Abstract
Rho-associated kinase (Rho-kinase/ROCK/ROK) is an effector of the small GTPase Rho and belongs to the AGC family of kinases. Rho-kinase has pleiotropic functions including the regulation of cellular contraction, motility, morphology, polarity, cell division, and gene expression. Pharmacological analyses have revealed that Rho-kinase is involved in a wide range of diseases such as vasospasm, pulmonary hypertension, nerve injury, and glaucoma, and is therefore considered to be a potential therapeutic target. This review focuses on the structure, function, and modes of activation and action of Rho-kinase.
Keywords: Rho, kinase, cytoskeleton, actomyosin, polarity
Introduction
Rho-kinase, originally identified as an effector of the small GTPase Rho [Leung et al., 1995; Ishizaki et al., 1996; Matsui et al., 1996], plays a major role in mediating rearrangements of the actomyosin cytoskeleton downstream of Rho. Rho family small GTPases, such as Rho, Rac, and Cdc42, regulate cytoskeletal reorganization in different ways [Kaibuchi et al., 1999; Jaffe and Hall, 2005]. The functions of these GTPases have been primarily investigated with regard to their effects of actin filaments. Rho regulates stress fiber formation and cell contraction, whereas Rac and Cdc42 regulate the formation of lamellipodia and filopodia, respectively, and promote protrusive activities [Hall, 2005]. Rho family GTPases also modulate microtubule dynamics and cell polarity. Furthermore, in addition to Rho-kinase, many other Rho effectors with various functions have been identified, including the myosin phosphatase-targeting subunit 1 (MYPT1) of myosin light chain (MLC) phosphatase, mDia, Protein kinase N (PKN), Citron, Citron-kinase, Rhotekin, and Rhophilin, and many act in concert following Rho activation. For example, Rho-kinase functions together with MYPT1 and mDia to achieve stress fiber and focal adhesion formation downstream of Rho [Amano et al., 2000; Narumiya et al., 2009]. In this review, we will focus on the structure, function, and modes of action of Rho-kinase.
Structure and Modes of Activation and Inhibition
Domain structure
Rho-kinase is a serine/threonine kinase belonging to the AGC family of protein kinases, which are structurally related to myotonic dystrophy kinase (DMPK) and myotonic dystrophy kinase-related CDC42-binding kinase (MRCK). There are two Rho-kinase members, Rho-kinase α/ROCK2/ROKα and Rho-kinase β/ROCK1/ROKβ: we will refer to them collectively as Rho-kinase. Rho-kinase is composed of an N-terminal catalytic domain, a central coiled-coil domain, and a C-terminal PH domain interrupted by a Cys-rich region (Fig. 1). Rho-kinase requires both N- and C-terminal extension segments in addition to the core catalytic domain for its activity, which is conserved among the DMPK subgroup (see below). Rho activates Rho-kinase by binding to the C-terminal portion of the coiled-coil.
Fig. 1. Structure of Rho-kinase/ROCK/ROK.
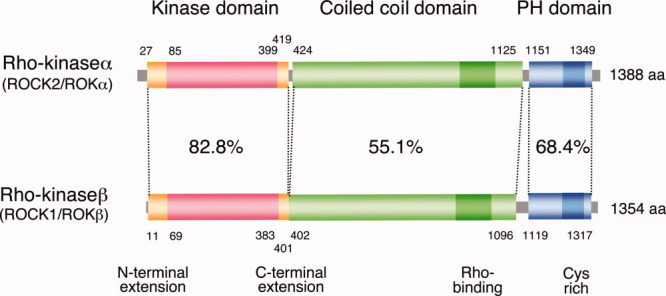
Schematic diagrams of the domain structure of Rho-kinases. Amino acid sequence identities for each domain are indicated.
Crystal structure
Unique features of the Rho-kinase catalytic domain have been revealed by studies of its crystal structures. Rho-kinase has N- and C-terminal extension segments outside the catalytic domain that form an intermolecular head-to-head homodimer, keeping it in an active conformation [Yamaguchi et al., 2006a; Jacobs et al., 2006] (Fig. 2). Interestingly, phosphorylation at the activation loop or C-terminal hydrophobic motif, which is necessary for most of other AGC kinases such as PKC and Akt, is absent from the Rho-kinase catalytic domain in its dimerized active conformation [Yamaguchi et al., 2006a]. The Rho-binding region of Rho-kinase also forms a parallel coiled-coil structure [Shimizu et al., 2003; Dvorsky et al., 2004], supporting the idea that Rho-kinase dimerizes in a parallel manner. Consistent with this, Rho-kinase is found as a multimer in the cell [Chen et al., 2002].
