Abstract
We characterized the distribution of AMPA receptor (AMPAR) subunits and the transmembrane AMPA receptor regulatory proteins (TARPs) γ-2 and γ-4 in adult rat nucleus accumbens (NAc) using a method that separates plasma membranes into synaptic membrane-enriched and extrasynaptic membrane-enriched fractions. We also measured GluA1 phosphorylated at serine 845 (pS845 GluA1) and serine 831 (pS831 GluA1). GluA1–3 protein levels and pS831 GluA1/total GluA1 were higher in synaptic membranes. However, pS845 GluA1/total GluA1 was higher in extrasynaptic membranes, consistent with a role for S845 phosphorylation in GluA1 insertion at extrasynaptic sites. Homeric GluA1 receptors were detected in extrasynaptic membranes, consistent with evidence for extrasynaptic Ca2+-permeable AMPARs in other systems. The TARP γ-2 was enriched in synaptic membranes, whereas γ-4 was mainly found in extrasynaptic membranes, suggesting distinct roles for these proteins in the NAc. These experiments provide fundamental information that will aid in the interpretation of studies on AMPAR-related plasticity in the NAc.
Keywords: addiction, Ca2+-permeable AMPA receptor, NMDA receptor, plasticity, subcellular fractionation, TARP
Introduction
It is well established that alterations in glutamate transmission in the nucleus accumbens (NAc) contribute to consequences of long-term cocaine exposure in animal models of addiction. Recent work has focused on cocaine-induced changes in surface expression and subunit composition of AMPA-type glutamate receptors (AMPARs) in the NAc [40] because of the role these that such adaptations play in activity-dependent synaptic plasticity [36]. The interpretation of studies on cocaine-induced AMPAR plasticity would be aided by a better understanding of AMPAR properties in the NAc of adult, drug-naïve rats.
Recent studies have provided information about AMPAR subunit composition in the NAc. Electrophysiological results indicate that synaptic AMPARs in the NAc are primarily GluA2-containing (e.g., [4,21]). Supporting this, biochemical studies suggest that GluA1A2 receptors are the most common subtype in the NAc [4,33], similar to the hippocampus [24]. AMPARs lacking the GluA2 subunit, hereafter termed Ca2+-permeable AMPARs (CP-AMPARs), comprise less than 10% of AMPARs in NAc membrane preparations; these CP-AMPARs may be homomeric GluA1, homomeric GluA3 or GluA1A3 receptors [4,33]. We are particularly interested in the regulation of CP-AMPARs in the NAc because they mediate the intensified (“incubated”) cocaine craving that occurs after withdrawal from extended-access cocaine self-administration [4,26].
Despite recent interest in AMPAR subunit composition, there is no information about synaptic versus extrasynaptic AMPAR populations in the NAc. This is a significant gap in current knowledge because of the important role of extrasynaptic AMPARs, particularly CP-AMPARs, in supplying synaptic pools during plasticity (see Conclusions). There is also no information available regarding transmembrane AMPA receptor regulatory proteins (TARPs) in the NAc, despite their important role in regulating AMPAR trafficking, channel properties and glutamate affinity [19]. The goal of this study was to characterize the synaptic versus extrasynaptic distribution of AMPAR subunits and the TARPs y-2 and γ-4 in the adult rat NAc using a previously described subcellular fractionation method [6,7,13].
