Abstract
An outbred rat model of the novelty-seeking phenotype is used to study nicotine vulnerability, where experimentally naïve rats were phenotype screened as high or low responders (HRs or LRs, ranking in the upper or lower 1/3rd of the population respectively) based on locomotor activity displayed in a novel environment. Following nicotine training and abstinence, HR animals pre-trained with nicotine showed expression of locomotor sensitization to nicotine challenge along with enhanced social anxiety-like behavior in the social interaction test compared to saline pre-trained controls. HR rats also showed a downregulation in neuropeptide Y (NPY) mRNA levels in the medial nucleus of amygdala and the CA1 field of the hippocampus, an upregulation in Y2 mRNA levels in the CA3 field of the hippocampus, and an upregulation in the corticotropin releasing factor (CRF) mRNA levels in the central nucleus of the amygdala. These findings implicate dysregulations in the NPY-CRF systems in the HR hippocampus and amygdala associated with the emergence of social anxiety-like behavior, and a novel Y2R-mediated pathway in nicotine relapse.
Keywords: central nucleus of amygdala, medial nucleus of amygdala, hippocampus, novelty-seeking phenotype, locomotor sensitization, anxiety-like behavior
The novelty-seeking phenotype is an outbred rat model of individual differences that has predictive value for nicotine vulnerability. Some rats display high locomotor reactivity in novel environments and are identified as high responders or HRs, whereas some display low locomotor reactivity in novel environments and are identified as low responders or LRs. HR rats are known to acquire nicotine self-administration more readily than LR rats and work more to obtain the drug when tested under the progressive ratio schedule of reinforcement [23]. Our laboratory reported that adolescent HRs develop locomotor sensitization to a low dose nicotine challenge following chronic intermittent nicotine training and 1 wk of abstinence [1, 2], and even a single nicotine training with a mild dose is sufficient for the expression of locomotor sensitization to a low dose challenge [1], further validating this phenotype in the study of individual differences in vulnerability to nicotine.
Nicotine withdrawal-associated anxiety has been hypothesized as an important factor contributing to smoking maintenance in humans [11], and is studied in rodents using several tests of anxiety-like behavior [4, 15]. In the present experiment we assess whether repeated nicotine training in the nicotine vulnerable HR phenotype leads to the emergence of negative affect in the form of anxiety-like behavior in response to a low dose nicotine challenge following abstinence. We are particularly interested in anxiety measured on the social interaction test as this test is different from traditional indices of anxiety such as performance on the elevated plus maze (EPM) or the light/dark box (LDB) in that it measures a behavioral correlate of social anxiety akin to social phobia [5].
Repeated drug exposure and withdrawal mediate adaptations in the extended amygdala that is hypothesized to control the switch from homeostasis to pathophysiology associated with drug abuse [13]. Nicotine withdrawal following chronic nicotine exposure increases CRF release in the central nucleus of amygdala (CeA), which is shown to mediate defensive burying [6], suggesting that increased CRF function in the amygdala may underlie behavioral aspects of anxiety associated with nicotine abstinence [12]. In addition to CRF, hippocampal and amygdalar NPY also play an important role in the neurocircuitry controlling anxiety [8]. In the amygdala, NPY and CRF produce opposing actions to conserve a balanced emotional state with CRF being anxiogenic and NPY being anxiolytic [7]. Brain anti-anxiety system, in particular NPY in the amygdala, is dysregulated during the development of dependence, which compromises a mechanism for restoring homeostasis [14]. In line with this, rats exposed to chronic nicotine during adolescence show a decreased ratio of NPY to CRF immunoreactivity in the amygdala long after discontinuation of the drug [21]. Administration of NPY or a Y1 receptor agonist during acute nicotine withdrawal is sufficient to suppress somatic withdrawal signs [18]; however, unlike the Y1 receptors, Y2 receptors have not been previously implicated in psychostimulant taking behavior.