Fig. 2. Ribbon diagram of the dimmer structure of catalytic domain of Rho-kinase.
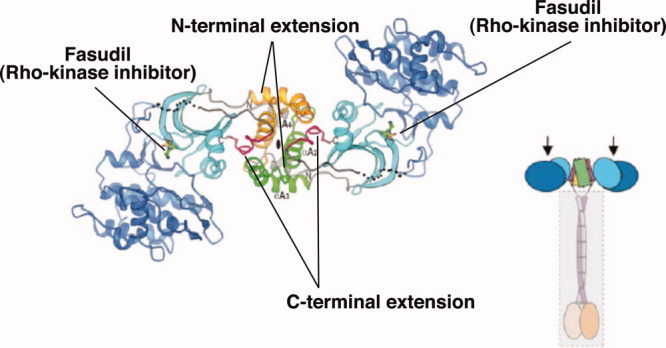
Rho-kinase forms a head-to head homodimer through its N- and C-terminal extensions. Fasudil binds to the ATP-binding cleft. Predicted whole structure of Rho-kinase is also shown, in which Rho-kinase forms parallel dimmer through both the extensions outside of catalytic domain and central coiled-coil regions. Arrows indicate the active centers of Rho-kinase. Reprinted from Structure, Vol 14(3), 2006, Yamaguchi et al., DOI: 10.1016/j.str.2005.11.024; ©2005, with permission from Elsevier.
Activation and inactivation
The C-terminal segment containing the Rho-binding and PH domains acts as the negative regulatory region of Rho-kinase. That the interaction between active Rho (Rho·GTP) and the Rho-binding domain releases this inhibition is based on several lines of evidence: (1) deletion of the C-terminal portion results in constitutive activation of Rho-kinase [Leung et al., 1996; Amano et al., 1997; Ishizaki et al., 1997], (2) the C-terminal segment interacts with and inhibits the constitutively active catalytic fragment both in vitro and in vivo [Amano et al., 1999], and (3) an antibody against the Rho-binding domain of Rho-kinase enhances its kinase activity in vitro (unpublished observation). In addition to Rho, certain lipids such as arachidonic acid can activate Rho-kinase via its PH domain [Araki et al., 2001]. Proteolytic cleavage at the C-terminus by caspase-3 and granzyme B is also reported to activate ROCK1 and ROCK2, leading to plasma membrane blebbing during apoptosis [Coleman et al., 2001; Sebbagh et al., 2001; Sebbagh et al., 2005]. Other small GTPases besides Rho - Rnd3/RhoE, Gem and Rad - can bind Rho-kinase outside of the Rho-binding region and inhibit its function [Ward et al., 2002; Riento et al., 2003; Komander et al., 2008]. It has also been reported that PDK1 directly binds to ROCK1 in competition with RhoE, leading to the release of ROCK1 from RhoE and activation [Pinner and Sahai, 2008].
ROCK1 and ROCK2
The structures of ROCK1 and ROCK2 are conserved with 64% overall amino acid identity (Fig. 1). The kinase domain containing both extension segments is more highly conserved between these two proteins (83% identical), suggesting that they may have similar substrate specificity. The consensus phosphorylation sequence for Rho-kinase is R/KXS/T or R/KXXS/T (X is any amino acid). Both Rho-kinase proteins are ubiquitously expressed in most tissues; however, higher levels of ROCK2 are found in brain and muscles whereas higher levels of ROCK1 are found in non-neuronal tissues including liver, lung and testis [Leung et al., 1996; Nakagawa et al., 1996]. So far, several functional differences between ROCK1 and ROCK2 have been reported. ROCK1 is specifically cleaved by caspase-3, whereas ROCK2 is cleaved by granzyme B [Coleman et al., 2001; Sebbagh et al., 2001; Sebbagh et al., 2005]. RhoE preferentially binds ROCK1, but not ROCK2, whereas MYPT1 binds only ROCK2 [Komander et al., 2008; Wang et al, 2009]. Gene silencing experiments also suggest different cellular functions for these two proteins, ROCK1 appears to be essential for the formation of stress fibers, whereas ROCK2 appears to be necessary for phagocytosis and cell contraction, both of which are dependent on MLC phosphorylation [Yoneda et al., 2005; Wang et al., 2009]. Most if not all mice carrying a homozygous deletion of the either the ROCK2 or ROCK1 allele show embryonic or postnatal lethality, respectively, whereas heterozygous mice are fertile and appear normal [Thumkeo et al., 2003; Shimizu et al., 2005; Thumkeo et al., 2005]. However, the question of whether these two Rho-kinase proteins are qualitatively different or expressed at different abundances in tissues remains unanswered.