Materials and methods
Experiments were approved by our institutional Animal Care and Use Committee. For studies of TARP distribution, brain regions were dissected from male Sprague Dawley rats (>PD60; Harlan Laboratories, Indianapolis, IN, USA; 275–300g), wild-type mice, and stargazer mutant mice which lack γ-2 (B6C3Fe-a/a-Cacng2stg/J, Jackson Laboratory), and homogenized in lysis buffer [10] prior to SDS-PAGE. All experiments compared fractions from three independent samples, each obtained by pooling NAc tissue from two rats, except for GluA2/3 immunodepletion experiments, which used four samples. Each sample was processed to obtain fractions enriched for synaptic and extrasynaptic membranes, as described previously [6,7,13]. This method relies on the insolubility of postsynaptic densities (PSD) and synaptic junctions in Triton X-100. A crude membrane fraction (P2) can therefore be separated, based on Triton X-100 solubility, into an insoluble fraction enriched for synaptic membranes (TxP) and a soluble fraction enriched for extrasynaptic membranes (TxS; Fig. 1A). Briefly, each NAc sample was homogenized in 6ml of sucrose homogenization buffer (10mM HEPES, 0.32M sucrose, 1mM Na3VO4, 5mM NaF, 2mM EDTA, pH 7.4) in a glass grinding vessel with rotating Teflon pestle (Wheaton Overhead Stirrer; 3000 RPM, 12 passes). The homogenate was centrifuged (800 × g, 10 min, 4°C). The resulting supernatant (S1) was centrifuged (10,000 × g, 15 min, 4°C) to yield a crude membrane pellet (P2). In preliminary experiments, the supernatant from the P2 fraction (S2) was collected and centrifuged (100,000 × g, 60 min) to yield the intracellular light membrane fraction (P3), which was used for initial characterization studies. The P2 pellet was washed twice with sucrose homogenization buffer and re-suspended in 4ml of sucrose homogenization buffer containing Triton X-100 (0.5%v/v) using a motorized pellet pestle mixing/grinding rod (Kontes, Vineland, NJ). The suspension was then incubated with gentle rotation (4°C, 20 min) and centrifuged (32,000 × g, 20 min) to yield the insoluble fraction (TxP; this pellet was washed twice before use) and the soluble fraction (TxS). The TxS fraction was concentrated by adding 8 volumes of cold acetone, incubating overnight (−20°C), and centrifuging (3000 × g). The concentrated TxS pellet was solubilized in sucrose-Triton buffer containing 1% SDS. The TxP fraction was solubilized in 200µl of the same buffer using 30 passes of the motorized pestle. Samples were stored at −80°C.
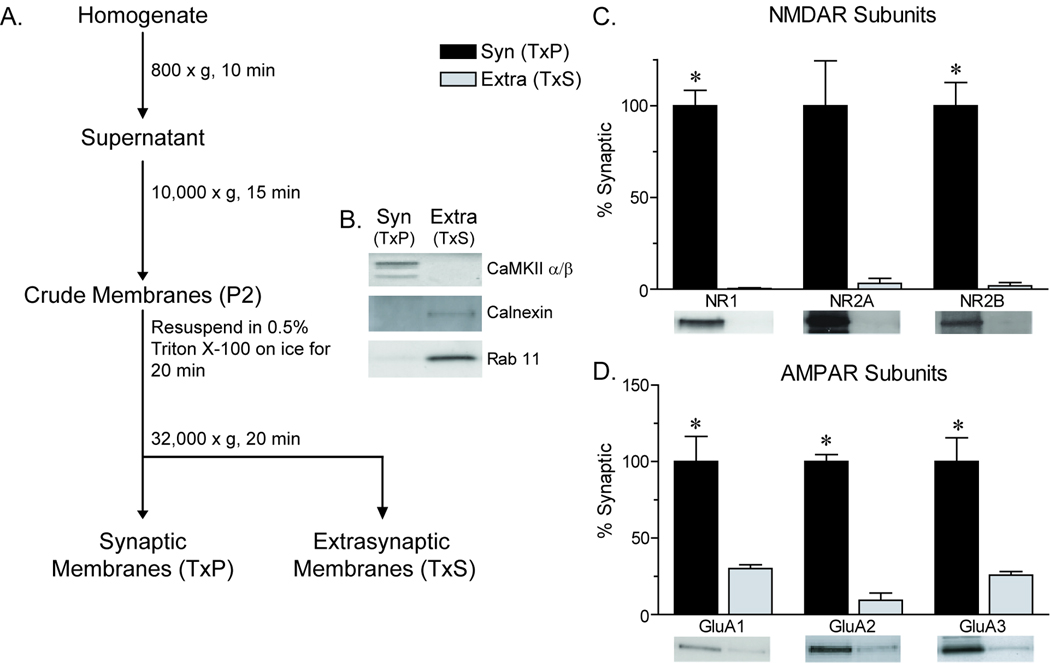
Figure 1.
Characterization of fractions enriched for synaptic membranes (TxP) and extrasynaptic membranes (TxS) and distribution of NMDAR and AMPAR subunits in the adult rat NAc. A) Schematic of the fractionation procedure. B) Representative Western blots demonstrating levels of marker proteins in TxP and TxS fractions. C) Relative abundance of NMDAR subunits in each fraction. NR1 and NR2B were significantly more abundant in synaptic membranes (*p≤0.05). A similar difference, which approached significance, was seen for NR2A (p=0.06). D) Relative abundance of AMPAR subunits in each fraction. GluA1–3 were detected in both fractions, but were significantly enriched in synaptic membranes (*p≤0.05). Data are presented as mean ± SEM (N=3) normalized to values for the synaptic membrane fraction, with representative blots shown below each bar. Protein loaded per lane: 5µg.
