Abstract
Cognitive decline can occur with aging; however, some individuals experience less cognitive decline than do others. Secretion of ovarian hormones is reduced post-menopause and may contribute to cognitive function. The extent to which hormonal effects may be parsed out from other age-related factors to influence cognition is of interest. Middle aged (12-month-old) female rats that were retired breeders were categorized as maintaining or declining reproductive function based upon their estrous cyclicity (regular 4-5 day cycles), fertility (> 60 % successful pregnancy) and fecundity (> 10 pups/litter). Performance in object recognition, Y-maze, water maze, inhibitory avoidance, and contextual/cued fear conditioning was evaluated. Estradiol, progesterone (P4), dihydroprogesterone, and 5α-pregnan-3α-ol-20-one (3α,5α-THP) were assessed in medial prefrontal cortex (mPFC) and hippocampus: corticosterone was assessed in plasma. Rats maintaining reproductive function performed significantly better on the object recognition, Y-maze, water maze, inhibitory avoidance, and cued fear conditioning tasks, than did rats with declining reproductive function. Steroid concentrations varied greatly within groups. Higher levels of P4 in mPFC and hippocampus were associated with better Y-maze performance. In mPFC, higher levels of P4 were associated with poorer inhibitory avoidance performance: greater levels of 3α,5α-THP were associated with better object memory. Neither estradiol, nor corticosterone, levels significantly contributed to cognitive performance. Thus, the capacity for cortico-limbic P4 utilization may influence cognitive performance in aging.
Keywords: 5α-reductase, Allopregnanolone, Amygdala, Estropause, Memory, Menopause
1. Introduction
Cognitive decline, independent of dementia, can occur with aging; however, some individuals experience less cognitive decline than do others (Colsher and Wallace, 1991; Wilson et al., 2002). For example, some centenarians experience no measureable cognitive decline (Motta et al., 2008). Various factors, including neuronal loss (Schochet, 1998), decrements in cell signaling (Zoli et al., 1999), changes in survival processes at the cellular level (Johnson et al., 1999; Von Zglinicki et al., 2001; Xiong et al., 2002), and physical health may be contributors (Bergman et al., 2007). Similarly, physical and psychological factors experienced across the lifespan can impact other faculties, including affective status (Petkus et al., 2009). Thus, it is important to understand the neurobiological factors that underlie individual differences in aging.
Steroid hormones are involved in the function and maintenance of cognition, and other processes, and can be markedly influenced by age. Among women, the climacteric is associated with decline of ovarian steroids, including estradiol (E2) and progestogens (progesterone and its metabolites), which may contribute to changes in cognitive function (Utian et al., 2008). However, cognitive decline also occurs with aging and the relative contribution of hormone decline versus other age-related processes is not well-understood.
Steroid hormones are developmentally-regulated and have pleiotropic effects to modulate a number of higher-order processes across the lifespan. In particular, progestogens, such as progesterone (P4) and/or its 5α-reduced metabolites, dihydroprogesterone (DHP) and 5α-pregnan-3α-ol-20-one (3α,5α-THP, a.k.a. allopregnanolone), are critical for the maintenance of pregnancy throughout neurodevelopment to gestation (Albano et al, 1998; Edwards et al., 1980; Smitz et al., 1988; Tavaniotou et al., 2001). The enhancement of ovarian hormones, including E2 and progestogens, characterize the pubertal transition to young adulthood. In women, cyclical enhancements of E2 and progestogens are associated with reduced anxiety (Le Mellédo and Baker, 2004). Similar effects occur concomitant with enhanced cognitive performance in female rodents (Archer, 1975; Gray and Levine, 1964; Johnston and File, 1991; Paris and Frye, 2008; Rhodes and Frye, 2004; Walf et al., 2009a). The hippocampus is an important target for progestogens’ actions to reduce anxiety (Bitran et al., 1999; Frye et al., 2000) and, in addition to prefrontal cortex, to mediate cognition in rodents (Walf et al., 2006). Notably, the prefrontal cortex (Jutapakdeegul et al., 2010; Murmu et al., 2006), and hippocampus (Swaab et al., 2005; Schmitz et al., 2002), are sensitive structures to neurodegenerative effects of stress and neurological insults, which increase with age. Indeed, developmental stressors which promote neurodegeneration in prefrontal cortex and/or hippocampus are associated with reduced dendritic spine density and/or progestogen formation (Murmu et al., 2006; Paris and Frye 2010a,b). In rodents, administration of P4 or 3α,5α-THP is neuroprotective and can promote synaptic connectivity in hippocampus (Brinton and Wang, 2006; Charalampopoulos et al., 2008; Djebaili et al., 2005; He et al., 2004a,b; Rhodes et al., 2004; Sayeed et al., 2005; Schumacher et al., 2007). Thus, ovarian steroids influence cognitive and affective processes and can have trophic/neuroprotective effects, which may play an important functional role in the aging brain.
Steroid-based interventions have been used to manage the sequelae of ovarian cessation due to natural, or surgical, menopause. Despite some evidence that hormone therapy may reduce cognitive deficits (Sherwin, 1988, 2007), such as the Cache County Study which found E2 to reduce the rate of cognitive decline (Carlson et al., 2001), results of clinical trials have not all consistently reported beneficial effects of ovarian steroids (Sherwin, 2007). In the Women’s Health Initiative Memory Study (WHIMS), a number of factors may have belied potential beneficial effects of steroid-based treatments. For instance, most participants were over 60 years of age and were not put on hormone therapy until 10-20 years after menopause. Effects of E2 and/or progestins may be more favorable when compromise is not already present, which implies that there may be a “window of opportunity” for beneficial effects of hormones. For example, preclinical studies demonstrate that aged rats only respond favorably to E2-based treatments when E2 is administered in temporal proximity to ovarian decline (Daniel and Bohacek, 2010; Gibbs and Gabor, 2003; Walf et al., 2009b). Given the considerable individual variability among women that experience age-related cognitive decline, it is critical to begin to parse out the contributions of aging-related changes versus hormone-related changes for these effects.
It is important to understand the effects and mechanisms by which steroid hormones may influence cognition. Among rodents, removal of the ovaries impairs performance in the object recognition task: administration of E2 and/or P4, reinstates high levels of performance, akin to that of natural steroid enhancement (Walf et al., 2006). Moreover, formation of neuroactive progestogens may be critical, given that systemic 3α,5α-THP is as efficacious as E2 and/or P4 administration, at enhancing object memory (Frye et al., 2007a; Walf et al., 2006). Indeed, multiparous rodents, that are exposed to elevated levels of hormones for longer than are their nulliparous and/or primiparous counterparts, demonstrate life-long enhancements of cognitive and affective performance (Kinsley and Lambert, 2008; Lambert et al., 2005; Macbeth and Luine, 2010; Paris and Frye, 2008). Synthetic progestins that do not form natural P4 metabolites, fail to produce beneficial cognitive and/or neuroprotective effects (Greendale et al., 1998; Ciriza et al., 2006). As well, the neuroprotective effects of P4 are diminished in rats that are 14-18 months old (Toung et al., 2004; Murphy et al., 2002), which implies the importance of changes in metabolic function with age. Thus, factors that promote formation of progestogen metabolites, such as 3α,5α-THP, may be key components in maintenance of higher-order function of the aging brain.
In the present investigation, we examined cognitive performance of 12-month-old female, retired breeder rats. In rodents, age-related cognitive impairments are consistently noted (Decker et al., 1988; Fischer et al., 1992; Frick et al., 1995, 2000) and have been observed as early as 11 months of age (Frick et al., 1995). Some of our 12-month-old rats were experiencing reproductive decline (based upon cyclicity, fertility and fecundity-see methods for detailed characterization of reproductive status). Other 12-month-old rats, with a commensurate breeding history, were maintaining reproductive function. In order to assess how reproductive status influenced cognitive performance, the behavior of all rats was examined. Performance in prefrontal cortex- and hippocampus-mediated tasks (object recognition, Y-maze), hippocampus-mediated paradigms (water maze), hippocampus- and amygdala-mediated behaviors (inhibitory avoidance, conditioned contextual fear), were examined. Concentrations of E2 and progestogens (P4, DHP, 3α,5α-THP) were assessed in medial prefrontal cortex (mPFC) and hippocampus, as well as the circulatory stress hormone, corticosterone, among a subset of rats that were tested and among a non-tested control group. We hypothesized that (1) rats maintaining reproductive function would perform better on cognitive tasks compared to rats experiencing reproductive status decline and (2) these differences would be influenced by formation of P4 and its metabolites in mPFC and/or hippocampus.
2. Results
Prior to behavioral analyses, middle-aged rats were evaluated for reproductive endocrine function. Rats with regular, 4-5 day estrous cycles, fertility greater than 60 % successful pregnancies upon mating, and fecundity of more than 10 pups per litter, were considered to be maintaining reproductive function. Rats with irregular estrous cycles, fewer than 60 % of mating resulting in pregnancy, and litter sizes lower than 10, were considered to have declining reproductive function. A more detailed description of how subjects were categorized can be found in the Experimental Procedure section. All rats were cycled daily and then behaviorally tested in one of the following tasks, in the following order (object recognition, Y-maze, water maze, inhibitory-avoidance, conditioned fear). The estrous cycle phase at the time of each testing occasion was recorded, as cycle phase can influence performance in these tasks (Walf et al., 2006; Frye, 1995; Llaneza and Frye, 2009; Rhodes and Frye, 2004). Chi-square analyses revealed no significant differences in the representation of estrous cycle phases among rats in the maintaining, vs. declining, reproductive status groups on any testing occasion. As such, cycle phases were not inordinately distributed among groups on any testing occasion. For each task, regression analyses were utilized to ascertain the amount of variance in behavioral performance that could be accounted for by cycle phase and these data are described with behavioral outcomes below.