Inhibitors
To elucidate the physiological roles of Rho-kinase, small molecule inhibitors have been developed and investigated in various cell types and animal models [Mueller et al., 2005; Shimokawa and Rashid, 2007]. Fasudil (HA-1077) and Y-27632 have been broadly used as Rho-kinase selective inhibitors and function in an ATP-competitive manner. Fasudil, composed of the isoquinoline ring and the pendant ring of the seven-membered homopiperazine, is used clinically for cerebral vasospasm after subarachnoid hemorrhage in Japan. H-1152 has been developed by optimization of a series of isoquinoline compounds. Y-27632 was identified by its ability to inhibit phenylephrine-induced contraction of a rabbit aortic strip and contains a 4-aminopyridine ring [Uehata et al., 1997]. Structural analyses of the Rho-kinase (ROCK2)-Fasudil complex [Yamaguchi et al., 2006a], Rho-kinase (ROCK2)-Y-27632 complex [Yamaguchi et al., 2006b], and ROCK1-Y-27632 complex [Jacobs et al., 2006] revealed the inhibitors cause an induced-fit conformational change to increase contacts with Rho-kinase phosphate loop, which may account for their specificity. Several pharmaceutical companies are investing in the development of Rho-kinase inhibitors for the treatment of certain diseases, such as glaucoma (see [Hahmann and Schroeter, 2010] for a review). Notably, the ROCK2-selective inhibitor SLx-2119 [Boerma et al., 2008] is now in clinical trials.
Functions
Rho-kinase members play roles in various cellular functions through phosphorylation of their specific substrates (Fig. 3 and Table I)
Fig. 3. Substrates of Rho-kinase.
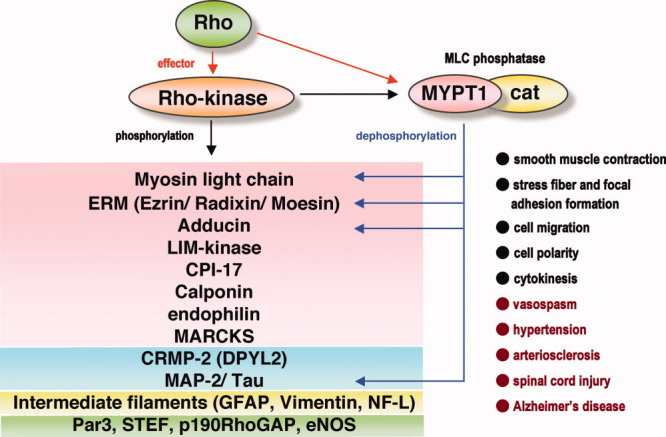
Rho-kinase inhibits the MLC phosphatase activity through both phosphorylation of MYPT1 of MLC phosphatase and phosphorylation of CPI17, an inhibitory protein of myosin phosphatase. Rho-kinase and MLC phosphatase share their substrates, such as MLC, ERM proteins, adducin and MAPs, and thought to regulate the level of phosphorylation. The substrates reported to be phosphorylated by Rho-kinase are illustrated; actin-bindig/regulating proeins (red), MT-binding/regulating proteins (blue), intermediate filaments (yellow), and proteins in other signaling pathways (green). Physiological (black) and pathological (red) processes in which Rho-kinase is involved are listed at the bottom right.
Table I.