To assess homomeric GluA1 receptors in the extrasynaptic fraction, GluA2 and GluA3 were depleted from the sample using immunoprecipitation (IP). For these experiments, we used the TxS fraction without acetone concentration. First, 3µg of GluA2/3 antibody (AB1506; Millipore, Billerica, MA) or normal rabbit IgG (12–370; Millipore) was incubated (4 h, 4°C) with 10µl of 50% protein A/G-agarose beads (20421; Thermo Scientific/Pierce, Rockford, IL). Then, antibody-coated beads were added to an aliquot of TxS (~80µg in 400µl) and incubated overnight (4°C) with constant rotation. The sample was then centrifuged, the supernatant was collected, and the process was repeated to ensure complete removal of GluA2 and GluA3. The supernatant, after the second round of IP, was termed the “unbound fraction”.
Samples were heated (70°C, 10 min) in Laemmli sample treatment buffer with 100mM dithiothreitol and then processed for SDS-PAGE and immunoblotting [10]. Protein concentration was determined using the BioRad kit (BioRad, Hercules, CA). The following antibodies were used: GluA1 (1:1000, Thermo Scientific/Pierce; PA1-37776), pS831 GluA1 (1:500, PhosphoSolutions, Aurora, CO; p1160-831), pS845 GluA1 (1:500, PhosphoSolutions; p1160-845), GluA2 (1:200, UC Davis/NIH NeuroMab Facility, Davis, CA; 75-002), GluA3 (1:500, Cell Signaling Technology, Danvers, MA; 3437), NR1 (1:300, Novus Biologicals, Littleton, CO; NB300-118), NR2A (1:500, Santa Cruz Biotechnology, Santa Cruz, CA; SC-1468), NR2B (1:1000, Calbiochem-EMD Chemicals, Gibbstown, NJ; 454582), y2 (1:1000, PhosphoSolutions; 1505-STAR), y4 (1:500, Millipore; AB5795), PSD-95 (1:30,000, UC Davis/NIH NeuroMab Facility; 75-028), Calnexin (1:300, Santa Cruz Biotechnology; SC-11397), CaMKIIα (1:20,000, Millipore; MAB8699), CaMKIIβ (1:500, Abcam, Cambridge, MA; AB34703), Rab11 (1:500, Invitrogen, Carlsbad, CA; 71-5300). Paired t-tests (two-tailed) were used to compare the relative abundance of proteins in synaptic versus extrasynaptic fractions.
Results and discussion
Representative immunoblots from the synaptic membrane-enriched and extrasynaptic membrane-enriched fractions are shown in Fig 1B. Consistent with previous results using this approach [6,7,13], the synaptic markers CaMKII and PSD-95 were concentrated in synaptic membranes and virtually undetectable in extrasynaptic membranes, whereas calnexin was detected in extrasynaptic but not synaptic membranes (Fig. 1B), although it was most enriched in the P3 fraction (data not shown). Calnexin’s presence in extrasynaptic membranes is consistent with its expression in the plasma membrane (see [6]). We also evaluated rab11 because of its role in the trafficking of recycling endosomes containing AMPARs to the plasma membrane [31]. In cultured NAc neurons, AMPARs are initially inserted into extrasynaptic regions of the plasma membrane [38]. Consistent with this, rab11 was enriched in extrasynaptic membranes (Fig. 1B). Finally, the NMDAR subunits NR1, NR2A and NR2B were predominantly found in synaptic membranes (Fig. 1C), consistent with prior results [6,7,13]. These results confirm that our fractions are enriched for synaptic and extrasynaptic membranes.
Next, we evaluated the relative abundance of AMPAR subunits GluA1–3 in synaptic and extrasynaptic membrane fractions from the adult rat NAc. All AMPAR subunits were significantly more abundant in synaptic than extrasynaptic membrane fractions (Fig. 1D). Because of the potential role of NAc GluA1 phosphorylation in motivated behaviors (e.g., [5]), we used phosphospecific antibodies to evaluate levels of GluA1 phosphorylated on serine 845 (pS845 GluA1) or serine 831 (pS831 GluA1). Serine 845 is phosphorylated by PKA while serine 831 is phosphorylated by CaMKII and PKC [1,27,34,]. Fig. 2 (panels A and B) shows the relative abundance of pS845 GluA1 and pS831 GluA1 and the ratio of phosphorylated to total GluA1 protein in synaptic and extrasynaptic membranes. pS845 GluA1/total GluA1 was significantly higher in extrasynaptic membranes (Fig. 2A), as found in hippocampus [7]. These results are consistent with evidence that PKA phosphorylation of GluA1 facilitates AMPAR insertion into extrasynaptic membranes (see Conclusions). The ratio of pS831 GluA1/total GluA1 was significantly higher in synaptic than extrasynaptic membranes (Fig. 2B), consistent with evidence that synaptic GluA1 is phosphorylated at serine 831 [1].