Present study investigates the regulation of the brain stress (e.g., CRF) and its opponent anti-stress/anti-anxiety (e.g., NPY) systems following an intermittent behavioral sensitization to nicotine regimen in the LRHR phenotype. We assess if there are phenotype-specific alterations in mRNA levels of CRF, NPY and its receptors Y1 and Y2 in the hippocampus and the amygdala following an intermittent behavioral sensitization to nicotine regimen in the LRHR rats. The central hypothesis is that the emergence of a nicotine-induced dysregulation in the CRF and NPY systems in the HR rats is associated with the development of social anxiety-like behavior during nicotine abstinence.
Fifty-four male Sprague-Dawley rats (Charles River, Wilmington, MA) arrived at weaning (postnatal day, PN 22), were housed 3 per cage on a 12 h light/dark cycle (lights on at 7:00 A.M.). On PN 25, animals were screened for locomotor reactivity to a novel environment for 60 min using commercially available, square-shaped locomotion chambers (San Diego Instruments, San Diego, CA). Activity was monitored by means of photocells (a total of X = 16 by Y = 16 photocells) 2.5 cm above the grid floor and equally spaced along the sides of the box. Total locomotor activity (i.e., X, Y, and Z locomotion) was pooled and the rats were ranked as HRs [i.e., rats with scores in the highest third of the sample, n=18, mean=3254.27 ± 136.61] or LRs [i.e., rats with scores in the lowest third of the sample, n=18, mean=1404.94 ± 97.56]. Following phenotype screening, rats were assigned to saline (1 ml/kg; s.c.; n=9) or nicotine (0.35 mg/kg; s.c.; n=9) training groups. Nicotine hydrogen (Sigma) was dissolved in 0.9% NaCl and the pH was adjusted to 7.4 using NaOH. On injection days, rats were given 60 min to habituate to the locomotor chambers before they received an injection of the assigned drug. Their locomotor response was recorded for 90 min. This procedure was repeated four times at a 3-d interval. Following 1 wk of abstinence, on PN 44, all rats were challenged with a low dose of nicotine (0.1 mg/kg; s.c.) and their locomotor response was monitored for 45 min. Subsequently, all animals were tested on the LDB, EPM and the social interaction (SI) tests with the order of tests presented in a randomized fashion. Animals returned to their home cages between each test and were allowed to rest.
LDB
The test was conducted in a 30 × 60 × 30 cm Plexiglas shuttle box that was divided into two equal size compartments by a wall with an open door. One compartment was brightly illuminated (60 lux) while the other compartment was painted black with very dim light. Time spent in each compartment-having the four paws on the same side-was monitored for 5 min.
EPM
The apparatus is constructed of black-painted Plexiglas, with four elevated arms (70cm from the floor, 45 cm long and 12 cm wide). The arms were arranged in a cross, with two opposite arms being enclosed by 45-cm high walls. The two other arms were open, having at their intersection a central 12 × 12 cm square platform giving access to all arms. The illumination above the central platform was 85 lux. Each rat was placed in the central square facing an open arm, and the time spent (with the four paws) in every arm was recorded for 5 min.
SI
A modified version of a previously described protocol was used [19]. Testing took place in an open topped, rectangular and transparent box. The resident rat was placed in the box 8 min prior to placement of the experimental rat. The resident rat was a naive rat of similar weight that was received and housed under identical conditions as but had no previous contact with the experimental rat. No aggressive behaviors such as biting were detected. The amount of time the experimental rat spent engaging in social interaction (i.e., grooming, sniffing, following, crawling over or under) directed at the resident rat was measured for 5 min.
Following testing, rats were rapidly decapitated; brains were immediately frozen in isopentane at − 30°C. Brains were coronally sectioned on a cryostat at 20 µm and mounted on slides which were kept at −80°C until processed. The in situ hybridization protocol is identical to our published protocol [9]. cDNA probes for rat NPY, Y1R, Y2R and CRF were antisense linearized and transcribed by using polymerases, and were 35S labeled separately in reaction mixtures consisting of 1 ml of linearized plasmid, 1× transcription buffer, 125 mCi [35S ]UTP, 125 mCi [35S ]CTP, 150 mM each of ATP, and GTP, 12.5 mM dithiothreitol, 20 U RNAase inhibitor and 6 U polymerase. Section images were captured digitally from x-ray films with a CCD camera, and optical densities were determined relative to the background using a macro, which subtracts any labeling below 3.5X the background [9]. Background measurements were taken from cell soma free areas within the structure of interest (i.e., hippocampus) or from the neighboring regions, and were kept consistent across sections. Integrated density was calculated as optical density multiplied by the area of the signal. For each brain region, data from multiple sections (6–8) were averaged to obtain a mean integrated density for each animal; and grand means were compared statistically between groups. Messenger RNA levels for NPY, Y1 and Y2 receptors were quantified in the hippocampus, the medial nucleus of amygdala (MeA) and the basolateral amygdala (BLA). CRF mRNA was quantified in the CeA.
