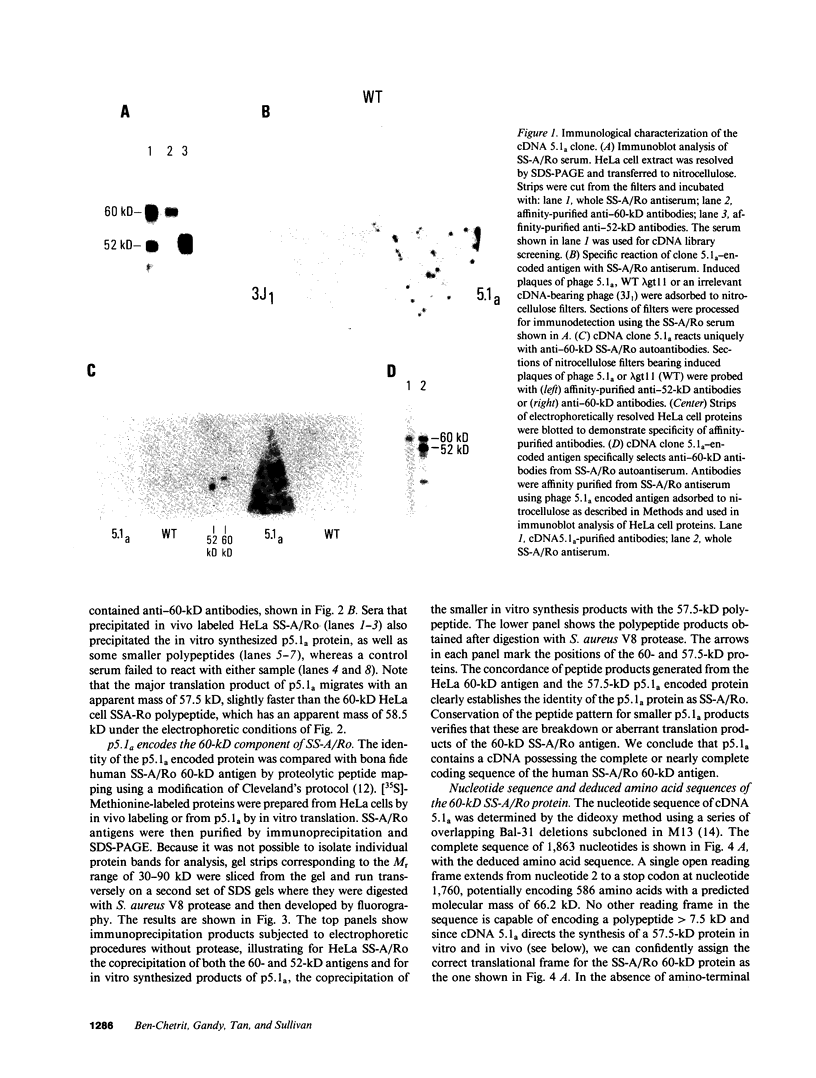
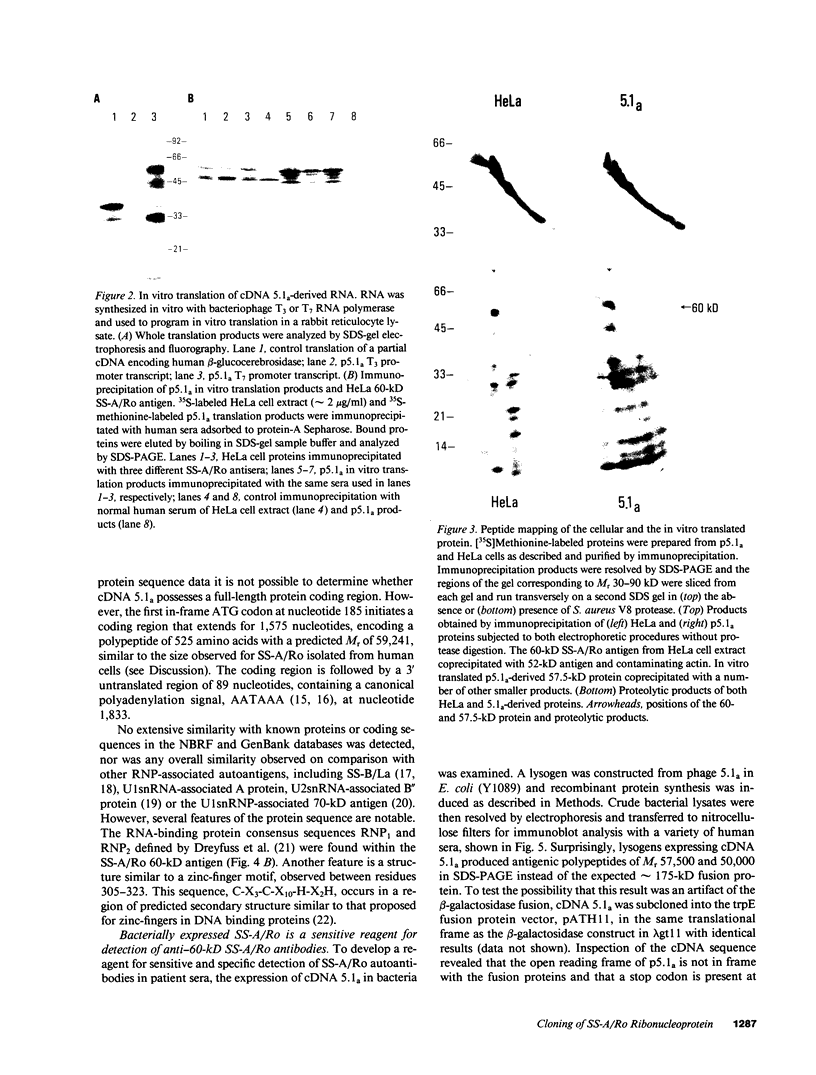
Abstract
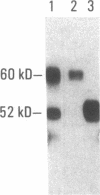
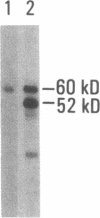
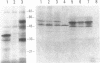
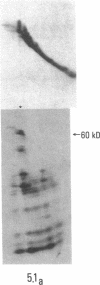
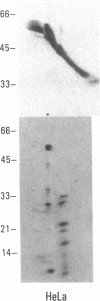
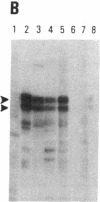
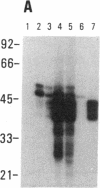
SS-A/Ro is a nucleocytoplasmic ribonucleoprotein (RNP) particle that is a common target of autoimmune response in Sjögren's syndrome (SS) and systemic lupus erythematosus (SLE). Previously, SS-A/Ro has been shown to be composed of at least two polypeptide antigens of 60 and 52 kD noncovalently associated with a set of small RNAs, designated Y1-Y5. A serum from an SS patient was selected to screen a lambda gt11 cDNA library constructed from human T cell lymphoblastic leukemia (MOLT-4) mRNA. An immunoreactive clone was isolated that possessed a 1.8-kb cDNA insert. In vitro transcription and translation of the cDNA resulted in the synthesis of a 57.5-kD polypeptide which was specifically immunoprecipitated by SS-A/Ro antisera. The identity of the cDNA encoded protein as the 60-kD SS-A/Ro antigen was established by proteolytic peptide mapping of the cDNA-encoded protein and the 60-kD HeLa cell antigen. The sequence of the cDNA shows that the 60-kD SS-A/Ro protein possesses both RNA binding protein consensus sequences and a single zinc-finger motif. Recombinant SS-A/Ro antigen produced in bacteria proved to be a sensitive and specific reagent for detection of anti-SS-A/Ro antibodies in patient sera. The availability of the 60-kD SS-A/Ro cDNA will enable detailed analysis of the molecular structure and function of the SS-A/Ro RNP particle and its role in autoimmune pathology.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann M., Mayet W. J., Schröder H. C., Pfeifer K., Meyer zum Büschenfelde K. H., Müller W. E. Identification of the Ro and La antigens in the endoribonuclease VII--ribonucleoprotein complex. Biochem J. 1987 Apr 1;243(1):189–194. doi: 10.1042/bj2430189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Chetrit E., Chan E. K., Sullivan K. F., Tan E. M. A 52-kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med. 1988 May 1;167(5):1560–1571. doi: 10.1084/jem.167.