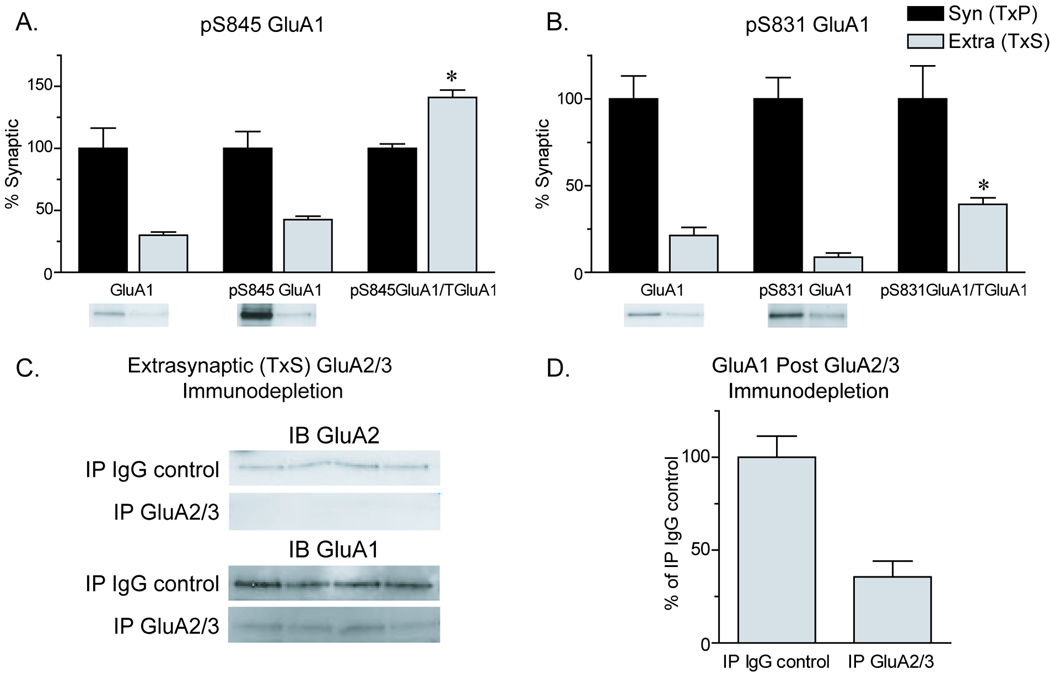
Figure 2.
GluA1 phosphorylation in synaptic and extrasynaptic membrane fractions and detection of extrasynaptic CP-AMPARs in the adult rat NAc. A) Relative levels of GluA1, pS845 GluA1 and pS845 GluA1/total GluA1. The ratio of pS845 GluA1/total GluA1 was significantly greater in extrasynaptic membranes compared to synaptic membranes (*p≤0.05). B) Relative levels of GluA1, pS831 GluA1, and pS831 GluA1/total GluA1. The ratio of pS831 GluA1/total GluA1 showed the opposite pattern to pS845 GluA1/total GluA1, with significantly lower relative phosphorylation in extrasynaptic versus synaptic membranes (*p≤0.05). C) Using NAc extrasynaptic membranes as starting material, an immunodepletion strategy was used to determine if homomeric GluA1 receptors are present in extrasynaptic membranes. Immunoprecipitation (IP) was performed with GluA2/3 antibody or control IgG antibody (IP IgG control). The unbound fraction remaining after IP was immunoblotted (IB) to detect remaining AMPAR subunits. IP with GluA2/3 antibody removed >95% of GluA2 (upper blots) and GluA3 (data not shown) but a portion of GluA1 remained (lower blots). D) The amount of GluA1 remaining in the unbound fraction after IP with GluA2/3 antibody shown as percentage of the IP IgG control. Approximately 35% (±8.5%) of GluA1 protein remained after depletion of GluA2-and GluA3-containing AMPARs, consistent with an extrasynaptic population of homomeric GluA1 AMPARs. Data are presented as mean ± SEM (N=3 for panels A and B; N=4 for panels C and D), with representative blots shown beneath each bar. Protein loaded per lane: 5µg (panels A–B); 7.5µg (panels C–D).