Nicotine training data were analyzed by repeated-measures ANOVAs: Phenotype (LR, HR) X Pre-training (SAL, NIC) X Injection Days (INJ 1, INJ 2, INJ 3, INJ 4). Locomotor response to nicotine challenge, percent time spent in social interaction, percent time spent in light compartment (LDB) and percent time spent in open arms (EPM) were analyzed by two-way ANOVAs with Phenotype (LR, HR) X Pre-training (SAL, NIC). Integrated density measures for mRNA quantification were also analyzed using two-way ANOVAs with Phenotype (LR, HR) X Pre-training (SAL, NIC). Significant main effects and interactions were followed by post-hoc comparisons and significance was set at p = 0.05.
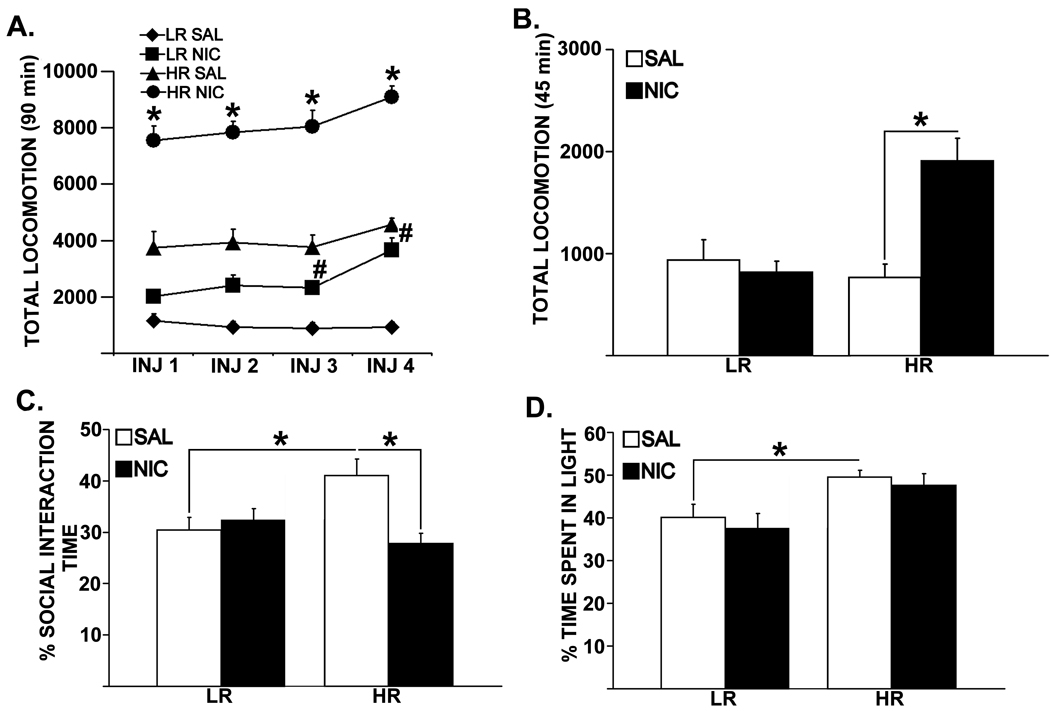
Figure 1 shows total locomotor reactivity to four intermittent nicotine or saline training injections (1A) and the low dose nicotine challenge following 1 wk of abstinence (1B) in LRHR rats. Repeated-measures ANOVA revealed a significant three-way interaction between Phenotype, Pre-training and Injection Days [F(3,32) = 12.11, p = 0.002] and main effects of Phenotype [LR, HR; F(1,32) = 29.99, p = 0.0001] and Pre-training [SAL, NIC; F(1,32) = 39.43, p = 0.0001]. Specific post-hoc comparisons showed that at all injection days, nicotine pre-trained HRs exhibited higher locomotor reactivity compared to saline pre-trained controls [ps < 0.028]; while in LRs, such nicotine-induced elevations in locomotor reactivity were observed only on INJ 3 [p = 0.031] and on INJ 4 [p = 0.001]. A two-way ANOVA showed a significant interaction between Phenotype and Pre-training in locomotor reactivity to challenge nicotine [1B; F(1,32) = 9.09, p = 0.029], and a main effect of Pre-training [SAL, NIC; F(1,32) = 7.76, p = 0.028]. Post-hoc comparisons further showed that HRs pre-trained with nicotine exhibited increased locomotor reactivity to the challenge dose of nicotine compared to saline pre-trained controls [p = 0.0005]. Such challenge nicotine-induced differences in locomotor reactivity were not observed between saline pre-trained LRs and HRs.
