 10.1038/embor.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kobori T, Smith GD, Sandford R, Edwardson JM. The Transient Receptor Potential Channels TRPP2 and TRPC1 Form a Heterotetramer with a 2:2 Stoichiometry and an Alternating Subunit Arrangement. J. Biol. Chem. 2009;284:35507–35513. doi: 10.1074/jbc.M109.060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Staruschenko A, Jeske NA, Akopian AN. Contribution of TRPV1-TRPA1 Interaction to the Single Channel Properties of the TRPA1 Channel. J. Biol. Chem. 2010;285:15167–15177. doi: 10.1074/jbc.M110.106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, Rudnik-Schoneborn S, Mrug M, Sweeney W, Avner ED, Zerres K, Guay-Woodford LM, Somlo S, Germino GG. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am. J. Hum. Genet. 2002;70:1305–1317. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat. Genet. 2002;30:259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- [90].Nagasawa Y, Matthiesen S, Onuchic LF, Hou X, Bergmann C, Esquivel E, Senderek J, Ren Z, Zeltner R, Furu L, Avner E, Moser M, Somlo S, Guay-Woodford L, Buttner R, Zerres K, Germino GG. Identification and characterization of Pkhd1, the mouse orthologue of the human ARPKD gene. J. Am. Soc. Nephrol. 2002;13:2246–2258. doi: 10.1097/01.asn.0000030392.19694.9d. [DOI] [PubMed] [Google Scholar]
- [91].Hogan MC, Griffin MD, Rossetti S, Torres VE, Ward CJ, Harris PC. PKHDL1, a homolog of the autosomal recessive polycystic kidney disease gene, encodes a receptor with inducible T lymphocyte expression. Hum. Mol Genet. 2003;12:685–698. [PubMed] [Google Scholar]
- [92].Menezes LF, Cai Y, Nagasawa Y, Silva AM, Watkins ML, Da Silva AM, Somlo S, Guay-Woodford LM, Germino GG, Onuchic LF. Polyductin, the PKHD1 gene product, comprises isoforms expressed in plasma membrane, primary cilium, and cytoplasm. Kidney Int. 2004;66:1345–1355. doi: 10.1111/j.1523-1755.2004.00844.x. [DOI] [PubMed] [Google Scholar]
- [93].Wang S, Luo Y, Wilson PD, Witman GB, Zhou J. The Autosomal Recessive Polycystic Kidney Disease Protein Is Localized to Primary Cilia, with Concentration in the Basal Body Area. J. Am. Soc. Nephrol. 2004;15:592–602. doi: 10.1097/01.asn.0000113793.12558.1d. [DOI] [PubMed] [Google Scholar]
- [94].Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum. Mol. Genet. 2003;12:2703–2710. doi: 10.1093/hmg/ddg274. [DOI] [PubMed] [Google Scholar]
- [95].Zhang MZ, Mai W, Li C, Cho SY, Hao C, Moeckel G, Zhao R, Kim I, Wang J, Xiong H, Wang H, Sato Y, Wu Y, Nakanuma Y, Lilova M, Pei Y, Harris RC, Li S, Coffey RJ, Sun L, Wu D, Chen XZ, Breyer MD, Zhao ZJ, McKanna JA, Wu G. PKHD1 protein encoded by the gene for autosomal recessive polycystic kidney disease associates with basal bodies and primary cilia in renal epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2311–2316. doi: 10.1073/pnas.0400073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kim I, Fu Y, Hui K, Moeckel G, Mai W, Li C, Liang D, Zhao P, Ma J, Chen XZ, George AL, Jr., Coffey RJ, Feng ZP, Wu G. Fibrocystin/polyductin modulates renal tubular formation by regulating polycystin-2 expression and function. J. Am. Soc. Nephrol. 2008;19:455–468. doi: 10.1681/ASN.2007070770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol. Cell Biol. 2007;27:3241–3252. doi: 10.1128/MCB.00072-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Israeli S, Amsler K, Zheleznova N, Wilson PD. Abnormalities in focal adhesion complex formation, regulation, and function in human autosomal recessive polycystic kidney disease epithelial cells. Am. J. Physiol. Cell Physiol. 2010;298:C831–C846. doi: 10.1152/ajpcell.00032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Sweeney WE, Chen Y, Nakanishi K, Frost P, Avner ED. Treatment of polycystic kidney disease with a novel tyrosine kinase inhibitor. Kidney Int. 2000;57:33–40. doi: 10.1046/j.1523-1755.2000.00829.x. [DOI] [PubMed] [Google Scholar]