In light of the importance of extrasynaptic CP-AMPARs in the hippocampus [15,16,22], we assessed their existence in the NAc. Using the extrasynaptic membrane fraction as starting material, we used GluA2/3 antibody to IP AMPARs that contain GluA2 or GluA3, leaving homomeric GluA1 receptors in the unbound fraction. Immunoblotting of the unbound fraction confirmed that less than 5% of GluA2 and GluA3 remained after 2 rounds of IP (Fig. 2C). Our previous work in a crude membrane fraction (containing synaptic and extrasynaptic membranes) showed that ~7% of GluA1 in the NAc is not physically associated with GluA2 or GluA3, and is therefore present either in homomeric GluA1 receptors (tetramers) or in GluA1 monomers or dimers [33]. Here we found that ~35 ± 8% of the GluA1 protein originally present in the extrasynaptic membrane fraction remained after IP of GluA2 and GluA3, consistent with the presence of homomeric GluA1 AMPARs at extrasynaptic sites (Fig. 2D). Compared to the 7% value obtained in a crude membrane fraction, this suggests an enrichment of homomeric GluA1 AMPARs in extrasynaptic membranes. We also compared pS845 GluA1 levels in the extrasynaptic membrane fraction (starting material for IP) and the unbound fraction that remained after GluA2/3 IP. Some pS845 GluA1 signal was detected in the unbound fraction, although it was too low to quantify reliably (data not shown). Nevertheless, these data suggest the existence of extrasynaptic homomeric GluA1 AMPARs that are phosphorylated on S845.
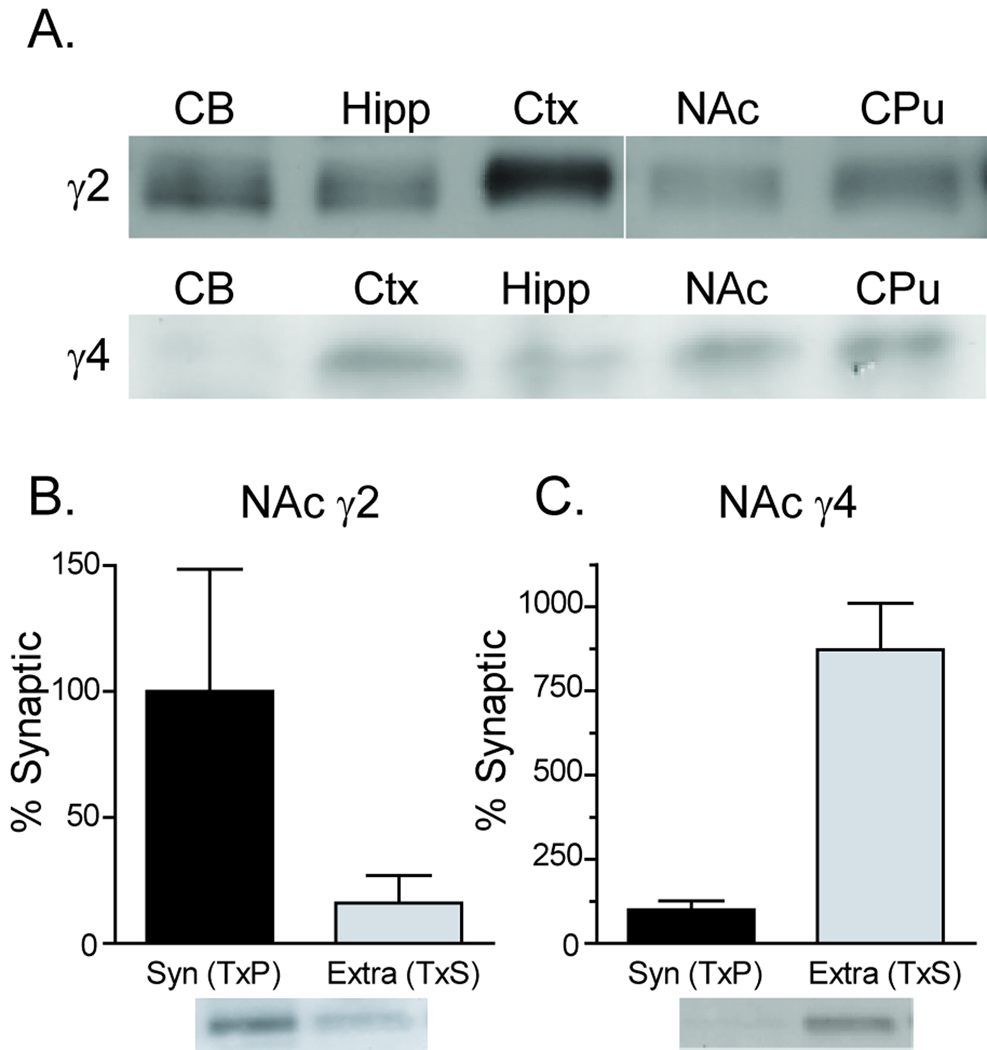
Next we evaluated TARP distribution, focusing on γ-2 because it is the prototypical TARP and is expressed in the NAc and on γ-4 because it is abundant in the striatum; γ-3 is also expressed in the striatum, but a reliable antibody was not available [11,20,35,39]. Specificity of the γ-2 antibody was confirmed in tissue from stargazer mutant mice which lack γ-2 (data not shown). As expected, γ-2 was found in homogenates from all regions, with high expression in cerebellum and cortex (Fig. 3A). We could not obtain γ-4 knockout mice; however, the γ-4 antibody recognized a band slightly larger than γ-2, as predicted (e.g. [35]). Consistent