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J. C., Kenan D., Martin B. J., Keene J. D. Genomic structure and amino acid sequence domains of the human La autoantigen. J Biol Chem. 1988 Dec 5;263(34):18043–18051. [PubMed] [Google Scholar]
- Chan E. K., Francoeur A. M., Tan E. M. Epitopes, structural domains, and asymmetry of amino acid residues in SS-B/La nuclear protein. J Immunol. 1986 May 15;136(10):3744–3749. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dreyfuss G., Swanson M. S., Piñol-Roma S. Heterogeneous nuclear ribonucleoprotein particles and the pathway of mRNA formation. Trends Biochem Sci. 1988 Mar;13(3):86–91. doi: 10.1016/0968-0004(88)90046-1. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Sullivan K. F., Machlin P. S., Cooke C. A., Kaiser D. A., Pollard T. D., Rothfield N. F., Cleveland D. W. Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J Cell Biol. 1987 Apr;104(4):817–829. doi: 10.1083/jcb.104.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K. B., Jankowski P. W. Fine specificities of autoantibodies directed against the Ro, La, Sm, RNP, and Jo-1 proteins defined by two-dimensional gel electrophoresis and immunoblotting. J Immunol. 1985 Jun;134(6):3819–3824. [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Kato N., Hoshino H., Harada F. Nucleotide sequence of 4.5S RNA (C8 or hY5) from HeLa cells. Biochem Biophys Res Commun. 1982 Sep 16;108(1):363–370. doi: 10.1016/0006-291x(82)91875-7. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Grabowski P. J., Padgett R. A., Sharp P. A. Characterization of the branch site in lariat RNAs produced by splicing of mRNA precursors. Nature. 1985 Feb 14;313(6003):552–557. doi: 10.1038/313552a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff S. E., Rosenfeld M. G., Evans R. M. Complex transcriptional units: diversity in gene expression by alternative RNA processing. Annu Rev Biochem. 1986;55:1091–1117. doi: 10.1146/annurev.bi.55.070186.005303. [DOI] [PubMed] [Google Scholar]