2.1. Endocrine Milieu
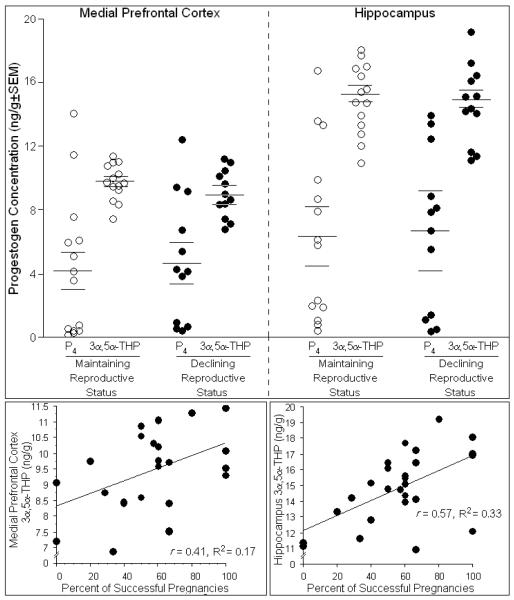
There was considerable individual variation in hormone levels among rats. Neither P4 or 3α,5α-THP in mPFC (Fig. 1, top left) or hippocampus (Fig. 1, top right), nor mean concentrations of DHP, E2, corticosterone, or P4 turnover to its metabolites (Table 1), significantly differed between groups among tested or non-tested rats. Among tested rats, the percentage of prior successful pregnancies positively predicted 3α,5α-THP concentrations in mPFC [t(24) = 14.48, p < 0.05] (Fig. 1, bottom left) and hippocampus [t(24) = 13.84, p < 0.05] (Fig. 1, bottom right) and accounted for a significant proportion of variance in 3α,5α-THP levels [R2mPFC = 0.17, F(1,24) = 4.89, p < 0.05; R2hippo = 0.33, F(1,24) = 11.66, p < 0.05]. As well, progestogen milieu influenced cognitive behavior of rats in several tasks examined (described below).
Fig. 1.
Depicts concentrations of progesterone (P4) and 3α,5α-THP in medial prefrontal cortex (top, left) and hippocampus (top, right) of middle-aged rats whose reproductive status was in decline (n=12) or was maintained (n=14). The percentage of prior pregnancies positively correlated with concentrations of 3α,5α-THP in the medial prefrontal cortex (bottom, left) and hippocampus (bottom, right) and accounted for a significant proportion of variance in these levels.
Table 1.
Steroid Concentrations among Non-Tested and Tested Same-aged rats with Maintaining or Declining Reproductive Status
|
Non-Tested Middle-Aged Control Rats |
Maintaining (n=3) |
Declining (n=3) |
|---|---|---|
| Corticosterone (μg/dl±SEM) | 0.8 ± 0.2 | 0.8 ± 0.3 |
| Medial Prefrontal Cortex | ||
| E2 (pg/g±SEM) | 3.9 ± 1.8 | 3.5 ± 1.0 |
| P4 (ng/g±SEM) | 3.5 ± 0.3 | 6.0 ± 1.7 |
| DHP (ng/g±SEM) | 2.2 ± 0.3 | 3.4 ± 0.7 |
| 3α,5α-THP (ng/g±SEM) | 5.4 ± 2.7 | 8.6 ± 1.4 |
| P4 Turnover (DHP+3α,5α-THP:P ratio) |
2.3 ± 1.0 | 2.2 ± 0.4 |
| Hippocampus | ||
| E2 (pg/g±SEM) | 4.7 ± 3.4 | 4.4 ± 4.0 |
| P4 (ng/g±SEM) | 7.1 ± 2.4 | 8.6 ± 2.3 |
| DHP (ng/g±SEM) | 3.9 ± 0.4 | 4.8 ± 0.8 |
| 3α,5α-THP (ng/g±SEM) | 12.5 ± 2.5 | 13.4 ± 5.3 |
| P4 Turnover (DHP+3α,5α-THP:P4 ratio) |
2.8 ± 0.9 | 2.1 ± 0.4 |
| Tested Middle-Aged Rats | Maintaining (n=14) |
Declining (n=12) |
|---|---|---|
| Corticosterone (μg/dl±SEM) | 1.4 ± 0.2 | 1.3 ± 0.3 |
| Medial Prefrontal Cortex | ||
| E2 (pg/g±SEM) | 4.1 ± 1.4 | 2.3 ± 0.9 |
| DHP (ng/g±SEM) | 1.2 ± 0.1 | 1.9 ± 0.6 |
| P4 Turnover (DHP+3α,5α-THP:P ratio) |
16.5 ± 6.1 | 7.9 ± 3.0 |
| Hippocampus | ||
| E2 (pg/g±SEM) | 6.2 ± 1.8 | 7.0 ± 2.2 |
| DHP (ng/g±SEM) | 1.9 ± 0.2 | 2.5 ± 0.8 |
| P4 Turnover (DHP+3α,5α-THP:P4 ratio) |
9.6 ± 3.7 | 8.0 ± 2.9 |
Depicts concentrations of corticosterone, estradiol (E2), progesterone (P4), dihydroprogesterone (DHP), 3α,5α-THP, and/or P4 turnover to its 5α-reduced metabolites (DHP and 3α,5α-THP) in medial prefrontal cortex and hippocampus among a subset of same-aged, 12-month-old rats that were non-tested controls or were tested in the behavioral battery described.
2.2. Object recognition
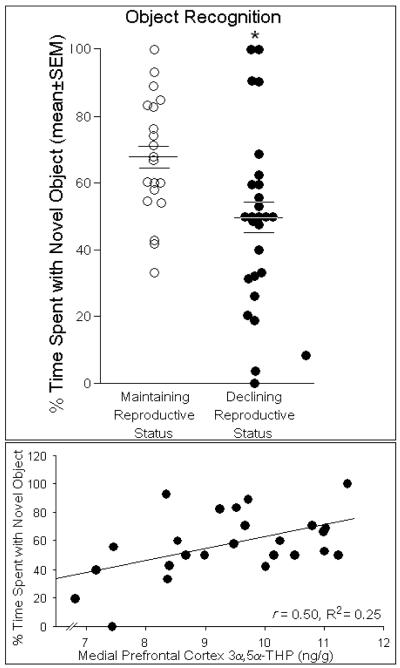
The object recognition task is a working memory task that primarily relies on cortical and, to a lesser extent, hippocampal function (Akirav and Maroun 2006; Broadbent et al., 2004; Ennaceur et al., 1997). Reproductive status significantly influenced performance in the object recognition task [F(1,44) = 6.93, p < 0.05]. Middle-aged rats maintaining reproductive function spent significantly greater percentage of time with the novel object than did same-aged rats that had declining reproductive function (Fig. 2, top). Among rats, higher concentrations of 3α,5α-THP in the prefrontal cortex significantly predicted greater percentages of time spent with a novel object [t(24) = 2.78, p < 0.05] and accounted for a significant proportion of variance in this behavior [R2 = 0.25, F(1,24) = 7.72, p < 0.05], (Fig. 2, bottom). Estrous cycle phase tended to contribute to the variance observed in time spent with the novel object [R2 = 0.07, F(1,44) = 3.40, p = 0.07, n.s.]. No other steroids investigated significantly influenced performance in the object recognition task.
Fig. 2.
Middle-aged rats whose reproductive status was maintained (n=20) spent a significantly greater percentage of time (indicated by mean bar ± SEM bars) investigating a novel object compared to same-aged rats that whose reproductive status was in decline (n=26; top panel). Concentrations of 3α,5α-THP (ng/g) in the medial prefrontal cortex positively correlated with the percentage of time that rats spent with a novel object and accounted for a significant proportion of variance in this behavior (bottom panel). * indicates significant difference between groups, p < 0.05.