- [100].Sweeney WE, Jr., Avner ED. Functional activity of epidermal growth factor receptors in autosomal recessive polycystic kidney disease. Am J Physiol. 1998;275:F387–F394. doi: 10.1152/ajprenal.1998.275.3.F387. [DOI] [PubMed] [Google Scholar]
- [101].Sweeney WE, Jr., Avner ED. Molecular and cellular pathophysiology of autosomal recessive polycystic kidney disease (ARPKD) Cell Tissue Res. 2006;326:671–685. doi: 10.1007/s00441-006-0226-0. [DOI] [PubMed] [Google Scholar]
- [102].Nakanishi K, Sweeney W, Jr., Avner ED. Segment-specific c-ErbB2 expression in human autosomal recessive polycystic kidney disease. J. Am. Soc. Nephrol. 2001;12:379–384. doi: 10.1681/ASN.V122379. [DOI] [PubMed] [Google Scholar]
- [103].Torres VE, Sweeney WE, Jr., Wang X, Qian Q, Harris PC, Frost P, Avner ED. Epidermal growth factor receptor tyrosine kinase inhibition is not protective in PCK rats. Kidney Int. 2004;66:1766–1773. doi: 10.1111/j.1523-1755.2004.00952.x. [DOI] [PubMed] [Google Scholar]
- [104].Sweeney WE, Jr., von Vigier RO, Frost P, Avner ED. Src inhibition ameliorates polycystic kidney disease. J. Am. Soc. Nephrol. 2008;19:1331–1341. doi: 10.1681/ASN.2007060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat. Rev. Mol. Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- [106].Schlessinger J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science. 2004;306:1506–1507. doi: 10.1126/science.1105396. [DOI] [PubMed] [Google Scholar]
- [107].Waterman H, Yarden Y. Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett. 2001;490:142–152. doi: 10.1016/s0014-5793(01)02117-2. [DOI] [PubMed] [Google Scholar]
- [108].Wilson PD, Du J, Norman JT. Autocrine, endocrine and paracrine regulation of growth abnormalities in autosomal dominant polycystic kidney disease. Eur. J. Cell Biol. 1993;61:131–138. [PubMed] [Google Scholar]
- [109].MacRae DK, Nemo R, Sweeney WE, Jr., Avner ED. EGF-related growth factors in the pathogenesis of murine ARPKD. Kidney Int. 2004;65:2018–2029. doi: 10.1111/j.1523-1755.2004.00623.x. [DOI] [PubMed] [Google Scholar]
- [110].Lowden DA, Lindemann GW, Merlino G, Barash BD, Calvet JP, Gattone VH. Renal cysts in transgenic mice expressing transforming growth factor-alpha. J. Lab. Clin. Med. 1994;124:386–394. [PubMed] [Google Scholar]
- [111].Richards WG, Sweeney WE, Yoder BK, Wilkinson JE, Woychik RP, Avner ED. Epidermal growth factor receptor activity mediates renal cyst formation in polycystic kidney disease. J. Clin. Invest. 1998;101:935–939. doi: 10.1172/JCI2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J. Biol. Chem. 1999;274:8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- [113].Cooper JA, Howell B. The when and how of Src regulation. Cell. 1993;73:1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- [114].Orellana SA, Sweeney WE, Neff CD, Avner ED. Epidermal growth factor receptor expression is abnormal in murine polycystic kidney. Kidney Int. 1995;47:490–499. doi: 10.1038/ki.1995.62. [DOI] [PubMed] [Google Scholar]
- [115].Goilav B, Satlin LM, Wilson PD. Pathways of apoptosis in human autosomal recessive and autosomal dominant polycystic kidney diseases. Pediatr. Nephrol. 2008;23:1473–1482. doi: 10.1007/s00467-008-0851-9. [DOI] [PubMed] [Google Scholar]