- Lieu T. S., Newkirk M. M., Capra J. D., Sontheimer R. D. Molecular characterization of human Ro/SS-A antigen. Amino terminal sequence of the protein moiety of human Ro/SS-A antigen and immunological activity of a corresponding synthetic peptide. J Clin Invest. 1988 Jul;82(1):96–101. doi: 10.1172/JCI113607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Sanger F. Determination of nucleotide sequences in DNA. Science. 1981 Dec 11;214(4526):1205–1210. doi: 10.1126/science.7302589. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillekens P. T., Habets W. J., Beijer R. P., van Venrooij W. J. cDNA cloning of the human U1 snRNA-associated A protein: extensive homology between U1 and U2 snRNP-specific proteins. EMBO J. 1987 Dec 1;6(12):3841–3848. doi: 10.1002/j.1460-2075.1987.tb02721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgess A. D., Peterson M. G., McNeilage L. J., Whittingham S., Coppel R. L. Characteristics and epitope mapping of a cloned human autoantigen La. J Immunol. 1988 May 1;140(9):3212–3218. [PubMed] [Google Scholar]
- Sullivan K. F., Machlin P. S., Ratrie H., 3rd, Cleveland D. W. Sequence and expression of the chicken beta 3 tubulin gene. A vertebrate testis beta-tubulin isotype. J Biol Chem. 1986 Oct 5;261(28):13317–13322. [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Theissen H., Etzerodt M., Reuter R., Schneider C., Lottspeich F., Argos P., Lührmann R., Philipson L. Cloning of the human cDNA for the U1 RNA-associated 70K protein. EMBO J. 1986 Dec 1;5(12):3209–3217. doi: 10.1002/j.1460-2075.1986.tb04631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. The biology and detection of immune complexes. Adv Immunol. 1979;28:89–220. doi: 10.1016/s0065-2776(08)60800-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1996–2000. doi: 10.1073/pnas.81.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]