2.3. Y-maze
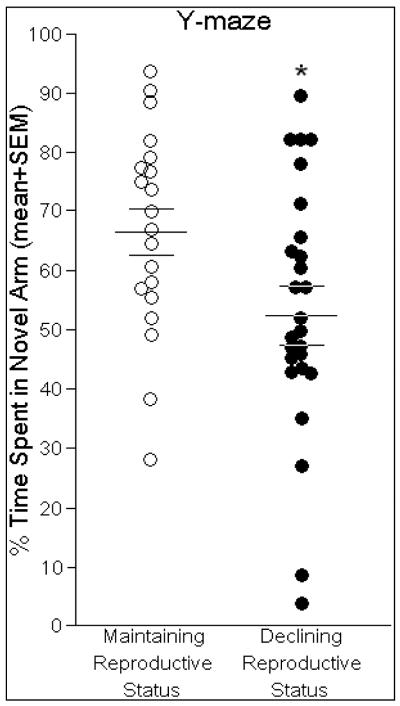
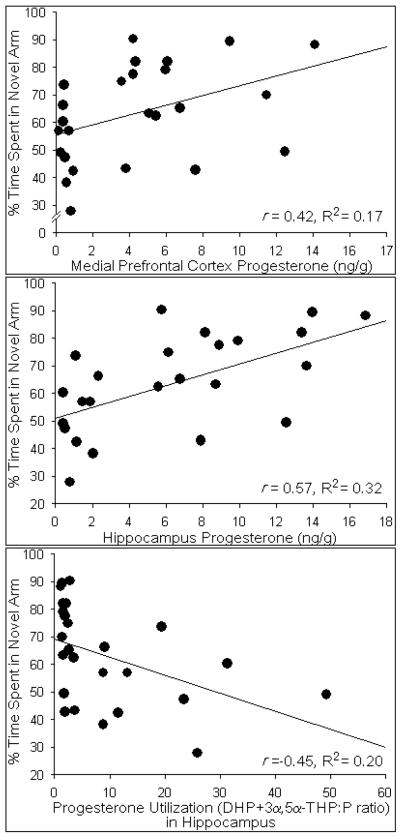
The Y-maze is a working memory task that is cortically- and hippocampally-mediated (Dillon et al., 2008; Featherby et al., 2008; Wagner et al., 2007). Performance in the Y-maze was significantly influenced by reproductive function [F(1,44) = 5.25, p < 0.05]. Middle-aged rats maintaining reproductive function spent a significantly greater percentage of time investigating the novel arm of the Y-maze than did same-aged rats with reproductive status in decline (Fig. 3). A greater percentage of time spent investigating the novel arm was significantly predicted by higher concentrations of progesterone in the prefrontal cortex [t(24) = 2.20, p < 0.05] and hippocampus [t(24) = 3.32, p < 0.05]. Progesterone in prefrontal cortex [R2 = 0.17, F(1,24) = 4.82, p < 0.05], (Fig. 4, top) and hippocampus [R2 = 0.32, F(1,24) = 11.02, p < 0.05], (Fig. 4, middle) accounted for a significant proportion of variance in this behavior. Notably, higher ratios of P4 turnover to its 5α-reduced metabolites (DHP+3α,5α-THP:P4) significantly predicted lower percentages of time spent exploring the novel arm [t(24) = −2.41, p < 0.05] and accounted for a significant proportion of variance [R2 = 0.20, F(1,24) = 5.79, p < 0.05], (Fig. 4, bottom). Estrous cycle phase did not significantly contribute to the variance observed in time spent in the novel arm [R2 = 0.02, F(1,44) = 0.73, p = 0.40, n.s.]. No other steroids investigated significantly influenced performance in the Y-maze.
Fig. 3.
Middle-aged rats whose reproductive status was maintained (n=20) spent a significantly greater percentage of time (indicated by mean bar ± SEM bars) investigating the novel arm of the Y-maze compared same-aged rats that whose reproductive status was in decline (n=26). * indicates significant difference between groups, p < 0.05.
Fig. 4.
Concentrations of progesterone (ng/g) in the medial prefrontal cortex (top panel) and hippocampus (middle panel) positively correlated with the percentage of time that rats spent in the novel arm of the Y-maze and accounted for a significant proportion of variance in this behavior. Progesterone metabolism to its 5α-reduced metabolites (DHP+3α,5α-THP:P ratio) in the hippocampus (bottom panel) negatively correlated with the percentage of time that rats spent in the novel arm of the Y-maze and accounted for a significant proportion of variance in this behavior.
2.4. Water maze
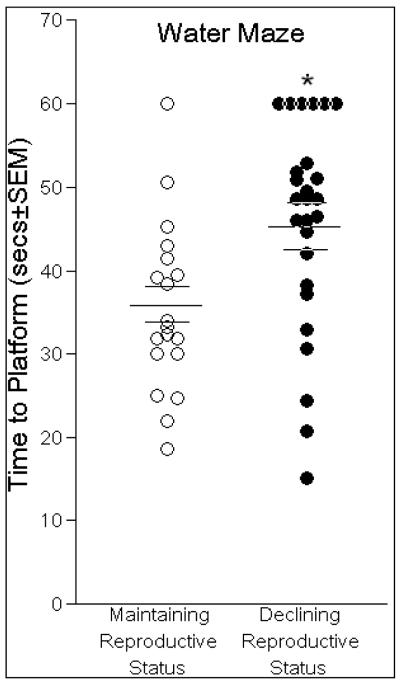
The water maze task is a spatial task, which is dependent upon a functioning hippocampus (Morris et al., 1986, 2003). Middle-aged rats that maintained reproductive status located the hidden platform in significantly less time than did same-aged rats whose reproductive status was in decline [F(1,44) = 9.48, p < 0.05], (Fig. 5). Reproductively-maintaining rats also swam less of a distance (119 ± 9 cm) to find the platform than did same-aged rats in reproductive decline (131 ± 10 cm), albeit, these differences were not significant. Swim speed was not significantly different between reproductively-maintaining (3.4 ± 0.5 cm/sec) and declining rats (2.8 ± 0.5 cm/sec). Estrous cycle phase was not a significant contributor to the variance observed in time to find the hidden platform [R2 = 0.01, F(1,44) = 0.01, p = 0.94, n.s.]. None of the steroids investigated significantly contributed to the variance in performance on the water maze.
Fig. 5.
Middle-aged rats whose reproductive status was maintained (n=20) had significantly shorter latencies to find the hidden platform in the water maze (indicated by mean bar ± SEM bars) than same-aged rats that whose reproductive status was in decline (n=26). * indicates significant difference between groups, p < 0.05.
2.5. Inhibitory avoidance
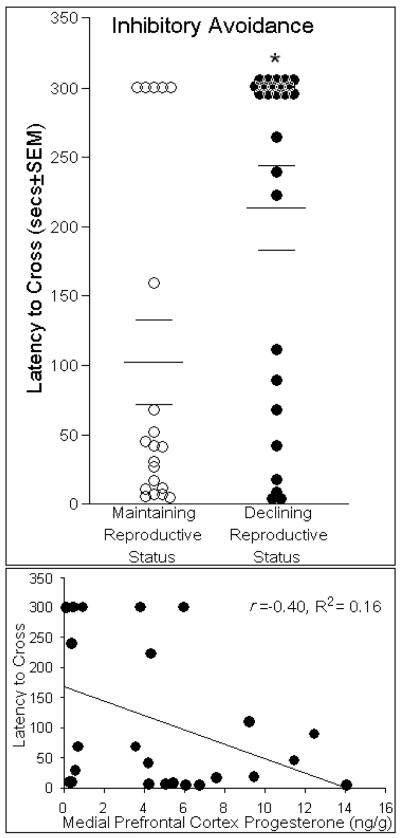
Inhibitory avoidance is a cognitive task that utilizes aversive stimuli to assess long term memory and primarily relies upon dorsal hippocampus (Lorenzini et al., 1996; Izquierdo and Medina, 1997) and basolateral amygdala (Cammarota et al., 2008; Izquierdo et al., 1992; Rossato et al., 2006). Middle-aged rats that had maintaining reproductive status had significantly shorter latencies to cross to the dark, shock-associated side, compared to same-aged rats in reproductive decline [F(1,44) = 9.83, p < 0.05], (Fig. 6, top). Nociceptive indices, such as flinch and jump responses to shock did not differ between rats with maintaining (2.7 ± 0.3) or declining reproductive status (2.5 ± 0.3). Greater concentrations of P4 in the mPFC significantly predicted lower latencies to cross over to the shock-associated side [t(24) = −2.11, p < 0.05] and accounted for a significant proportion of variance [R2 = 0.16, F(1,24) = 4.47, p < 0.05], (Fig. 6, bottom). Estrous cycle phase did not contribute to the variance observed in time to cross to the shock-associated side [R2 = 0.06, F(1,44) = 0.16, p = 0.69, n.s.]. No other steroids investigated were observed to significantly influence performance on inhibitory avoidance.
Fig. 6.
Middle-aged rats whose reproductive status was maintained (n=20) had shorter latencies to cross over to the shock-associated compartment of the inhibitory avoidance chamber (indicated by mean bar ± SEM bars) than did same-aged rats that whose reproductive status was in decline (n=26; top panel). Concentrations of progesterone (ng/g) in the medial prefrontal cortex negatively correlated with shorter latencies to cross to the shock associated side and accounted for a significant proportion of variance in this behavior (bottom panel). * indicates significant difference between groups, p < 0.05.
2.6. Conditioned contextual fear
Conditioned contextual fear is mediated by the amygdala and hippocampus (Phillips and LeDoux, 1994; Kim et al., 1993). Conditioned fear, performed in a subset of rats from each group, demonstrated a notable, but non-significant, difference wherein middle-aged rats that maintained reproductive status spent less time freezing in each trial of the amygdala-mediated, cued learning task, than did same-aged rats whose reproductive status was in decline (Table 2). As well, reproductively-maintained rats spent equal or less time freezing in the hippocampus-mediated contextual learning task compared to same-aged rats whose reproductive status was in decline; albeit, these differences did not reach statistical significance (Table 3). Estrous cycle phase was not a significant contributor to the variance observed in time spent freezing during the cued [R2 = 0.09, F(1,44) = 2.30, p = 0.14, n.s.] or contextual learning [R2 = 0.01, F(1,44) = 0.17, p = 0.69, n.s.] aspects of this task. Differences in steroid concentrations examined did not significantly contribute to the variability of performance in this task.
Table 2.