- [116].Ryan S, Verghese S, Cianciola NL, Cotton CU, Carlin CR. Autosomal recessive polycystic kidney disease epithelial cell model reveals multiple basolateral epidermal growth factor receptor sorting pathways. Mol. Biol. Cell. 2010;21:2732–2745. doi: 10.1091/mbc.E09-12-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wang Z, Zhang L, Yeung TK, Chen X. Endocytosis deficiency of epidermal growth factor (EGF) receptor-ErbB2 heterodimers in response to EGF stimulation. Mol. Biol. Cell. 1999;10:1621–1636. doi: 10.1091/mbc.10.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Hazan R, Margolis B, Dombalagian M, Ullrich A, Zilberstein A, Schlessinger J. Identification of autophosphorylation sites of HER2/neu. Cell Growth Differ. 1990;1:3–7. [PubMed] [Google Scholar]
- [119].Nauta J, Sweeney WE, Rutledge JC, Avner ED. Biliary epithelial cells from mice with congenital polycystic kidney disease are hyperresponsive to epidermal growth factor. Pediatr. Res. 1995;37:755–763. doi: 10.1203/00006450-199506000-00014. [DOI] [PubMed] [Google Scholar]
- [120].Spencer KS, Graus-Porta D, Leng J, Hynes NE, Klemke RL. ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J. Cell Biol. 2000;148:385–397. doi: 10.1083/jcb.148.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Graus-Porta D, Beerli RR, Hynes NE. Single-chain antibody-mediated intracellular retention of ErbB-2 impairs Neu differentiation factor and epidermal growth factor signaling. Mol. Cell Biol. 1995;15:1182–1191. doi: 10.1128/mcb.15.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Beerli RR, Graus-Porta D, Woods-Cook K, Chen X, Yarden Y, Hynes NE. Neu differentiation factor activation of ErbB-3 and ErbB-4 is cell specific and displays a differential requirement for ErbB-2. Mol. Cell Biol. 1995;15:6496–6505. doi: 10.1128/mcb.15.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wilson SJ, Amsler K, Hyink DP, Li X, Lu W, Zhou J, Burrow CR, Wilson PD. Inhibition of HER-2(neu/ErbB2) restores normal function and structure to polycystic kidney disease (PKD) epithelia. Biochim. Biophys. Acta. 2006;1762:647–655. doi: 10.1016/j.bbadis.2006.04.006. [DOI] [PubMed] [Google Scholar]
- [124].Butterworth MB. Regulation of the epithelial sodium channel (ENaC) by membrane trafficking, Biochimica Et Biophysica Acta (BBA) - Molecular Basis of Disease In Press. Corrected Proof. doi: 10.1016/j.bbadis.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol. Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- [126].Alvarez de la Rosa D, Canessa CM, Fyfe GK, Zhang P. Structure and regulation of amiloride-sensitive sodium channels. Annu. Rev. Physiol. 2000;62:573–594. doi: 10.1146/annurev.physiol.62.1.573. [DOI] [PubMed] [Google Scholar]
- [127].Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- [128].Schild L. The epithelial sodium channel: from molecule to disease. Rev. Physiol Biochem. Pharmacol. 2004;151:93–107. doi: 10.1007/s10254-004-0023-7. [DOI] [PubMed] [Google Scholar]
- [129].Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J. Am. Soc. Nephrol. 2008;19:1845–1854. doi: 10.1681/ASN.2008020225. [DOI] [PubMed] [Google Scholar]
- [130].Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The Contribution of Epithelial Sodium Channels to Alveolar Function in Health and Disease. An. Rev. Physiol. 2009;71:403–423. doi: 10.1146/annurev.physiol.010908.163250. [DOI] [PubMed] [Google Scholar]
- [131].Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- [132].Avner ED, Sweeney WE., Jr. Renal cystic disease: new insights for the clinician. Pediatr. Clin. North Am. 2006;53:889–909. ix. doi: 10.1016/j.pcl.2006.08.012. [DOI] [PubMed] [Google Scholar]
- [133].Wilson PD. Epithelial cell polarity and disease. Am J Physiol. 1997;272:F434–F442. doi: 10.1152/ajprenal.1997.272.4.F434. [DOI] [PubMed] [Google Scholar]
- [134].Guay-Woodford LM. Murine models of polycystic kidney disease: molecular and therapeutic insights. Am. J. Physiol. Renal Physiol. 2003;285:F1034–F1049. doi: 10.1152/ajprenal.00195.2003. [DOI] [PubMed] [Google Scholar]