Figure 1.
Total locomotor reactivity to 0.35 mg/kg dose of nicotine or saline training injections (A), a low dose nicotine challenge (B; 0.1 mg/kg; s.c), percent time spent interacting with the resident rat in the SI test (C) and percent time spent in the light compartment in the LDB (D) following 1 wk of injection free period. Group means±SEMs are plotted in line (A) and bar (B, C, D) graphs. * represents the significant effects in total locomotor reactivity to nicotine in HRs compared to saline-injected controls, whereas # represents significant effects in nicotine-injected LRs compared to saline-injected controls. Significance is set at p = 0.05.
A significant interaction between Phenotype and Pre-training was observed in percent time spent in social interaction [Figure 1C; F(1,32)= 10.01, p = 0.007], along with significant main effects of Phenotype [LR, HR; F(1,32) = 9.53, p = 0.012] and Pre-training [SAL, NIC; F(1,32) = 9.88, p = 0.05]. A significant main effect of only Phenotype [Figure 1D; F(1,32) = 12.92, p = 0.001] was detected in percent time spent in light compartment in the LDB. Post-hoc comparisons showed that nicotine pre-trained HRs spent less time interacting with the resident rat in the SI test [p = 0.0003] compared to saline pre-trained controls, while such nicotine-induced effects were not detected in time spent in light compartment in the LDB. Moreover, saline pre-trained HRs that received a single challenge nicotine injection showed significantly higher percent time spent in social interaction [p = 0.004] and percent time spent in light compartment in the LDB [Figure 1D; p = 0.020] compared to saline pre-trained and nicotine challenged LRs, suggesting that a naïve dose of nicotine (i.e., the low dose challenge) results in inhibited anxiety in this phenotype compared to LR counterparts. However, with prior nicotine training, HRs showed a dramatic decrease in percent time spent in social interaction compared to saline pre-trained HRs, indicating that repeated nicotine training selectively switches HR rats into an anxiogenic state, and this effect is unique to social anxiety-like behavior. No significant effects were detected in performance measures in the EPM test (data not shown).