Conditioned Contextual Fear – Cued Learning
|
Reproductive Status |
Trial 1 |
Trial 2 |
Trial 3 |
Trial 4 |
Trial 5 |
Trial 6 |
Trial 7 |
Trial 8 |
Mean Freezing |
|---|---|---|---|---|---|---|---|---|---|
| Maintaining | 39 ± 4 | 37 ± 4 | 37 ± 5 | 33 ± 5 | 31 ± 5 | 30 ± 5 | 27 ± 5 | 22 ± 5 | 32 ± 4 |
| Declining | 46 ± 3 | 46 ±3 | 41 ± 5 | 38 ± 5 | 35 ± 5 | 33 ± 5 | 31 ± 4 | 28 ± 4 | 37 ± 4 |
Depicts time spent freezing among same-aged rats whose reproductive status was in decline (n=15) or was maintained (n=10) on the amygdala-mediated cued learning aspect of the conditioned contextual fear task (secs ± SEM).
Table 3.
Conditioned Contextual Fear – Contextual Learning
|
Reproductive Status |
Trial 1 |
Trial 2 |
Trial 3 |
Trial 4 |
Trial 5 |
Trial 6 |
Trial 7 |
Trial 8 |
Mean Freezing |
|---|---|---|---|---|---|---|---|---|---|
| Maintaining | 38 ± 4 | 33 ± 3 | 34 ± 5 | 29 ± 5 | 25 ± 3 | 24 ± 4 | 24 ± 5 | 24 ± 4 | 29 ± 3 |
| Declining | 39 ± 4 | 39 ± 4 | 38 ± 4 | 29 ± 5 | 26 ± 5 | 22 ± 5 | 23 ± 5 | 24 ± 5 | 30 ± 4 |
Depicts time spent freezing among same-aged rats whose reproductive status was in decline (n=15) or was maintained (n=10) on the hippocampus-mediated contextual learning aspect of the conditioned contextual fear task (secs ± SEM).
3. Discussion
3.1. Maintaining reproductive function in middle age was associated with improved cognitive performance on mPFC- and hippocampus-mediated tasks
The present findings supported our hypothesis that middle-aged rats that maintained reproductive function would perform better on cognitive tasks compared to those that had declining reproductive status, and these differences were influenced by formation of P4 and its metabolites in mPFC and/or hippocampus. Indeed, great variability was observed in steroid concentrations within middle-aged groups, and the percentage of successful prior pregnancies was associated with greater 3α,5α-THP concentrations in mPFC and hippocampus. Among rats in each group, task performance was associated with utilization of progestogens in mPFC and hippocampus. Rats that maintained their reproductive status had better cognitive performance on the prefrontal cortex- and hippocampus-mediated tasks, object recognition, Y-maze, and water maze, compared to rats that were in reproductive decline. However, rats in reproductive decline outperformed reproductively-maintained rats on amygdala-mediated tasks that were associated with aversive stimuli, such as inhibitory avoidance. This pattern, albeit not statistically-significant, was also observed on the amygdala-mediated cued learning portion of the contextual conditioned fear task wherein rats in reproductive decline spent more time freezing than did reproductively-maintained rats on every trial. Neither flinch and jump responses, nor swim speed, significantly differed between groups, suggesting that observed effects were not due to age-related differences in nociceptive and/or motor behavior.
Some caveats of the present study are important to consider. First, was the necessity of testing rats across a number of behavioral tasks. All rats were tested in the same battery of tasks in the same order. Rats may have been less reactive when assessed for amygdala-mediated tasks than they would otherwise have been if they were naïve to other behavioral tasks. However, despite this limitation, divergent cognitive profiles can be discerned between rats that were maintaining, or declining, in reproductive status. Second, estrous cycle stage can influence performance in prefrontal cortex- or hippocampus-mediated tasks. Although we were unable to control cycle phase during testing, we saw modest effects of estrous cycle stage to influence variability in performance in the present study. Despite the limitation of testing rats through a battery of tasks, and not being able to control estrous cyclicity, we found that middle-aged rats maintaining reproductive function had better cognitive performance and greater progestogen formation in mPFC and hippocampus than did their same-aged counterparts in reproductive decline.
These indications of cognitive and reproductive decline confirm prior observations wherein performance is poorer on tasks that are mediated by the prefrontal cortex and hippocampus (see Daniel and Bohacek, 2010). It is notable that deficits in memory among reproductively-declining rats were prevalent on both object recognition and Y-maze, which are considered spatial, working memory tasks (predominantly prefrontal cortex-mediated), and water maze, a spatial reference memory task (predominantly hippocampus-mediated). Indeed, the prefrontal cortex receives direct projections from the CA1 region of the hippocampus (Jay and Witter, 1991; Jay et al., 1991); however, independent age-related deterioration of these different memory constructs have been observed in rats (Gage et al., 1989; Markowska et al., 1989), particularly on frontal cortex-dependent tasks (Aggleton et al., 1989; Frick et al., 1995; Gallagher and Rapp, 1997; Winocur, 1992; Winocur and Moscovitch, 1990). The present findings suggest a more consistent decline in these functions, which may indicate a general vulnerability for spatial memory associated with age. In support, 5-month-old mice perform better on a spatial water maze than do 25-month-old mice, but significant differences are not observed on a non-spatial water maze test (Frick et al., 2000). Together, these data suggest that spatial impairments, potentially-mediated by the prefrontal cortex and/or hippocampus, may be disrupted with age; however, this decline does not occur in all individuals, and may be related to differences in steroid metabolism in the brain. It is important to understand what factors may contribute to the maintenance of spatial cognition in some individuals but not others.
3.2. Natural progestogen enhancement in adulthood may influence cognitive performance in later life
Differences in the cognitive performance of age-matched, 12-month-old rats may be better explained by individual differences in steroid utilization, rather than mean differences between reproductively-transitioning groups. In the current study, there was great individual variation in concentrations of E2, P4, DHP, 3α,5α-THP, and corticosterone among same-aged rats that had, or had not, transitioned to reproductive decline. As such, significant differences were not observed in mean levels of P4 turnover to its 5α-reduced metabolites. However, steroid utilization was significantly associated with cognition; wherein P4 and its conversion to 5α-reduced metabolites differentially affected task performance within individual rats. Greater P4 concentrations in mPFC and hippocampus, and reduced metabolism of P4 in hippocampus, positively accounted for 17-32% of the variance in performance on the Y-maze task. Others have found that intracerebroventricular (i.c.v.) administration of a P4 precursor to male mice can enhance Y-maze performance; however, i.c.v. enhancement of P4’s 5α-reduced metabolites is associated with decreased performance in this task (Ladurelle et al., 2000). These data are informing in light of recent findings that P4 can promote neurogenesis in dentate gyrus independent of 5α-reduction (Zhang et al., 2010). In the mPFC, 3α,5α-THP positively accounted for 25% of the variance in object recognition. We have previously observed that systemic 3α,5α-THP administration, or natural 3α,5α-THP enhancement, can enhance performance in the object recognition and object placement tasks among rats (Frye et al., 2007a; Paris and Frye, 2008; Walf et al., 2006). Thus, progestogen formation in the brain may influence cognitive performance.
It is notable that, although rats in both groups had a commensurate number of matings in their lifetime, a greater percentage of successful pregnancies were associated with higher 3α,5α-THP concentrations in mPFC and hippocampus. Prior pregnancy accounted for 17% of the variance of 3α,5α-THP in mPFC and 33% of the variance of 3α,5α-THP in hippocampus. Changes in P4 metabolism and/or 3α,5α-THP formation in corticolimbic structures in response to lifetime reproductive experience may be critical for healthy neurodevelopmental aging. Indeed, a history of prior progestogen exposure, via pregnancy to term, is associated with greater object, and spatial, memory (Frye et al., 2007a; Kinsley and Lambert, 2006; Love et al., 2005; Paris and Frye, 2008; Pawluski et al., 2006a,b). Prenatal stress, which is associated with neurodegeneration, reduces central 3α,5α-THP formation in hippocampus of dams throughout life (Frye and Walf, 2004a). As well, in a transgenic mouse model of Alzheimer’s disease, 3α,5α-THP formation is blunted (Frye and Walf, 2009). Together, these findings suggest that inter-individual variability in progestogen utilization/metabolism in brain is influenced by lifetime reproductive experience with consequences for cognition in aging.
3.3. Progestogen formation in later life may influence emotional/stress responding
Age-related changes in emotional memory may be influenced by steroid utilization independently of changes in spatial memory. Among young rats, ovarian hormones influence amygdala-mediated processes (Day et al., 2005; Frye and Walf, 2004b; Gupta et al., 2001; Holzbauer, 1976; Jasnow et al., 2006; Luine and McEwen, 1977; Shors et al., 1998; Walf and Frye, 2003; Wood et al., 2001). In the current investigation, reproductively-declining rats performed better than did reproductively-maintained rats in tasks that utilize shock stimuli and are known to be dependent on the amygdala (Schütz and Izquierdo, 1979), a region associated with storage of emotional memory (reviewed in LeDoux, 2007). Indeed, clinical literature details robust changes in amygdala function and affective behavior with aging (Almaguer et al., 2002; Tessitore et al., 2005; Wright et al., 2008). Moreover, mood-related pathologies that occur later in life, such as depression, are typically intractable and comorbid with cognitive impairment (reviewed in Alexopoulos, 2005). We have previously categorized the reproductive transition among cycling rats and have observed greater anxiety-like behavior among those in reproductive decline compared to those maintaining reproductive status (Walf et al., 2009c). In these experiments, rats in reproductive decline spent less time in on the open arms of an elevated plus maze, less time in the open quadrants of an elevated zero maze, and made fewer licks of an electrified water bottle in a Vogel drinking-conflict task compared to rats that maintained reproductive senescence (Walf et al., 2009c). As such, considering age-related changes in affective and stress response may be important when evaluating task performance.