- [135].Ma R, Sansom SC. Epidermal growth factor activates store-operated calcium channels in human glomerular mesangial cells. J. Am. Soc. Nephrol. 2001;12:47–53. doi: 10.1681/ASN.V12147. [DOI] [PubMed] [Google Scholar]
- [136].Li WP, Tsiokas L, Sansom SC, Ma R. Epidermal growth factor activates store-operated Ca2+ channels through an inositol 1,4,5-trisphosphate-independent pathway in human glomerular mesangial cells. J. Biol. Chem. 2004;279:4570–4577. doi: 10.1074/jbc.M304334200. [DOI] [PubMed] [Google Scholar]
- [137].Kiselyov K, Patterson RL. The integrative function of TRPC channels. Front Biosci. 2009;14:45–58. doi: 10.2741/3230. [DOI] [PubMed] [Google Scholar]
- [138].Dietrich A, Chubanov V, Gudermann T. Renal TRPathies. J. Am. Soc. Nephrol. 2010;21:736–744. doi: 10.1681/ASN.2009090948. [DOI] [PubMed] [Google Scholar]
- [139].Goel M, Sinkins WG, Zuo CD, Estacion M, Schilling WP. Identification and localization of TRPC channels in the rat kidney. Am. J. Physiol. Renal Physiol. 2006;290:F1241–F1252. doi: 10.1152/ajprenal.00376.2005. [DOI] [PubMed] [Google Scholar]
- [140].Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat. Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- [141].Karpushev A, Pavlov T, Staruschenko A. Regulation of the epithelial sodium channels (ENaC) by small G proteins and phosphatidylinositides. Biochemistry (Moscow) Supplemental Series A: Membrane and Cell Biology. 2009;3:261–274. [Google Scholar]
- [142].Pochynyuk O, Medina J, Gamper N, Genth H, Stockand JD, Staruschenko A. Rapid translocation and insertion of the epithelial Na+ channel in response to RhoA signaling. J. Biol. Chem. 2006;281:26520–26527. doi: 10.1074/jbc.M603716200. [DOI] [PubMed] [Google Scholar]
- [143].Pochynyuk O, Staruschenko A, Bugaj V, Lagrange L, Stockand JD. Quantifying RhoA Facilitated Trafficking of the Epithelial Na+ Channel toward the Plasma Membrane with Total Internal Reflection Fluorescence-Fluorescence Recovery after Photobleaching. J. Biol. Chem. 2007;282:14576–14585. doi: 10.1074/jbc.M701348200. [DOI] [PubMed] [Google Scholar]
- [144].Staruschenko A, Nichols A, Medina JL, Camacho P, Zheleznova NN, Stockand JD. Rho small GTPases activate the epithelial Na(+) channel. J. Biol. Chem. 2004;279:49989–49994. doi: 10.1074/jbc.M409812200. [DOI] [PubMed] [Google Scholar]
- [145].Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat. Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- [146].Schlingmann KP, Weber S, Peters M, Niemann NL, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- [147].Groenestege WM, Thebault S, van der WJ, van den BD, Janssen R, Tejpar S, van den Heuvel LP, van CE, Hoenderop JG, Knoers NV, Bindels RJ. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J. Clin. Invest. 2007;117:2260–2267. doi: 10.1172/JCI31680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Ikari A, Okude C, Sawada H, Yamazaki Y, Sugatani J, Miwa M. TRPM6 expression and cell proliferation are up-regulated by phosphorylation of ERK1/2 in renal epithelial cells. Biochem. Biophys. Res. Commun. 2008;369:1129–1133. doi: 10.1016/j.bbrc.2008.03.002. [DOI] [PubMed] [Google Scholar]
- [149].Ikari A, Sanada A, Okude C, Sawada H, Yamazaki Y, Sugatani J, Miwa M. Up-regulation of TRPM6 transcriptional activity by AP-1 in renal epithelial cells. J. Cell Physiol. 2010;222:481–487. doi: 10.1002/jcp.21988. [DOI] [PubMed] [Google Scholar]