Figure 2 shows NPY mRNA expression in the MeA (A) and the hippocampus (C), Y2R mRNA expression in the CA3 field of the hippocampus (E) and CRF mRNA expression in the CeA (G) in saline and nicotine pre-trained HRs. Two-way ANOVAs revealed significant interactions between Phenotype (LR, HR) and Pre-training (SAL, NIC) in NPY mRNA levels in the MeA [F(1,32)= 5.16, p = 0.032], the CA1 field and the dentate gyrus (DG, data not shown) of the hippocampus [Fs(1.32) ≥ 6.71, ps ≤ 0.019]; Y2R mRNA levels in the CA3 field of the hippocampus [F(1,32) = 4.39, p =0.044] and CRF mRNA levels in the CeA [F(1,32) = 6.71, p = 0.014]. Significant main effects of Pre-training were detected in the NPY mRNA levels in the MeA [F(1,32)= 5.77, p = 0.024] and CRF mRNA levels in the CeA [F(1,32) = 16.27, p = 0.0003]. Moreover, significant main effects of Phenotype were detected in NPY mRNA levels in the CA1 field of the hippocampus [F(1,32) = 8.02, p = 0.011] and the MeA [F(1,32) = 21.99, p = 0.0001]. Specific post-hoc comparisons showed that compared to saline pre-trained controls, nicotine pre-trained HRs had significant downregulation of NPY mRNA in the MeA [Figure 2B; p = 0.003] the CA1 field [Figure 2D; p = 0.009] and the DG of the hippocampus [data not shown, p = 0.023]. Moreover, an upregulation of Y2R mRNA levels in the CA3 field of the hippocampus [Figure 2F; p = 0.012] along with an upregulation of the CRF mRNA levels in the CeA [Figure 2H; p = 0.0001] were detected in nicotine pre-trained HRs compared to saline pre-trained controls following 1 wk of abstinence and a low dose nicotine challenge. No significant effects were seen in the Y2 or NPY mRNA levels in the BLA, NPY mRNA levels in the CA3 field of the hippocampus or Y1R mRNA levels in the hippocampus or in the amygdala (data not shown). Y2 as opposed to Y1 receptors and particularly those in the hippocampus are implicated in the possible dysregulation of the NPY system in the nicotine vulnerable rats. In sum, a deficit in the NPY levels in the hippocampus and the amygdala and overactivation of the CRF in the CeA were observed in the HR phenotype in response to challenge nicotine following the intermittent nicotine training and 1 wk of abstinence.
Figure 2.
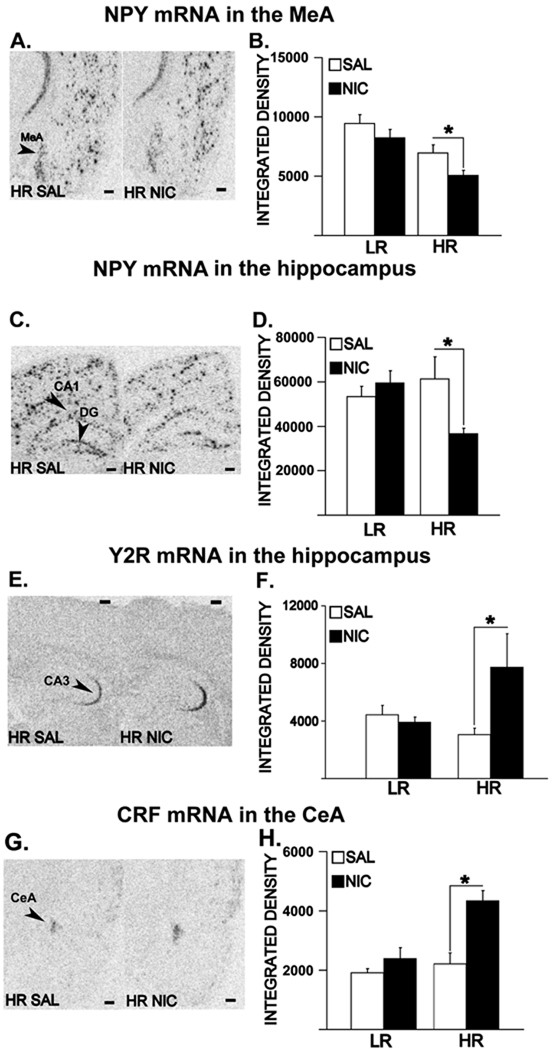
Alterations in NPY mRNA expression in the MeA (A, B), CA1 field of the hippocampus (C, D); Y2R mRNA expression in the CA3 field of the hippocampus (E, F) and CRF mRNA expression in the CeA (G, H) in HRs pre-trained with nicotine or saline. Panels A, C, E and G show images of representative coronal hemisections of the amygdala and the hippocampus that were radioactively labeled with an antisense cRNA probe against NPY (A, C), Y2R (E) and CRF (G) mRNA, and exposed on an x-ray film. Means of quantification results for integrated densities ± SEMs are plotted with bar graphs (B, D, F, H; *: p ≤ 0.05). Scale bar = 250 µm.