In the present study, steroid concentrations were assessed in tested and non-tested rats. While significant differences were not observed between rats that were maintaining or declining in reproductive status, rats that were non-tested had notably lower concentrations of corticosterone in circulation and P4 turnover to its metabolites in mPFC and hippocampus. As such, mild stressors, such as testing in the behavioral battery described, may activate hypothalamic-pituitary-adrenal (HPA) function, promoting formation of P4’s 5α-reduced metabolites. Acute stressors, including footshock or cold-water swim, enhance central 3α,5α-THP synthesis (Barbaccia et al., 1996; Drugan et al., 1994; Purdy et al., 1991), which may promote trophic actions in the brain. In particular, 3α,5α-THP is demonstrated to dampen HPA activity (Patchev et al., 1996), reduce cell death and promote positive prognosis in models of brain injury/degeneration (Baulieu and Schumacher, 2000; Frye, 2009; Sayeed and Stein, 2009), and improve cognitive status in healthy models and models of neurodegeneration (Mellon, 2007). As such, steroid-promoted changes in cellular structure may be a mechanism by which observed effects are partly mediated.
3.4. Steroids actions to influence cognition may involve structural remodeling in the brain
It is likely that the observed outcomes of this study reflect differences in plasticity with highly variable inter-individual changes in observed in steroid utilization during aging, but this needs to be examined further. Ovarian steroids alter dendritic spines density (Kinsley et al., 2006; Pawluski and Galea, 2006; Woolley et al., 1990; Woolley and McEwen, 1993) and cognitive performance (Frye et al., 2007a; Paris and Frye, 2008; Walf et al., 2006) of young rats. Among rats, ovariectomy or prenatal stress can significantly reduce dendritic spinal density in frontal cortex and hippocampus concomitant with reduction in object recognition performance (Gould et al., 1990; Paris and Frye, 2010a; Wallace et al., 2006). As well, it has been suggested that timing of E2-replacement therapy following reproductive decline is critical for the beneficial effects of hormone therapy (Daniel et al., 2006; Klaiber et al., 1997; Sherwin, 2009). Recent investigations demonstrate that the efficacy of E2 to promote synaptic density of CA1 apical spines and memory are dependent on the timing of steroid administration following the transition to reproductive decline (Daniel and Bohacek, 2010; McLaughlin et al., 2008; Rodgers et al., 2010).
While actions of E2 can enhance 3α,5α-THP formation in brain (Cheng and Karavolas, 1973; Frye et al., 1998; Micevych et al., 2008), activation of extranuclear estrogen receptors also enhances expression of the progestin receptor, which in rat hippocampus, is largely located in the extranuclear regions of cells (Waters et al., 2008). Extranuclear progestin receptors may comprise a rapid signaling mechanism for P4’s actions. In mouse hippocampus, extranuclear estrogen and progestin receptor expression peaks at different times throughout the estrous cycle in synaptic dendrites and axons, respectively (Mitterling et al., 2010), which may confer the temporal nature for E2 to regulate mnemonic improvement in the climacteric. Less is known about extranuclear expression of progestin receptor in prefrontal cortex. However, GABAA and other neurotransmitter receptors, rather than progestin receptors, are a target for 3α,5α-THP’s actions (Callachan et al., 1987; Frye, 2009) and may partly underlie P4’s effects in this region. Indeed, we have found that middle-aged mice lacking progestin receptors have improved cognitive performance following P4 (Frye and Walf, 2010), suggesting progestin receptors may not be necessary for progestogens’ mnemonic effects.
3.5. Conclusions
In summary, we observed enhanced cognitive performance on spatial memory tasks (object recognition, Y-maze, water maze) among middle-aged rats maintaining reproductive status compared to their same-aged, reproductively-declining counterparts. Emotional memory, such as that observed on the inhibitory avoidance task was greater in reproductively senescent, compared to reproductively competent, rats. Individual differences in progestogen formation in prefrontal cortex and/or hippocampus were associated with improved performance on these tasks. These data support the notion that progestogen metabolism in prefrontal cortex and hippocampus of rats in later life may influence the propensity for cognitive decline and these factors can be influenced by lifetime reproductive experience.
4. Experimental Procedure
These methods were pre-approved by the Institutional Animal Care and Use Committee at The University at Albany-SUNY and were conducted in accordance with the ethical guidelines defined by The National Institutes of Health (NIH Publication No. 85-23).
4.1. Animals and housing
The present study compared cognitive behavior of middle-aged (12 months old) female Long-Evans rats (N=46). Rats in Cohorts 1 and 2 were obtained from Taconic Farms (Germantown, NY; n=30) at 2 months of age. These animals comprised an age-matched cohort of new breeders that were purchased to begin the rat colony in the Life Sciences Research Building, which was completing construction at the University at Albany-SUNY (and was subject to new building renovations). As such, all rats were of the same age and had a commensurate breeding history (described below). Rats in cohorts 3 and 4 were obtained from in-house breeding (derived from offspring of rats in cohorts 1 and 2; n=16) and were also comprised of age-matched breeders in our new Life Sciences Research Building. Notably, no differences were noted between cohorts for behavioral indices and, thus, these cohorts were combined in the final data analyses.
Rats were group-housed (3-4 per cage) in polycarbonate cages (45 × 24 × 21 cm) in a temperature-controlled room (21 ± 1 °C) in the Laboratory Animal Care Facility core at The University at Albany-SUNY. Rats lived under reversed-lighting conditions (lights off between 08:00 and 20:00 h), with ad libitum access to rodent chow and water in their homecages.
4.2. Reproductive status characterization
We investigated cognitive behavior among same-aged rats with varying degrees of reproductive-viability, which would be associated with changes in ovarian function (i.e. estropause). Although, natural reproductive senescence among aged rodents is not marked by exactly the same type of gradual decline in ovarian function as is observed in the menopause of women, reproductive function does decline with age in rodents (Wise, 1999). Declining reproductive status is characterized by changes in feedback of the hypothalamic-pituitary-adrenal stress and gonadal axes and is marked by abnormal estrous cyclicity in female rodents (Clemens and Meites, 1971; Dudley, 1982; Huang et al., 1978). Rats were characterized as maintaining reproductive status or as being in reproductive decline based upon measures of estrous cyclicity (regular 4-5-day versus irregular/longer cycles), fertility (whether there was successful pregnancy on their last mating with a male and their overall percentage of successful pregnancies throughout life), and fecundity (the average number of pups/litter). All rats had a commensurate number of lifetime matings (5.4 ± 0.1 matings). A detailed explanation of how independent variables can be derived has been described (Walf et al., 2009b,c). Briefly, rats whose reproductive status was considered to be maintained had regular 4-5 day cycles, ≥ 60 % successful pregnancies (66.0 ± 6.7 %), and an average of ≥ 10 pups per litter (10.3 ± 0.9) at the time of testing. Rats whose reproductive status was considered to be in decline had irregular cycles that were greater than 5 days, < 60 % successful pregnancies (46.0 ± 7.6 %), an average of < 10 pups per litter (3.7 ± 1.3) at the time of testing.
4.3. Behavioral testing
Estrous cycle phase was determined per prior methods (Frye et al., 2000) and rats were tested in one of the following behavioral tasks by an experimenter blind to the hypothesized outcome of the study. Data were simultaneously collected by hand using stopwatches and by the ANY-maze video-tracking system (Stoelting Co., Wood dale, IL) with 95%+ concordance between these methods. Testing in the following tasks was conducted in order presented below for all rats (with the exception of conditioned fear which was conducted only in a subset of rats) and no more than one test was conducted per day, every 1-4 days. As well, all rats were assessed in a standard mating and paced mating task after completing Y-maze and prior to testing in water maze. The results of data from the standard and paced mating tasks are reported in Walf et al. (submitted to this issue). While testing all rats in the same order of tasks introduces a concern that prior experience in one task may interfere with following tasks, a greater confound may be produced when subjects are exposed to noxious tasks involving swim stress or shock prior to less noxious tasks. As such, a counterbalanced Latin square testing design would not have been appropriate. As well, the rigorous requirements for inclusion in this study (age-matched, middle-aged breeders with a commensurate breeding history undergoing natural reproductive senescence) precluded a fully between-subjects design given that number of subjects required would have exceeded capacity. We have previously used similar batteries of behavioral tests (Edinger & Frye, 2004; Frye et al., 2007) and have systematically demonstrated that testing in conflict tasks without noxious stimuli, such as shock, do not result in changes in central neurosteroidogenesis (Frye et al., 2007). As such, it was necessary to test rats, first in the least noxious tasks (object recognition, Y-maze) and to test rats last in the most noxious tasks (inhibitory avoidance, conditioned fear) that may influence steroid concentrations.
4.3.1. Object recognition
This task was used per published methods (Ennaceur and Delacour, 1988; Frye and Lacey 2000; Luine et al., 2003; Paris and Frye, 2008; Walf et al., 2006). Briefly, during the acquisition trial, rats were placed in a white open field (76 × 57 cm with 35 cm walls) in a brightly-lit testing room and allowed to explore two objects (plastic toys in the shape of oranges). Following a four hour inter-trial interval, rats’ performance in a retention trial assessed the time they spent exploring a novel object when exposed to the object used in the acquisition trial and one that they had never been exposed to (a plastic toy in the shape of a buoy). A greater percentage of time spent exploring the novel object, as a function of the total amount of time spent exploring both objects during the retention trial, is considered an index of enhanced cognitive performance in this task.