- [150].Thebault S, Alexander RT, Tiel Groenestege WM, Hoenderop JG, Bindels RJ. EGF Increases TRPM6 Activity and Surface Expression. J. Am. Soc. Nephrol. 2009;20:78–85. doi: 10.1681/ASN.2008030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Dimke H, van der WJ, Alexander TR, Meijer IM, Mulder GM, van GH, Tejpar S, Hoenderop JG, Bindels RJ. Effects of the EGFR Inhibitor Erlotinib on Magnesium Handling. J. Am. Soc. Nephrol. 2010;21:1309–1316. doi: 10.1681/ASN.2009111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Ma R, Li WP, Rundle D, Kong J, Akbarali HI, Tsiokas L. PKD2 Functions as an Epidermal Growth Factor-Activated Plasma Membrane Channel. Mol. Cell. Biol. 2005;25:8285–8298. doi: 10.1128/MCB.25.18.8285-8298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- [154].Rundle DR, Gorbsky G, Tsiokas L. PKD2 interacts and co-localizes with mDia1 to mitotic spindles of dividing cells: role of mDia1 IN PKD2 localization to mitotic spindles. J Biol. Chem. 2004;279:29728–29739. doi: 10.1074/jbc.M400544200. [DOI] [PubMed] [Google Scholar]
- [155].Bai CX, Kim S, Li WP, Streets AJ, Ong AC, Tsiokas L. Activation of TRPP2 through mDia1-dependent voltage gating. EMBO J. 2008;27:1345–1356. doi: 10.1038/emboj.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Danto SI, Borok Z, Zhang XL, Lopez MZ, Patel P, Crandall ED, Lubman RL. Mechanisms of EGF-induced stimulation of sodium reabsorption by alveolar epithelial cells. Am. J. Physiol. 1998;275:C82–C92. doi: 10.1152/ajpcell.1998.275.1.C82. [DOI] [PubMed] [Google Scholar]
- [157].Khurana S, Nath SK, Levine SA, Bowser JM, Tse CM, Cohen ME, Donowitz M. Brush border phosphatidylinositol 3-kinase mediates epidermal growth factor stimulation of intestinal NaCl absorption and Na+/H+ exchange. J. Biol. Chem. 1996;271:9919–9927. doi: 10.1074/jbc.271.17.9919. [DOI] [PubMed] [Google Scholar]
- [158].Muto S, Furuya H, Tabei K, Asano Y. Site and mechanism of action of epidermal growth factor in rabbit cortical collecting duct. Am. J. Physiol. 1991;260:F163–F169. doi: 10.1152/ajprenal.1991.260.2.F163. [DOI] [PubMed] [Google Scholar]
- [159].Vehaskari VM, Hering-Smith KS, Moskowitz DW, Weiner ID, Hamm LL. Effect of epidermal growth factor on sodium transport in the cortical collecting tubule. Am. J. Physiol. 1989;256:F803–F809. doi: 10.1152/ajprenal.1989.256.5.F803. [DOI] [PubMed] [Google Scholar]
- [160].Vehaskari VM, Herndon J, Hamm LL. Mechanism of sodium transport inhibition by epidermal growth factor in cortical collecting ducts. Am J Physiol. 1991;261:F896–F903. doi: 10.1152/ajprenal.1991.261.5.F896. [DOI] [PubMed] [Google Scholar]
- [161].Warden DH, Stokes JB. EGF and PGE2 inhibit rabbit CCD Na+ transport by different mechanisms: PGE2 inhibits Na(+)-K+ pump. Am. J. Physiol. 1993;264:F670–F677. doi: 10.1152/ajprenal.1993.264.4.F670. [DOI] [PubMed] [Google Scholar]
- [162].Falin R, Veizis IE, Cotton CU. A role for ERK1/2 in EGF- and ATP-dependent regulation of amiloride-sensitive sodium absorption. Am. J. Physiol. Cell Physiol. 2005;288:C1003–C1011. doi: 10.1152/ajpcell.00213.2004. [DOI] [PubMed] [Google Scholar]
- [163].Shen JP, Cotton CU. Epidermal growth factor inhibits amiloride-sensitive sodium absorption in renal collecting duct cells. Am. J. Physiol. Renal Physiol. 2003;284:F57–F64. doi: 10.1152/ajprenal.00028.2002. [DOI] [PubMed] [Google Scholar]
- [164].Cao L, Owsianik G, Becq F, Nilius B. Chronic exposure to EGF affects trafficking and function of ENaC channel in cystic fibrosis cells. Biochem. Biophys. Res. Commun. 2005;331:503–511. doi: 10.1016/j.bbrc.2005.03.201. [DOI] [PubMed] [Google Scholar]
- [165].Tong Q, Stockand JD. Receptor tyrosine kinases mediate epithelial Na(+) channel inhibition by epidermal growth factor. Am. J. Physiol. Renal Physiol. 2005;288:F150–F161. doi: 10.1152/ajprenal.00261.2004. [DOI] [PubMed] [Google Scholar]