Our results showed that a chronic intermittent nicotine training followed by 1 wk of abstinence results in locomotor sensitization to nicotine challenge in HR rats, along with increased anxiety-like behavior in the social interaction test, downregulated NPY mRNA expression in the MeA and the hippocampus, as well as upregulated Y2R mRNA levels in the CA3 field of the hippocampus and CRF mRNA levels in the CeA. These results suggest that increased anxiety, particularly social anxiety due to nicotine abstinence may be a factor associated with the development of locomotor sensitization to nicotine challenge in the HR phenotype, possibly due to enhanced amygdalar CRF levels, a molecular mediator of the stress system, along with inhibited amygdalar and hippocampal NPY levels, a molecular mediator of the anti-anxiety system. Moreover a novel, presynaptic Y2 receptor [16] mechanism may be involved in nicotine-induced deficits in NPY in the hippocampus of nicotine vulnerable HR phenotype.
Our findings show that HRs exhibit augmented locomotor reactivity to all nicotine training injections compared to saline pre-trained controls. HRs respond rapidly to this dose of nicotine, reaching a ceiling response as early as the first nicotine injection and maintaining that level at the end of training. Conversely, increased locomotor response to nicotine emerges only at the 3rd nicotine injection in LRs compared to saline injected controls. During nicotine challenge, HRs but not LRs pre-trained with nicotine express locomotor sensitization to the low dose nicotine challenge indicating a phenotype-specific vulnerability to the sensitizing effects of nicotine following abstinence. The phenotype-specific expression of sensitization implicates that there are behavioral and neural adaptations induced by repeated nicotine unique to the HR phenotype. Although the expression of behavioral sensitization is mediated at the level of the nucleus accumbens (Nacc), hippocampus via its glutamatergic projections to the ventral tegmental area (VTA) can antagonize the inhibiting effects of the VTA dopamine at the Nacc [20]. Likewise, we have previously shown that reversible lesions of the dorsal hippocampus alter the behaviorally-sensitizing effects of nicotine in the HR phenotype [1]. In addition to the Nacc, VTA has dopaminergic projections to the amygdala also that play a role in the development of behavioral sensitization to amphetamine [3]. Therefore, in addition to the traditional reward circuitry, the hippocampus and amygdala are also critical in sustaining locomotor sensitization to psychostimulants.
We have shown that the expression of locomotor sensitization to nicotine in the HRs is associated with the development of negative affect in nicotine abstinence that manifests as a switch in the nicotine-induced social anxiety-like towards an anxiogenic state. Moreover anxiety is not uniformly affected with repeated nicotine exposure in the HR animals, hence molecular correlates of augmented social anxiety should help distinctly address a particular aspect of negative affect that may be critical in the course of developing vulnerability to nicotine relapse. Nicotine abstinence results in increased anxiety-like behavior in the defensive burying test which is associated with upregulated CRF signaling in the CeA [6]. Furthermore, a component of the brain anti-anxiety system, NPY, is released following an increase in CRF, producing opposing actions on the stress system and maintaining a balanced emotional state [7]. However, although alterations in central NPY signaling in anxiety-like behavior associated with alcohol withdrawal has been extensively studied [17], NPY signaling in nicotine withdrawal-induced anxiety remains to be fully elucidated. In the only published study, nicotine abstinence has been shown to produce lasting increases in anxiety-like behavior in the EPM and a decreased ratio of NPY to CRF in the amygdala, suggesting that neurobehavioral changes associated with nicotine exposure may be related to allostatic changes in stress and anti-stress neuropeptide systems [21]. The latter report overlaps with our findings in that expression of locomotor sensitization to nicotine challenge following 1 wk of abstinence observed in nicotine pre-trained HRs may be associated with increased social anxiety-like behavior that is mediated by an altered balance between the NPY and CRF signaling in the amygdala, rendering these animals vulnerable to the sensitizing effects of nicotine.