4.3.2. Y-maze
This task was carried out modified from previous methods (Frye and Lacey, 2000). The apparatus consists of a start arm (61 × 13 × 30 cm) and two goal boxes (46 × 15 × 30 cm each) with guillotine doors enclosing the start box, and each of the two goal arms. During acquisition, rats were placed in the start box and allowed 15 minutes to explore the start arm and one open goal arm while the other goal arm was closed. Arm entries and total time spent in each arm were recorded. After a 4-hr interval, rats were tested in the Y-maze by being placed in the start box and allowed 5 min exploration with both goal arms open. Entries and duration spent in each arm were recorded. A greater percentage of time spent exploring the novel arm during the retention trial, as a function of the total amount of time spent exploring goal arms, indicates enhanced performance.
4.3.3. Water maze
The water maze paradigm utilized was modified from previous methods (Frye and Lacey, 2000; Vongher and Frye, 1999) and was commensurate with that utilized by Rhodes and Frye (2004). In brief, the water maze tank (555 cm circumference, 71 cm deep) was filled with 25-30 °C water which was made opaque by the addition of non-dairy creamer and had many extra-maze visual cues. The hidden platform (5.3 × 5.3 × 33.5 cm) was placed approximately 2.5 cm below the water line and 60 cm from the inner edge of the water tank. On Day One, rats were habituated to the pool and swimming by spending two minutes in the pool, which did not contain the platform. On Day Two, rats were given two consecutive 2-min acquisition trials to find the hidden platform. After each trial, rats that did not find the platform were placed on the platform for 45 sec. On Day Three, rats were given four 2-min retention trials to find the platform when their starting location was varied to a different quadrant of the pool each time. Starting quadrants were completely counterbalanced across subjects and conditions. The average time and distance swam to find the platform was calculated across all four trials for each subject and lower latencies/distances to find the platform indicates better performance in this task and swim speed was calculated from these variables.
4.3.4. Inhibitory avoidance
Testing was conducted as previously reported (Edinger et al., 2004; Walf and Frye, 2007). Rats were placed in a stainless steel apparatus that is separated by a guillotine door to make two distinct compartments (24 × 18 × 11 cm each), one of which is painted white and brightly lit and the other is painted black and dark. Rats were habituated to the chamber. Following habituation, rats were placed in the white side for one minute before a guillotine door was lifted. The latency of the rat to cross to the black side is recorded, and rats are exposed to a brief mild shock through the floor bars of the chamber (0.25 mA for 2 sec). Twenty-four hours later, rats were given a retention trial in which they were confined to the white side of the apparatus for one min and then assessed for their latency to cross to the black side when the guillotine door is raised (300 sec max). A greater latency to cross to the dark, shock-associated side indicates enhanced cognitive performance. Flinch and jump in response to shock was calculated on a 4-point scale per previously described methods (Edinger et al., 2004; Walf and Frye, 2007).
4.3.5. Conditioned contextual fear
Conditioned fear was carried out in a representative subset of rats (N=25; reproductively-competent=9, transitioning n=8, reproductively-senescent n=8) per previously reported methods (Kim et al., 1993; Kjelstrup et al., 2002; Edinger et al., 2004). Briefly, rats were habituated for 4 min in the apparatus. Rats were then exposed to a tone followed by an electric footshock (0.5 mA for 2 sec) for three acquisition trials administered one min apart. Five days later, rats were tested in the amygdala-mediated cued task (different chamber and almond extract scented, same tone as during acquisition), or the hippocampus-mediated contextual memory task (same chamber, no tone; Kim et al., 1993; Sanders et al., 2003). The time spent freezing by rats during eight one-minute retention trials was recorded, following a four minute habituation period to the chamber. More time spent freezing indicates enhanced cognitive performance.
4.4. Radioimmunoassay for Steroid Concentrations
Some rats were euthanized via rapid decapitation, and whole brains were collected and flash frozen. Trunk blood was collected into chilled test tubes, spun in a refrigerated centrifuge, and plasma was decanted into clean 1.5 ml tubes and stored frozen until radioimmunoassay. At the time of assay, plasma and brains were thawed and mPFC and hippocampus were grossly dissected for assessment of E2 and progestogens in brain and corticosterone in plasma, per previously described methods (Frye et al., 2000, 2007b). In order to assess changes in the metabolism of P4 via the 5α-reductase enzyme that forms DHP, and subsequently 3α,5α-THP, the ratio P4 to its 5α-reduced metabolites was calculated for statistical analyses (see below).
4.5. Statistical analyses
One-way analyses of variance (ANOVAs) were utilized to determine behavioral and endocrine effects of reproductive status. To determine P4 turnover to its 5α-reduced metabolites, the ratio of DHP+3α,5α-THP:P4 was used per previous methods (Kellogg and Frye, 1999; Paris and Frye, 2010b). When main effects were present, Fisher’s protected LSD post-hoc tests were conducted to determine group differences. For conditioned contextual fear, repeated measures ANOVAs were utilized to determine effects of reproductive status and testing session on performance in the cued or contextual learning paradigms. Simple linear regressions were utilized to determine the amount of variance that endocrine analyses could account for in behaviors examined. To assess whether estrous cycle phase was disparately distributed among reproductively-maintaining or -declining rats on testing days, chi-square analyses were conducted with reproductive status (maintaining, declining) and cycle phase (prosetrus, estrus, metaestrus, diestrus) as factors. To assess the amount of variance that estrous cycle phase contributed to performance on each behavioral task, cycle phases were assigned a numeric value (proestrus=4, estrus=3, metaestrus=2, diestrus=1) and correlations were executed among these values and task dependent variables. Alpha level for significance was p < 0.05.
Acknowledgements
This research was supported in part by funding from the Department of Defense CDMRP Breast Cancer Research Program, National Science Foundation (IBN03-16083), and the National Institute of Mental Health (MH0676980). The technical assistance of Dr. Madeline Rhodes, Alicia Babson, and Irene Chin is greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Aggleton JP, Blindt HS, Candy JM. Working memory in aged rats. Behav Neurosci. 1989;103:975–983. doi: 10.1037//0735-7044.103.5.975. [DOI] [PubMed] [Google Scholar]
- Akirav I, Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb Cortex. 2006;16:1759–1765. doi: 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- Albano C, Grimbizis G, Smitz J, Riethmüller-Winzen H, Reissmann T, Van Steirteghem A, Devroey P. The luteal phase of nonsupplemented cycles after ovarian superovulation with human menopausal gonadotropin and the gonadotropin-releasing hormone antagonist. Cetrorelix Fertil Steril. 1998;70:357–359. doi: 10.1016/s0015-0282(98)00135-6. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- Almaguer W, Estupiñán B, Uwe Frey J, Bergado JA. Aging impairs amygdala-hippocampus interactions involved in hippocampal LTP. Neurobiol Aging. 2002;23:319–324. doi: 10.1016/s0197-4580(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Archer J. Rodent sex differences in emotional and related behavior. Behav Biol. 1975;14:451–479. doi: 10.1016/s0091-6773(75)90636-7. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Baulieu E, Schumacher M. Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination. Steroids. 2000;65:605–612. doi: 10.1016/s0039-128x(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Bergman I, Blomberg M, Almkvist O. The importance of impaired physical health and age in normal cognitive aging. Scand J Psychol. 2007;48:115–125. doi: 10.1111/j.1467-9450.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3α-OH-5β-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999;850:217–224. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Wang JM. Preclinical analyses of the therapeutic potential of allopregnanolone to promote neurogenesis in vitro and in vivo in transgenic mouse model of Alzheimer’s disease. Curr Alzheimer Res. 2006;3:11–17. doi: 10.2174/156720506775697160. [DOI] [PubMed] [Google Scholar]
- Broadbent N, Squire L, Clark R. Spatial memory, recognition memory, and the hippocampus. Neuroscience. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci. 1987;231:359–369. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Rossato JI, Lima RH, Medina JH, Izquierdo I. Parallel memory processing by the CA1 region of the dorsal hippocampus and the basolateral amygdala. Proc Natl Acad Sci USA. 2008;105:10279–10284. doi: 10.1073/pnas.0805284105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MC, Zandi PP, Plassman BL, Tschanz JT, Welsh-Bohmer KA, Steffens DC, Bastian LA, Mehta KM, Breitner JC, Cache County Study Group Hormone replacement therapy and reduced cognitive decline in older women: the Cache County Study. Neurology. 2001;57:2210–2216. doi: 10.1212/wnl.57.12.2210. [DOI] [PubMed] [Google Scholar]
- Charalampopoulos I, Remboutsika E, Margioris AN, Gravanis A. Neurosteroids as modulators of neurogenesis and neuronal survival. Trends Endocrinol Metab. 2008;19:300–307. doi: 10.1016/j.tem.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Cheng YJ, Karavolas HJ. Conversion of progesterone to 5α-pregnane-3, 20-dione and 3α-hydroxy-5α-pregnan-20-one by rat medical basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology. 1973;93:1157–1162. doi: 10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Carrero P, Frye CA, Garcia-Segura LM. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol. 2006;66:916–928. doi: 10.1002/neu.20293. [DOI] [PubMed] [Google Scholar]
- Clemens JA, Meites J. Neuroendocrine status of old constant-estrous rats. Neuroendocrinology. 1971;7:249–256. doi: 10.1159/000121973. [DOI] [PubMed] [Google Scholar]