- [166].Falin RA, Cotton CU. Acute downregulation of ENaC by EGF involves the PY motif and putative ERK phosphorylation site. J. Gen. Physiol. 2007;130:313–328. doi: 10.1085/jgp.200709775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Grossmann C, Freudinger R, Mildenberger S, Krug AW, Gekle M. Evidence for epidermal growth factor receptor as negative-feedback control in aldosterone-induced Na+ reabsorption. Am. J. Physiol. Renal Physiol. 2004;286:F1226–F1231. doi: 10.1152/ajprenal.00378.2003. [DOI] [PubMed] [Google Scholar]
- [168].Markadieu N, Crutzen R, Blero D, Erneux C, Beauwens R. Hydrogen peroxide and epidermal growth factor activate phosphatidylinositol 3-kinase and increase sodium transport in A6 cell monolayers. Am. J. Physiol. Renal Physiol. 2005;288:F1201–F1212. doi: 10.1152/ajprenal.00383.2004. [DOI] [PubMed] [Google Scholar]
- [169].Liu L, Duke BJ, Malik B, Yue Q, Eaton DC. Biphasic regulation of ENaC by TGF-{alpha} and EGF in renal epithelial cells. Am. J. Physiol. Renal Physiol. 2009;296:F1417–F1427. doi: 10.1152/ajprenal.90337.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Takemura Y, Goodson P, Bao HF, Jain L, Helms MN. Rac1-mediated NADPH oxidase release of OFormula regulates epithelial sodium channel activity in the alveolar epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;298:L509–L520. doi: 10.1152/ajplung.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Michlig S, Mercier A, Doucet A, Schild L, Horisberger JD, Rossier BC, Firsov D. ERK1/2 controls Na, K-ATPase activity and transepithelial sodium transport in the principal cell of the cortical collecting duct of the mouse kidney. J. Biol. Chem. 2004;279:51002–51012. doi: 10.1074/jbc.M405674200. [DOI] [PubMed] [Google Scholar]
- [172].Booth RE, Stockand JD. Targeted degradation of ENaC in response to PKC activation of the ERK1/2 cascade. Am. J. Physiol. Renal Physiol. 2003;284:F938–F947. doi: 10.1152/ajprenal.00373.2002. [DOI] [PubMed] [Google Scholar]
- [173].Staruschenko A, Patel P, Tong Q, Medina JL, Stockand JD. Ras activates the epithelial Na(+) channel through phosphoinositide 3-OH kinase signaling. J. Biol. Chem. 2004;279:37771–37778. doi: 10.1074/jbc.M402176200. [DOI] [PubMed] [Google Scholar]
- [174].Staruschenko A, Pochynyuk OM, Tong Q, Stockand JD. Ras couples phosphoinositide 3-OH kinase to the epithelial Na+ channel. Biochim. Biophys. Acta. 2005;1669:108–115. doi: 10.1016/j.bbamem.2005.01.005. [DOI] [PubMed] [Google Scholar]
- [175].Staruschenko A, Pochynyuk O, Vandewalle A, Bugaj V, Stockand JD. Acute Regulation of the Epithelial Na+ Channel by Phosphatidylinositide 3-OH Kinase Signaling in Native Collecting Duct Principal Cells. J. Am. Soc. Nephrol. 2007;18:1652–1661. doi: 10.1681/ASN.2007010020. [DOI] [PubMed] [Google Scholar]
- [176].Mastroberardino L, Spindler B, Forster I, Loffing J, Assandri R, May A, Verrey F. Ras Pathway Activates Epithelial Na+ Channel and Decreases Its Surface Expression in Xenopus Oocytes. Mol. Biol. Cell. 1998;9:3417–3427. doi: 10.1091/mbc.9.12.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Spindler B, Mastroberardino L, Custer M, Verrey F. Characterization of early aldosterone-induced RNAs identified in A6 kidney epithelia. Pflugers Arch. 1997;434:323–331. doi: 10.1007/s004240050403. [DOI] [PubMed] [Google Scholar]
- [178].Spindler B, Verrey F. Aldosterone action: induction of p21ras and fra-2 and transcription-independent decrease in myc, jun, and fos. Am. J. Physiol. Cell Physiol. 1999;276:C1154–C1161. doi: 10.1152/ajpcell.1999.276.5.C1154. [DOI] [PubMed] [Google Scholar]
- [179].Stockand JD, Spier BJ, Worrell RT, Yue G, Al-Baldawi N, Eaton DC. Regulation of Na+ Reabsorption by the Aldosterone-induced Small G Protein K-Ras2A. J. Biol. Chem. 1999;274:35449–35454. doi: 10.1074/jbc.274.50.35449. [DOI] [PubMed] [Google Scholar]