with prior results [35,39], γ-4 immunoreactivity was detected in homogenates from all regions except the cerebellum (Fig. 3A). In NAc synaptic and extrasynaptic membrane fractions, γ-2 and γ-4 showed opposite expression patterns. γ-2 was more abundant in synaptic membranes, whereas γ-4 was more abundant in extrasynaptic membranes (Fig. 3B,C). To confirm these results, we measured γ-4 in a classical PSD fraction from NAc [14] and found that γ-4 was nearly undetectable whereas the γ-2 signal was strong (data not shown). Our results are the first to link γ-4 to an extrasynaptic function. However, a prior study in hippocampal and cortical neuronal cultures indicated a predominantly synaptic role for γ-2 whereas γ-8 seemed to have both synaptic and extrasynaptic roles [18]. We have observed that γ-8 is not highly expressed in cultured NAc medium spiny neurons (data not shown). Although type Ia TARPs (γ-2 and γ-3) and Type Ib TARPS (γ-4 and γ-8) both enhance ion flow through AMPARs by altering glutamate affinity and channel properties, the Type Ib TARPs produce a more robust enhancement of AMPAR transmission [19]. Thus, the preferential expression of γ-2 synaptically and γ-4 extrasynaptically may suggest relatively greater ion flow through extrasynaptic AMPARs.
Figure 3.
Expression of the TARPs γ-2 and γ-4 in synaptic and extrasynaptic membranes. A) Immunoblots of γ-2 (upper) and γ-4 (lower) protein in homogenates from the indicated brain regions (note different order of brain regions for upper and lower images). γ-2 was abundant in cerebellum (CB), hippocampus (Hipp) and prefrontal cortex (PFC) and present in both nucleus accumbens (NAc) and caudate putamen (CPu) whereas γ-4 was not present in the CB but was found in all other regions examined. B) The relative abundance of γ-2 and γ-4 protein in each fraction was quantified for adult rat NAc tissue; γ-2 was more abundant in synaptic membranes whereas γ4 was more abundant in extrasynaptic membranes. Data are presented as mean ± SEM (N=3) normalized to values for the synaptic membrane fraction, with representative blots shown beneath each bar. Protein loaded per lane: 20µg (panel A) and 5µg (panel B).