- Colsher PL, Wallace RB. Epidemiologic considerations in studies of cognitive function in the elderly: methodology and nondementing acquired dysfunction. Epidemiol Rev. 1991;13:1–27. doi: 10.1093/oxfordjournals.epirev.a036065. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Bohacek J. The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochim Biophys Acta. 2010;1800:1068–1076. doi: 10.1016/j.bbagen.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Day M, Sung A, Logue S, Bowlby M, Arias R. estrogen receptor knockout (BERKO) mice present attenuated hippocampal CA1 long-term potentiation and related memory deficits in contextual fear conditioning. Behav Brain Res. 2005;164:128–131. doi: 10.1016/j.bbr.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Decker MW, Pelleymounter MA, Gallagher M. Effects of training on a spatial memory task on high affinity choline uptake in hippocampus and cortex in young adult and aged rats. J Neurosci. 1988;8:90–99. doi: 10.1523/JNEUROSCI.08-01-00090.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon GM, Qu X, Marcus JN, Dodart JC. Excitotoxic lesions restricted to the dorsal CA1 field of the hippocampus impair spatial memory and extinction learning in C57BL/6 mice. Neurobiol Learn Mem. 2008;90:426–433. doi: 10.1016/j.nlm.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22:106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- Drugan RC, Basile AS, Ha JH, Ferland RJ. The protective effects of stress control may be mediated by increased brain levels of benzodiazepine receptor agonists. Brain Research. 1994;661:127–136. doi: 10.1016/0006-8993(94)91189-4. [DOI] [PubMed] [Google Scholar]
- Dudley SD. Responsiveness to estradiol in central nervous system of aging female rats. Neurosci Biobehav Rev. 1982;6:39–45. doi: 10.1016/0149-7634(82)90005-7. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone’s analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5α-reduced metabolites in the hippocampus. Behav Neurosci. 2004;118:1352–11364. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Lee B, Frye CA. Mnemonic effects of testosterone and its 5α-reduced metabolites in the conditioned fear and inhibitory avoidance tasks. Pharmacol Biochem Behav. 2004;78:559–568. doi: 10.1016/j.pbb.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Edwards RG, Steptoe PC, Purdy JM. Establishing full-term pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol. 1980;87:737–756. doi: 10.1111/j.1471-0528.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–19. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Featherby T, van den Buuse M, Lubman DI, Lawrence AJ. Persistent downregulation of hippocampal CREB mRNA parallels a Y-maze deficit in adolescent rats following semi-chronic amphetamine administration. Br J Pharmacol. 2008;154:417–428. doi: 10.1038/bjp.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W, Chen KS, Gage FH, Björklund A. Progressive decline in spatial learning and integrity of forebrain cholinergic neurons in rats during aging. Neurobiol Aging. 1992;13:9–23. doi: 10.1016/0197-4580(92)90003-g. [DOI] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol Behav. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- Frye CA. Neurosteroids - from basic research to clinical perspectives. In: Rubin RT, Pfaff DW, editors. Hormone/Behavior Relations of Clinical Importance: Endocrine Systems Interacting with Brain and Behavior. Academic Press; San Diego: 2009. pp. 395–416. [Google Scholar]
- Frye CA, Bayon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3α,5α-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998;808:72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007a;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Lacey EH. Progestins influence performance on cognitive tasks independent of changes in affective behavior. Psychobiology. 2000;28:550–563. [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5α-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007b;133:663–674. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Hippocampal 3α,5α-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav. 2004a;78:531–540. doi: 10.1016/j.pbb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behav. Neurosci. 2004b;118:306–313. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Progesterone reduces depression-like behavior in a murine model of Alzheimer’s Disease. Age (Dordr) 2009;31:143–153. doi: 10.1007/s11357-009-9091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Progesterone enhances learning and memory of aged wildtype and progestin receptor knockout mice. Neurosci Lett. 2010;472:38–42. doi: 10.1016/j.neulet.2010.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Bjorklund A. Age-related impairments in spatial memory are independent of those in sensorimotor skills. Neurobiol Aging. 1989;10:347–352. doi: 10.1016/0197-4580(89)90047-x. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res. 2003;74:637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Levine S. Effect of induced cestrus on emotional behaviour in selected strains of rats. Nature. 1964;201:1198–1200. doi: 10.1038/2011198a0. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Reboussin BA, Hogan P, Barnabei VM, Shumaker S, Johnson S, Barrett-Connor E. Symptom relief and side effects of postmenopausal hormones: results from the Postmenopausal Estrogen/Progestin Interventions Trial. Obstet Gynecol. 1998;92:982–988. doi: 10.1016/s0029-7844(98)00305-6. [DOI] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats. Brain Res. 2001;888:356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- He J, Evans CO, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 2004a;189:404–412. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci. 2004b;22:19–31. [PubMed] [Google Scholar]
- Holzbauer M. Physiological aspects of steroids with anaesthetic properties. Med Biol. 1976;54:227–242. [PubMed] [Google Scholar]
- Huang HH, Steger RW, Bruni JF, Meites J. Patterns of sex steroid and gonadotropin secretion in aging female rats. Endocrinology. 1978;103:1855–1859. doi: 10.1210/endo-103-5-1855. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, da Cunha C, Rosat R, Jerusalinsky D, Ferreira MB, Medina JH. Neurotransmitter receptors involved in post-training memory processing by the amygdala, medial septum, and hippocampus of the rat. Behav Neural Biol. 1992;58:16–26. doi: 10.1016/0163-1047(92)90847-w. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH. Memory formation: The sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav. 2006;49:197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Jay TM, Thierry AM, Wiklund L, Glowinski J. Excitatory amino acid pathway from the hippocampus to the prefrontal cortex. Contribution of AMPA receptors in hippocampo-prefrontal cortex transmission. Eur J Neurosci. 1991;4:1285–1295. doi: 10.1111/j.1460-9568.1992.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Johnson FB, Sinclair DA, Guarente L. Molecular biology of aging. Cell. 1999;96:291–302. doi: 10.1016/s0092-8674(00)80567-x. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Jutapakdeegul N, Afadlal S, Polaboon N, Phansuwan-Pujito P, Govitrapong P. Repeated restraint stress and corticosterone injections during late pregnancy alter GAP-43 expression in the hippocampus and prefrontal cortex of rat pups. Int J Dev Neurosci. 2010;28:83–90. doi: 10.1016/j.ijdevneu.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Frye CA. Endogenous levels of 5α-reduced progestins and androgens in fetal vs. adult rat brains. Brain Res Dev Brain Res. 1999;115:17–24. doi: 10.1016/s0165-3806(99)00041-3. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Lambert KG. The maternal brain. Sci Am. 2006;294:72–79. doi: 10.1038/scientificamerican0106-72. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Lambert KG. Reproduction-induced neuroplasticity: natural behavioural and neuronal alterations associated with the production and care of offspring. J Neuroendocrinol. 2008;20:515–525. doi: 10.1111/j.1365-2826.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EA, Hester N, Morgan M, Kozub FJ, Lambert KG. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49:131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci USA. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaiber EL, Broverman DM, Vogel W, Peterson LG, Snyder MB. Relationships of serum estradiol levels, menopausal duration, and mood during hormonal replacement therapy. Psychoneuroendocrinology. 1997;22:549–558. doi: 10.1016/s0306-4530(97)00043-7. [DOI] [PubMed] [Google Scholar]
- Ladurelle N, Eychenne B, Denton D, Blair-West J, Schumacher M, Robel P, Baulieu E. Prolonged intracerebroventricular infusion of neurosteroids affects cognitive performances in the mouse. Brain Res. 2000;858:371–379. doi: 10.1016/s0006-8993(00)01953-3. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Berry AE, Griffins G, Amory-Meyers E, Madonia-Lomas L, Love G, Kinsley CH. Pup exposure differentially enhances foraging ability in primiparous and nulliparous rats. Physiol Behav. 2005;84:799–806. doi: 10.1016/j.physbeh.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Le Mellédo JM, Baker G. Role of progesterone and other neuroactive steroids in anxiety disorders. Expert Rev Neurother. 2004;4:851–860. doi: 10.1586/14737175.4.5.851. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17:R868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Llaneza DC, Frye CA. Progestogens and estrogen influence impulsive burying and avoidant freezing behavior of naturally cycling and ovariectomized rats. Pharmacol Biochem Behav. 2009;93:337–342. doi: 10.1016/j.pbb.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzini CA, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat’s passive avoidance response: A tetrodotoxin functional inactivation study. Brain Res. 1996;730:32–39. doi: 10.1016/0006-8993(96)00427-1. [DOI] [PubMed] [Google Scholar]