- [180].Woodall AJ, Richards MA, Turner DJ, Fitzgerald EM. Growth factors differentially regulate neuronal Cav channels via ERK-dependent signalling. Cell Calcium. 2008;43:562–575. doi: 10.1016/j.ceca.2007.10.001. [DOI] [PubMed] [Google Scholar]
- [181].Franch HA, Wang X, Sooparb S, Brown NS, Du J. Phosphatidylinositol 3-Kinase Activity Is Required for Epidermal Growth Factor to Suppress Proteolysis. J. Am. Soc. Nephrol. 2002;13:903–909. doi: 10.1681/ASN.V134903. [DOI] [PubMed] [Google Scholar]
- [182].Mazak I, Fiebeler A, Muller DN, Park JK, Shagdarsuren E, Lindschau C, Dechend R, Viedt C, Pilz B, Haller H, Luft FC. Aldosterone Potentiates Angiotensin II-Induced Signaling in Vascular Smooth Muscle Cells. Circulation. 2004;109:2792–2800. doi: 10.1161/01.CIR.0000131860.80444.AB. [DOI] [PubMed] [Google Scholar]
- [183].Huang S, Zhang A, Ding G, Chen R. Aldosterone-induced mesangial cell proliferation is mediated by EGF receptor transactivation. Am. J. Physiol. Renal Physiol. 2009;296:F1323–F1333. doi: 10.1152/ajprenal.90428.2008. [DOI] [PubMed] [Google Scholar]
- [184].Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat. Med. 2008;14:1370–1376. doi: 10.1038/nm.1879. [DOI] [PubMed] [Google Scholar]
- [185].Fujita T. Mineralocorticoid receptors, salt-sensitive hypertension, and metabolic syndrome. Hypertension. 2010;55:813–818. doi: 10.1161/HYPERTENSIONAHA.109.149062. [DOI] [PubMed] [Google Scholar]
- [186].Silva GB, Garvin JL. Rac1 mediates NaCl-induced superoxide generation in the thick ascending limb. Am J Physiol Renal Physiol. 2009;298:F421–F425. doi: 10.1152/ajprenal.00472.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Ma HP, Chou CF, Wei SP, Eaton DC. Regulation of the epithelial sodium channel by phosphatidylinositides: experiments, implications, and speculations. Pflugers Arch. 2007;455:169–180. doi: 10.1007/s00424-007-0294-3. [DOI] [PubMed] [Google Scholar]
- [188].Berdiev BK, Shlyonsky VG, Karlson KH, Stanton BA, Ismailov II. Gating of amiloride-sensitive Na(+) channels: subunit-subunit interactions and inhibition by the cystic fibrosis transmembrane conductance regulator. Biophys. J. 2000;78:1881–1894. doi: 10.1016/S0006-3495(00)76737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Burch LH, Talbot CR, Knowles MR, Canessa CM, Rossier BC, Boucher RC. Relative expression of the human epithelial Na+ channel subunits in normal and cystic fibrosis airways. Am. J. Physiol. 1995;269:C511–C518. doi: 10.1152/ajpcell.1995.269.2.C511. [DOI] [PubMed] [Google Scholar]
- [190].Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- [191].Stutts MJ, Rossier BC, Boucher RC. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. J Biol. Chem. 1997;272:14037–14040. doi: 10.1074/jbc.272.22.14037. [DOI] [PubMed] [Google Scholar]
- [192].Berdiev BK, Qadri YJ, Benos DJ. Assessment of the CFTR and ENaC association. Mol. Biosyst. 2009;5:123–127. doi: 10.1039/b810471a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat. Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- [194].Myerburg MM, Butterworth MB, McKenna EE, Peters KW, Frizzell RA, Kleyman TR, Pilewski JM. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: a mechanism for sodium hyperabsorption in cystic fibrosis. J. Biol. Chem. 2006;281:27942–27949. doi: 10.1074/jbc.M606449200. [DOI] [PubMed] [Google Scholar]
- [195].Hanaoka K, Devuyst O, Schwiebert EM, Wilson PD, Guggino WB. A role for CFTR in human autosomal dominant polycystic kidney disease. Am. J. Physiol. 1996;270:C389–C399. doi: 10.1152/ajpcell.1996.270.1.C389. [DOI] [PubMed] [Google Scholar]