We also reported an increase in Y2R levels in the hippocampal CA3 field in the HR rats pre-trained with nicotine compared to saline pre-trained controls. Y2 receptors within the hippocampus are expressed at the mossy fiber terminals, where their activation suppresses hippocampal glutamatergic transmission through presynaptic mechanisms [16]. We have previously reported that the identical intermittent nicotine sensitization regimen results in increased mossy fibre terminal field size in the HR rats, suggesting that nicotine-induced sprouting of mossy fibres may be responsible for elevated Y2 receptor pool in the HR phenotype [1]. As presynaptic auto-receptors on NPY-ergic terminals, Y2R inhibits the release of endogenous NPY [10], hence decrease the availability of NPY in the synaptic cleft. Studies have shown that Y2 receptor knockout mice display less anxiety-like behavior in several tests [22], and administration of a Y2R antagonist selectively suppresses ethanol self administration [24]. Causal studies targeting the Y2R are needed to definitively link modulation of this receptor in abstinence-induced negative affect and vulnerability to nicotine relapse in the LRHR rats.
In conclusion, these findings support the hypothesis that individual differences exist in behavioral and neuropeptidergic vulnerability to nicotine relapse. Increased social anxiety-like behavior observed in HRs following repeated nicotine training and abstinence is accompanied by distinct phenotype-specific alterations in the stress (i.e., CRF) and anti-anxiety (i.e., NPY) systems; namely HRs show activation of the stress system (CRF in CeA) coupled with deactivation of the anti-anxiety system (NPY in the amygdala and hippocampus). Targeting the NPY signaling possibly through the Y2R mechanism could be a useful strategy in an effort to reverse behavioral sensitization to nicotine in combating relapse in nicotine vulnerable individuals.
Research highlights
Nicotine trained HRs exhibited abstinence-induced social anxiety-like behavior
A chronic intermittent nicotine regimen resulted in increased CRF in CeA in HRs
NPY levels decreased in amygdala and hippocampus in nicotine trained HRs
Hippocampal Y2 receptor levels were upregulated in nicotine trained HRs
Acknowledgements
This research is supported by the NIH grant DA023675 awarded to Dr. Isgor.
Abbreviations
- BLA
basolateral nucleus of the amygdala
- CeA
central nucleus of the amygdala
- CRF
corticotropin releasing factor
- DG
dentate gyrus
- EPM
elevated plus maze
- HR
high responder
- LR
low responder
- LDB
light dark box
- MeA
medial nucleus of the amygdala
- Nacc
nucleus accumbens
- NPY
neuropeptide Y
- SI
social interaction
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhatti AS, Hall P, Tao R, Isgor C. Hippocampus modulates the behaviorally-sensitizing effects of nicotine in a rat model of novelty-seeking: potential role for mossy fibers. Hippocampus. 2007;17:922–933. doi: 10.1002/hipo.20310. [DOI] [PubMed] [Google Scholar]
- 2.Bhatti AS, Aydin C, Oztan O, Ma Z, Hall P, Tao R, Isgor C. Effects of a cannabinoid receptor (CB) 1 antagonist AM251 on behavioral sensitization to nicotine in a rat model of novelty-seeking behavior: correlation with hippocampal 5HT. Psychopharmacology (Berl) 2009;203:23–32. doi: 10.1007/s00213-008-1366-6. [DOI] [PubMed] [Google Scholar]
- 3.Bjijou Y, De Deurwaerdere P, Spampinato U, Stinus L, Cador M. D-amphetamine-induced behavioral sensitization: effects of lesioning dopaminergic terminals in the medial prefrontal cortex, amygdala and the entorhinal cortex. Neuroscience. 2002;109:499–516. doi: 10.1016/s0306-4522(01)00508-5. [DOI] [PubMed] [Google Scholar]
- 4.Bura SA, Burokas A, Martín-García E, Maldonado R. Effects of chronic nicotine on food intake and anxiety-like behaviour in CB(1) knockout mice. Eur Neuropsychopharmacol. 2010;6:369–378. doi: 10.1016/j.euroneuro.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Barsy B, Leveleki C, Zelena D, Haller J. The context specificity of anxiety responses induced by chronic psychosocial stress in rats: a shift from anxiety to social phobia? Stress. 2010;13:230–237. doi: 10.3109/10253890903296389. [DOI] [PubMed] [Google Scholar]