- Love G, Torrey N, McNamara I, Morgan M, Banks M, Hester NW, Glasper ER, Devries AC, Kinsley CH, Lambert KG. Maternal experience produces long-lasting behavioral modifications in the rat. Behav Neurosci. 2005;119:1084–1096. doi: 10.1037/0735-7044.119.4.1084. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN, McEwen BS. Effect of oestradiol on turnover of type A monoamine oxidase in brain. J Neurochem. 1977;28:1221–1227. doi: 10.1111/j.1471-4159.1977.tb12313.x. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, Luine VN. Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev. 2010;34:452–467. doi: 10.1016/j.neubiorev.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Stone WS, Ingram DK, Reynolds J, Gold PE, Conti LH, Pontecorvo MJ, Wenk GL, Olton DS. Individual differences in aging: Behavioral and neurobiological correlates. Neurobiol Aging. 1989;10:31–43. doi: 10.1016/s0197-4580(89)80008-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: Evidence that the duration of hormone deprivation after ovariectomy compromises 17β-estradiol effectiveness in altering CA1 spines. Horm Behav. 2008;54:386–395. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH. Neurosteroid regulation of central nervous system development. Pharmacol Ther. 2007;116:107–124. doi: 10.1016/j.pharmthera.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Soma KK, Sinchak K. Neuroprogesterone: key to estrogen positive feedback? Brain Res Rev. 2008;57:470–480. doi: 10.1016/j.brainresrev.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010;518:2729–2743. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O’Carroll C. Elements of a neurobiological theory of the hippocampus: The role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:773–786. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta M, Ferlito L, Magnolfi SU, Petruzzi E, Pinzani P, Malentacchi F, Petruzzi I, Bennati E, Malaguarnera M, IMUSCE Cognitive and functional status in the extreme longevity. Arch Gerontol Geriatr. 2008;46:245–252. doi: 10.1016/j.archger.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- Murphy SJ, Littleton-Kearney MT, Hurn PD. Progesterone administration during reperfusion, but not preischemia alone, reduces injury in ovariectomized rats. J Cereb Blood Flow Metab. 2002;22:1181–1188. doi: 10.1097/01.WCB.0000037990.07114.07. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Frye CA. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105–115. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Frye CA. Gestational exposure to variable stressors produces decrements in cognitive and neural development of juvenile male and female rats. Curr Top Med Chem. 2010a doi: 10.2174/156802611796117649. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Frye CA. Juvenile offspring of rats exposed to restraint stress in late gestation have impaired cognitive performance and dysregulated progestogen formation. Stress. 2010b doi: 10.3109/10253890.2010.512375. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Galea LA. Hippocampal morphology is differentially affected by reproductive experience in the mother. J Neurobiol. 2006;66:71–81. doi: 10.1002/neu.20194. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Vanderbyl BL, Ragan K, Galea LA. First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or ‘mothering’ alone. Behav Brain Res. 2006a;175:157–165. doi: 10.1016/j.bbr.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Walker SK, Galea LA. Reproductive experience differentially affects spatial reference and working memory performance in the mother. Horm Behav. 2006b;49:143–149. doi: 10.1016/j.yhbeh.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Petkus AJ, Gum AM, King-Kallimanis B, Wetherell JL. Trauma history is associated with psychological distress and somatic symptoms in homebound older adults. Am J Geriatr Psychiatry. 2009;17:810–818. doi: 10.1097/JGP.0b013e3181b20658. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem. 1994;1:34–44. [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr., Paul SM. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proceedings of the National Academy of Science U S A. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Estrogen has mnemonic-enhancing effects in the inhibitory avoidance task. Pharmacol Biochem Behav. 2004;78:551–558. doi: 10.1016/j.pbb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, McCormick CM, Frye CA. 3α,5α-THP mediates progestins’ effects to protect against adrenalectomy-induced cell death in the dentate gyrus of female and male rats. Pharmacol Biochem Behav. 2004;78:505–12. doi: 10.1016/j.pbb.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010;151:1194–203. doi: 10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Zinn CG, Furini C, Bevilaqua LR, Medina JH, Cammarota M, Izquierdo I. A link between the hippocampal and the striatal memory systems of the brain. An Acad Bras Cienc. 2006;78:515–523. doi: 10.1590/s0001-37652006000300011. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. Eur J Pharmacol. 2003;463:217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Stein DG. Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog Brain Res. 2009;175:219–237. doi: 10.1016/S0079-6123(09)17515-5. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Rhodes ME, Bludau M, Kaplan S, Ong P, Ueffing I, Vehoff J, Korr H, Frye CA. Depression: reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Mol Psychiatry. 2002;7:810–813. doi: 10.1038/sj.mp.4001118. [DOI] [PubMed] [Google Scholar]
- Schochet SS., Jr. Neuropathology of aging. Neurol Clin. 1998;16:569–580. doi: 10.1016/s0733-8619(05)70081-5. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Stein DG, De Nicola AF. Progesterone: therapeutic opportunities for neuroprotection and myelin repair. Pharmacol Ther. 2007;116:77–106. doi: 10.1016/j.pharmthera.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Schütz RA, Izquierdo I. Effect of brain lesions on rat shuttle behavior in four different tests. Physiol Behav. 1979;23:97–105. doi: 10.1016/0031-9384(79)90128-8. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The critical period hypothesis: Can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol. 2007;19:77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol. 2009;5:620–627. doi: 10.1038/nrendo.2009.193. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9:419–423. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- Smitz J, Devroey P, Camus M, Deschacht J, Khan I, Staessen C, Van Waesberghe L, Wisanto A, Van Steirteghem AC. The luteal phase and early pregnancy after combined GnRH-agonist/HMG treatment for superovulation in IVF or GIFT. Hum Reprod. 1988;3:585–590. doi: 10.1093/oxfordjournals.humrep.a136750. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Tavaniotou A, Smitz J, Bourgain C, Devroey P. Ovulation induction disrupts luteal phase function. Ann N Y Acad Sci. 2001;943:55–63. doi: 10.1111/j.1749-6632.2001.tb03790.x. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, Mattay VS. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Res. 2005;139:9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab. 2004;24:1160–1166. doi: 10.1097/01.WCB.0000135594.13576.D2. [DOI] [PubMed] [Google Scholar]
- Utian WH, Archer DF, Bachmann GA, Gallagher C, Grodstein F, Heiman JR, Henderson VW, Hodis HN, Karas RH, Lobo RA, Manson JE, Reid RL, Schmidt PJ, Stuenkel CA, North American Menopause Society Estrogen and progestogen use in postmenopausal women: July 2008 position statement of The North American Menopause Society. Menopause. 2008;15:584–602. doi: 10.1097/gme.0b013e31817b076a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Zglinicki T, Burkle A, Kirkwood TB. Stress, DNA damage and ageing: an integrative approach. Exp. Gerontol. 2001;36:1049–1062. doi: 10.1016/s0531-5565(01)00111-5. [DOI] [PubMed] [Google Scholar]
- Vongher JM, Frye CA. Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacol Biochem Behav. 1999;64:777–785. doi: 10.1016/s0091-3057(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Postal BA, Darrah SD, Chen X, Khan AS. Deficits in novelty exploration after controlled cortical impact. J Neurotrauma. 2007;24:1308–1320. doi: 10.1089/neu.2007.0274. [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res. 2009a;196:254–260. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Anti-nociception following exposure to trimethylthiazoline, peripheral or intra-amygdala estrogen and/or progesterone. Behav Brain Res. 2003;144:77–85. doi: 10.1016/s0166-4328(03)00067-6. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Estradiol decreases anxiety behavior and enhances inhibitory avoidance and gestational stress produces opposite effects. Stress. 2007;10:251–260. doi: 10.1080/00958970701220416. [DOI] [PubMed] [Google Scholar]
- Walf AA, Paris JJ, Frye CA. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology. 2009b;34:909–916. doi: 10.1016/j.psyneuen.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Paris JJ, Frye CA. Nociceptive and anxiety-like behavior in reproductively competent and reproductively senescent middle-aged rats. Gend Med. 2009c;6:235–246. doi: 10.1016/j.genm.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Paris JJ, Llaneza DC, Frye CA. Levels of 5α-reduced progesterone metabolite levels in the midbrain account for variability in reproductive behavior of middle-aged female rats. Brain Res. doi: 10.1016/j.brainres.2010.11.004. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126:176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Waters EM, Torres-Reveron A, McEwen BS, Milner TA. Ultrastructural localization of extranuclear progestin receptors in the rat hippocampal formation. J Comp Neurol. 2008;511:34–46. doi: 10.1002/cne.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- Winocur G. A comparison of normal old rats and young adult rats with lesions to the hippocampus or prefrontal cortex on a test of matching-to-sample. Neuropsychologia. 1992;30:769–781. doi: 10.1016/0028-3932(92)90081-v. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M. Hippocampal and prefrontal cortex contributions to learning and memory: Analysis of lesion and aging effects on maze learning in rats. Behav Neurosci. 1990;104:544–551. doi: 10.1037//0735-7044.104.4.544. [DOI] [PubMed] [Google Scholar]
- Wise PM. Neuroendocrine modulation of the “menopause”: Insights into the aging brain. Am J Physiol. 1999;277:E965–E970. doi: 10.1152/ajpendo.1999.277.6.E965. [DOI] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Wright CI, Negreira A, Gold AL, Britton JC, Williams D, Barrett LF. Neural correlates of novelty and face-age effects in young and elderly adults. Neuroimage. 2008;42:956–968. doi: 10.1016/j.neuroimage.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Verkhratsky A, Toescu EC. Changes in mitochondrial status associated with altered Ca2+ homeostasis in aged cerebellar granule neurons in brain slices. J Neurosci. 2002;22:10761–10771. doi: 10.1523/JNEUROSCI.22-24-10761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Yang R, Zhou R, Li L, Sokabe M, Chen L. Progesterone promotes the survival of newborn neurons in the dentate gyrus of adult male mice. Hippocampus. 2010;20:402–412. doi: 10.1002/hipo.20642. [DOI] [PubMed] [Google Scholar]
- Zoli M, Picciotto MR, Ferrari R, Cocchi D, Changeux JP. Increased neurodegeneration during ageing in mice lacking high-affinity nicotine receptors. EMBO J. 1999;18:1235–1244. doi: 10.1093/emboj/18.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]