- [196].Magenheimer BS, St John PL, Isom KS, Abrahamson DR, De Lisle RC, Wallace DP, Maser RL, Grantham JJ, Calvet JP. Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na(+),K(+),2Cl(−) Co-transporter-dependent cystic dilation. J. Am. Soc. Nephrol. 2006;17:3424–3437. doi: 10.1681/ASN.2006030295. [DOI] [PubMed] [Google Scholar]
- [197].Yang B, Sonawane ND, Zhao D, Somlo S, Verkman AS. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. 2008;19:1300–1310. doi: 10.1681/ASN.2007070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Nakanishi K, Sweeney WE, Jr., MacRae DK, Cotton CU, Avner ED. Role of CFTR in autosomal recessive polycystic kidney disease. J. Am. Soc. Nephrol. 2001;12:719–725. doi: 10.1681/ASN.V124719. [DOI] [PubMed] [Google Scholar]
- [199].Bens M, Chassin C, Vandewalle A. Regulation of NaCl transport in the renal collecting duct: lessons from cultured cells. Pflugers Arch. 2006;453:133–146. doi: 10.1007/s00424-006-0123-0. [DOI] [PubMed] [Google Scholar]
- [200].Hamm LL, Feng Z, Hering-Smith KS. Regulation of sodium transport by ENaC in the kidney. Curr. Opin. Nephrol. Hypertens. 2010;19:98–105. doi: 10.1097/MNH.0b013e328332bda4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Gray MA. Primary cilia and regulation of renal Na+ transport. Focus on “Heightened epithelial Na+ channel-mediated Na+ absorption in a murine polycystic kidney disease model epithelium lacking apical monocilia”. Am. J. Physiol. Cell Physiol. 2006;290:C947–C949. doi: 10.1152/ajpcell.00640.2005. [DOI] [PubMed] [Google Scholar]
- [202].Olteanu D, Yoder BK, Liu W, Croyle MJ, Welty EA, Rosborough K, Wyss JM, Bell PD, Guay-Woodford LM, Bevensee MO, Satlin LM, Schwiebert EM. Heightened epithelial Na+ channel-mediated Na+ absorption in a murine polycystic kidney disease model epithelium lacking apical monocilia. Am. J. Physiol. Cell Physiol. 2006;290:C952–C963. doi: 10.1152/ajpcell.00339.2005. [DOI] [PubMed] [Google Scholar]
- [203].Veizis EI, Carlin CR, Cotton CU. Decreased amiloride-sensitive Na+ absorption in collecting duct principal cells isolated from BPK ARPKD mice. Am. J. Physiol. Renal Physiol. 2004;286:F244–F254. doi: 10.1152/ajprenal.00169.2003. [DOI] [PubMed] [Google Scholar]
- [204].Veizis IE, Cotton CU. Abnormal EGF-dependent regulation of sodium absorption in ARPKD collecting duct cells. Am. J. Physiol. Renal Physiol. 2005;288:F474–F482. doi: 10.1152/ajprenal.00227.2004. [DOI] [PubMed] [Google Scholar]
- [205].Rohatgi R, Greenberg A, Burrow CR, Wilson PD, Satlin LM. Na transport in autosomal recessive polycystic kidney disease (ARPKD) cyst lining epithelial cells. J. Am. Soc. Nephrol. 2003;14:827–836. doi: 10.1097/01.asn.0000056481.66379.b2. [DOI] [PubMed] [Google Scholar]
- [206].Calvet JP, Grantham JJ. The genetics and physiology of polycystic kidney disease. Semin. Nephrol. 2001;21:107–123. doi: 10.1053/snep.2001.20929. [DOI] [PubMed] [Google Scholar]
- [207].Ye M, Grant M, Sharma M, Elzinga L, Swan S, Torres VE, Grantham JJ. Cyst fluid from human autosomal dominant polycystic kidneys promotes cyst formation and expansion by renal epithelial cells in vitro. J. Am. Soc. Nephrol. 1992;3:984–994. doi: 10.1681/ASN.V34984. [DOI] [PubMed] [Google Scholar]
- [208].Rohatgi R, Zavilowitz B, Vergara M, Woda C, Kim P, Satlin LM. Cyst fluid composition in human autosomal recessive polycystic kidney disease. Pediatr. Nephrol. 2005;20:552–553. doi: 10.1007/s00467-004-1728-1. [DOI] [PubMed] [Google Scholar]
- [209].Hogan MC, Masyuk TV, Page LJ, Kubly VJ, Bergstralh EJ, Li X, Kim B, King BF, Glockner J, Holmes DR, III, Rossetti S, Harris PC, LaRusso NF, Torres VE. Randomized Clinical Trial of Long-Acting Somatostatin for Autosomal Dominant Polycystic Kidney and Liver Disease. J. Am. Soc. Nephrol. 2010;21:1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]