In conclusion, our results demonstrate that GluA1–3 are mainly detected in synaptic membranes but are also present in extrasynaptic membranes. When we specifically analyzed GluA1 phosphorylated at the PKA site (S845), we found a relative enrichment in extrasynaptic membranes. In cultured NAc neurons, GluA1-containing AMPARs are incorporated into synapses through a two-step process involving insertion onto the cell surface at extrasynaptic sites followed by NMDAR-dependent translocation into the synapse; the first step is accelerated by PKA activation, most likely via phosphorylation of GluA1 at S845 [2,3,29,38,41]. These in vitro results are consistent with the observed in vivo enrichment of pS845 GluA1 in extrasynaptic membranes. Studies in other brain regions also support a role for extrasynaptic surface AMPARs in supplying the synapse (e.g., [17,23,25,32,42,44]) and similarly indicate that PKA phosphorylation of GluA1 primes AMPARs for synaptic insertion [8,12,28,30,37]. Interestingly, we found that homomeric GluA1 CP-AMPARs are present in extrasynaptic NAc membranes. Taken together with the enrichment of pS845 GluA1 in this fraction, these data suggest PKA phosphorylation of GluA1 located within homomeric GluA1 receptors. In the hippocampus, perisynaptic CP-AMPARs are important for increasing synaptic strength during LTP [15,43] and PKA phosphorylation of GluA1 stabilizes this pool of CP-AMPARs [16]. If the same regulatory mechanism operates in the NAc, then the stabilization of extrasynaptic CP-AMPARs (via increased PKA phosphorylation) could help explain their accumulation in NAc synapses after prolonged withdrawal from extended-access cocaine self-administration [4,26]. Consistent with this idea, we recently found increased pS845 GluA1 levels in extrasynaptic NAc membranes prepared on withdrawal day 45 from such a regimen [9]. Finally, our results raise the exciting prospect that, in the NAc, the TARPs γ-2 and γ-4 are preferentially involved in regulating synaptic and extrasynaptic AMPARs, respectively.
Research highlights.
AMPAR subunits are more abundant in synaptic than extrasynaptic rat NAc membranes
GluA1 in NAc extrasynaptic membranes shows relatively greater S845 phosphorylation
GluA1 in NAc synaptic membranes shows relatively greater S831 phosphorylation
Homomeric GluA1 AMPARs are found in NAc extrasynaptic membranes
TARP y2 is enriched in NAc synaptic membranes; y4 is predominantly extrasynaptic
Acknowledgements
These studies were supported by DA015835, DA000453 and DA029099 to M.E.W. and postdoctoral NRSA DA024502 to C.R.F. We thank Dr. Makoto Itakura (Kitasato University School of Medicine, Kanagawa, Japan) for generously supplying the γ-8 antibody and Dr. Susumu Tomita (Yale University, New Haven, CT) for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
C.R.F. and M.E.W. designed the study and wrote the paper. All authors performed the experiments and approved the final article. M.E.W. has no biomedical financial interests but has a patent on A Possible Therapy For Cue-Induced Cocaine Craving Leading to Relapse in Abstinent Cocaine Abusers Based on Blockade of GluR2-lacking AMPA Receptors in the Nucleus Accumbens. The other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 2.Chao SZ, Ariano MA, Peterson DA, Wolf ME. D1 dopamine receptor stimulation increases GluR1 surface expression in nucleus accumbens neurons. J. Neurochem. 2002;83:704–712. doi: 10.1046/j.1471-4159.2002.01164.x. [DOI] [PubMed] [Google Scholar]
- 3.Chao SZ, Lu W, Lee HK, Huganir RL, Wolf ME. D(1) dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J. Neurochem. 2002;81:984–992. doi: 10.1046/j.1471-4159.2002.00877.x. [DOI] [PubMed] [Google Scholar]
- 4.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng L, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crombag HS, Sutton JM, Takamiya K, Holland PC, Gallagher M, Huganir RL. A role for alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid GluR1 phosphorylation in the modulatory effects of appetitive reward cues on goal-directed behavior. Eur. J. Neurosci. 2008;27:3284–3291. doi: 10.1111/j.1460-9568.2008.06299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies KD, Alvestad RM, Coultrap SJ, Browning MD. αCaMKII autophosphorylation levels differ depending on subcellular localization. Brain Res. 2007;1158:39–49. doi: 10.1016/j.brainres.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies KD, Goebel-Goody SM, Coultrap SJ, Browning MD. Browning, Long-term synaptic depression that is associated with GluR1 dephosphorylation but not amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor internalization. J. Biol. Chem. 2008;283:33138–33146. doi: 10.1074/jbc.M803431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci. 2003;62:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- 9.Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galiñanes GL, Heng L-J, Tseng KY, Wolf ME. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacol. doi: 10.1016/j.neuropharm.2011.01.021. (in revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacol. 2010;35:818–833. doi: 10.1038/npp.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukaya M, Yamazaki M, Sakimura K, Watanabe M. Spatial diversity in gene expression for VDCCgamma subunit family in developing and adult mouse brains. Neurosci. Res. 2005;53:376–383. doi: 10.1016/j.neures.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Gao C, Sun X, Wolf ME. Activation of D1 dopamine receptors increases surface expression of AMPA receptors and facilitates their synaptic incorporation in cultured hippocampal neurons. J. Neurochem. 2006;98:1664–1677. doi: 10.1111/j.1471-4159.2006.03999.x. [DOI] [PubMed] [Google Scholar]