- 6.George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O’Dell LE, Richardson HN, Koob GF. CRF–CRF1 system activation mediates withdrawal induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci USA. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17:80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 8.Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–214. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Isgor C, Huang GC, Akil H, Watson SJ. Correlation of estrogen β-receptor messenger RNA with endogenous levels of plasma estradiol and progesterone in the female rat hypothalamus, bed nucleus of stria terminalis and the medial amygdala. Brain Res Mol Brain Res. 2002;106:30–41. doi: 10.1016/s0169-328x(02)00407-2. [DOI] [PubMed] [Google Scholar]
- 10.King PJ, Williams G, Doods H, Widdowson PS. Effect of a selective neuropeptide Y Y(2) receptor antagonist, BIIE0246 on neuropeptide Y release. Eur J Pharmacol. 2000;396:R1–R3. doi: 10.1016/s0014-2999(00)00230-2. [DOI] [PubMed] [Google Scholar]
- 11.Koob GF, Markou A, Weiss F, Schulteis G. Opponent process and drug dependence: neurobiological mechanisms. Seminars in Neuroscience. 1993;5:351–358. [Google Scholar]
- 12.Koob GF. Stress, corticotropin-releasing factor, and drug addiction. Ann N Y Acad Sci. 1999;897:27–45. doi: 10.1111/j.1749-6632.1999.tb07876.x. [DOI] [PubMed] [Google Scholar]
- 13.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manhães AC, Guthierrez MC, Filgueiras CC, Abreu-Villaça Y. Anxiety-like behavior during nicotine withdrawal predict subsequent nicotine consumption in adolescent C57BL/6 mice. Behav Brain Res. 2008;193:216–224. doi: 10.1016/j.bbr.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 16.McQuiston AR, Colmers WF. Neuropeptide Y2 receptors inhibit the frequency of spontaneous but not miniature EPSCs in CA3 pyramidal cells of rat hippocampus. J Neurophysiol. 1996;76:3159–3168. doi: 10.1152/jn.1996.76.5.3159. [DOI] [PubMed] [Google Scholar]
- 17.Pandey SC. Anxiety and alcohol abuse disorders: a common role for CREB and its target, the neuropeptide Y gene. Trends Pharmacol Sci. 2003;24:456–460. doi: 10.1016/S0165-6147(03)00226-8. [DOI] [PubMed] [Google Scholar]
- 18.Rylkova D, Boissoneault J, Isaac S, Prado M, Shah HP, Bruijnzeel AW. Effects of NPY and the specific Y1 receptor agonist [D-His(26)]-NPY on the deficit in brain reward function and somatic signs associated with nicotine withdrawal in rats. Neuropeptides. 2008;42:215–227. doi: 10.1016/j.npep.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sajdyk TJ, Schober DA, Gehlert DR. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacology. 2002;43:1165–1172. doi: 10.1016/s0028-3908(02)00234-4. [DOI] [PubMed] [Google Scholar]
- 20.Schmajuk NA, Tyberg M. Animal Models in Psychiatry I. Vol 18. Clifton, N. J: Humana Press; 1991. The hippocampal lesion model of schizophrenia, Neuromethods. [Google Scholar]
- 21.Slawecki CJ, Thorsell AK, El Khoury A, Mathe AA, Ehlers CL. Increased CRF-like and NPY-like immunoreactivity in adult rats exposed to nicotine during adolescence: relation to anxiety-like and depressive-like behavior. Neuropeptides. 2005;39:369–377. doi: 10.1016/j.npep.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Sorensen G, Lindberg C, Wortwein G, Bolwig TG, Woldbye DPD. Differential Roles for Neuropeptide Y Y1 and Y5 Receptors in Anxiety and Sedation. J Neurosci. 2004;77:723–729. doi: 10.1002/jnr.20200. [DOI] [PubMed] [Google Scholar]
- 23.Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat’s propensity to self-administer nicotine. Psychopharmacology (Berl) 2001;158:175–180. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- 24.Thorsell A, Rimondini R, Heilig M. Blockade of central neuropeptide Y (NPY) Y2 receptors reduces ethanol self-administration. Neurosci Lett. 2002;332:1–4. doi: 10.1016/s0304-3940(02)00904-7. [DOI] [PubMed] [Google Scholar]