- 13.Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-D-aspartate receptors in adult hippocampal slices. Neurosci. 2009;158:1146–1159. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee H-K. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat. Neurosci. 2006;9:1001–1003. doi: 10.1038/nn1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guire ES, Oh MC, Soderling TR, Derkach VA. Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I. J. Neurosci. 2008;28:6000–6009. doi: 10.1523/JNEUROSCI.0384-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee H-K. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc. Natl. Acad. Sci. USA. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heine M, Groc L, Frischknecht R, Béïque J-C, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface motility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inamura M, Itakura M, Okamoto H, Hoka S, Mizoguchi A, Fukazawa Y, Shigemoto R, Yamamori S, Takahashi M. Differential localization and regulation of stargazin-like protein, gamma-8 and stargazin in the plasma membrane of hippocampal and cortical neurons. Neurosci. Res. 2006;55:45–53. doi: 10.1016/j.neures.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Kato AS, Gill MB, Yu H, Nisenbaum ES, Bredt DS. TARPS differentially decorate AMPA receptors to specify neuropharmacology. Trends Neurosci. 2010;33:241–248. doi: 10.1016/j.tins.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Klugbauer N, Dai S, Specht V, Lacinová L, Marais E, Bohn G, Hofmann F. A family of gamma-like calcium channel subunits FEBS Lett. 2000;470:189–197. doi: 10.1016/s0014-5793(00)01306-5. [DOI] [PubMed] [Google Scholar]
- 21.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J. Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonoudakis D, Zhao P, Beattie EC. Rapid tumor necrosis factor α-induced exocytosis of glutamate receptor 2-lacking AMPA receptors to extrasynaptic plasma membrane potentiates excitotoxicity. J. Neurosci. 2008;28:2119–2130. doi: 10.1523/JNEUROSCI.5159-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin D-T, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL. Regulation of AMPA receptor extrasynaptic insertion by 4.1 N, phosphorylation and palmitoylation. Nat. Neurosci. 2009;12:879–887. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: The role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Lüscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat. Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 27.Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J. Biol. Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- 28.Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc. Natl. Acad. Sci. USA. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangiavacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J. Neurochem. 2004;88:1261–1271. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- 30.Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J. Biol. Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- 31.Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 32.Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat. Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- 33.Reimers JM, Milovanovic M, Wolf ME. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2010 doi: 10.1016/j.brainres.2010.10.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- 35.Sharp AH, Black JL, III, Dubel SJ, Sundarraj S, Shen J-P, Yunker AMR, Copeland TD, McEnery MW. Biochemical and anatomical evidence for specialized voltage-dependent calcium channel y isoform expression in the epileptic and ataxic mouse, stargazer. Neuroscience. 2001;105:599–617. doi: 10.1016/s0306-4522(01)00220-2. [DOI] [PubMed] [Google Scholar]
- 36.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 37.Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J. Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons co-cultured with prefrontal cortex neurons. J. Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J. Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci. Biobehav. Rev. 2010 doi: 10.1016/j.neubiorev.2010.01.013. Epub Jan 28. PMID: 20109488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf ME. Regulation of AMPA receptor trafficking in the nucleus accumbens by dopamine and cocaine. Neurotoxicol. Res. 2010;18:393–409. doi: 10.1007/s12640-010-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y, Wang XB, Frerking M, Zhou Q. Delivery of AMPA receptors to perisynaptic sites precedes the full expression of long-term potentiation. Proc. Natl. Acad. Sci. USA. 2008;105:11388–11393. doi: 10.1073/pnas.0802978105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y, Wang X-B, Zhou Q. Perisynaptic GluR2-lacking AMPA receptors control the reversibility of synaptic and spines modifications. Proc. Natl. Acad. Sci. USA. 2010;107:11999–12004. doi: 10.1073/pnas.0913004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yudowski GA, Puthenveedu MA, Leonoudakis D, Panicker S, Thorn KS, Beattie EC, von Zastrow M. Real-time imaging of discrete exocytotic events mediating surface delivery of AMPA receptors. J. Neurosci. 2007;27:11112–11121. doi: 10.1523/JNEUROSCI.2465-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]