List of Known Substrates and Effects of Phosphorylation
| Substrate | Effect of phosphorylation | Cellular function | Reference |
|---|---|---|---|
| Microfilament | |||
| MYPT1 | Inhibition of MLC phosphatase | Increase in cell contraction | [Kimura et al., 1996] |
| MLC | Activation of myosin ATPase | Increase in cell contraction | [Amano et al., 1996] |
| ERM | Maintenance of crosslinking | Microvilli formation? | [Fukata et al., 1998] [Oshiro et al., 1998] [Matsui et al., 1998] |
| Adducin | Enhancement of F-actin binding | Cell migration | [Fukata et al., 1999] |
| LIMK1/2 | Activation of cofilin phosphorylation | Stress fiber formation | [Maekawa et al., 1999] [Ohashi et al., 2000] |
| Calponin | Inhibition of F-actin binding | n.d. | [Kaneko et al., 2000] |
| CPI-17 | Inhibition of MLC phosphatase | Increase in cell contraction | [Koyama et al., 2000] |
| NHE1 | n.d. | Stress fiber formation | [Tominaga and Barber, 1998] [Denker et al., 2000] |
| MARCKS | Inhibition of F-actin binding | n.d. | [Nagumo et al., 2001] |
| EF1α | Inhibition of F-actin binding | n.d. | [Izawa et al., 2000] |
| Troponin I/T | n.d. | Inhibition of Ca2+ response in cardiac muscle | [Vahebi et al., 2005] |
| Profilin | Inhibition of G-actin binding | Decrease in polyQ aggregation | [Shao et al., 2008] [Bauer et al., 2009] |
| Microtubule | |||
| MAP2/Tau | Inhibition of tubulin polymerization | Inhibition of neurite elongation? | [Amano et al., 2003] |
| CRMP2 | Inhibition of tubulin polymerization | Growth cone collapse | [Arimura et al., 2000; Arimura et al., 2005] |
| Doublecortin | Inhibition of microtubule bundling | Neuronal migration? | [Amano et al., 2010] |
| Intermediate filament | |||
| GFAP | Depolymerization | Progression of cytokinesis | [Yasui et al., 1998] |
| Vimentin | Depolymerization | Progression of cytokinesis | [Goto et al., 1998] |
| Neurofilament (NF-L) | Depolymerization | Neurite retraction | [Hashimoto et al., 1998] |
| Signaling crosstalk | |||
| Par3 | Dissociation of Par3/Tiam1 from Par6/aPKC | Control of cell migration | [Nakayama et al., 2008] |
| STEF | Inhibition of GEF activity? | Inhibition of neurite outgrowth? | [Takefuji et al., 2007] |
| p190RhoGAP | Inhibition of Rnd binding | Positive feedback signal of Rho? | [Mori et al., 2009] |
| eNOS | Inactivation of eNOS | Cell contraction? | [Sugimoto et al., 2007] |
| PTEN | Activation of phosphatase activity? | Cell polarity? | [Li et al., 2005] |
| FilGAP | Activation of RacGAP activity | Lamella formation, cell polarity | [Ohta et al., 2006] |
| IRS1 | Positive/negative regulation of insulin signal? | [Furukawa et al., 2005] | |
| Endophilin | Inhibition of CIN85 binding | Inhibition of EGFR endocytosis | [Kaneko et al., 2005] |
| P300 | Increase in acetyltransferase activity | Enhancement of transcription | [Tanaka et al., 2006] |
| RhoE | Stabilization | Decrease of stress fibers | [Komander et al., 2008; Riento et al., 2003] |
n.d.: not defined.
Cell contraction and actin organization
Smooth muscle contraction
Vascular smooth muscle tone is regulated by MLC phosphorylation of myosin II, which is mediated by Ca2+-dependent MLC kinase and MLC phosphatase. The Rho/Rho-kinase signaling pathway is known to modulate the “Ca2+-sensitivity” of smooth muscle mainly through the suppression of MLC phosphatase activity. MLC phosphatase is composed of the catalytic subunit (PP1cδ) and two regulatory subunits, myosin phosphatase-targeting subunit 1 (MYPT1) and M20 [Ito et al., 2004]. Rho-kinase phosphorylates MYPT1 at two inhibitory sites, Thr853 and Thr696, resulting in a decrease in MLC phosphatase activity and an increase in phosphorylated MLC [Kimura et al., 1996]. Rho-kinase can also phosphorylate MLC at Ser19 to increases myosin ATPase activity at least in vitro [Amano et al., 1996], although a direct contribution of Rho-kinase to phospho-MLC levels in vivo is not yet proved. CPI-17 has an inhibitory effect on MLC phosphatase when it is phosphorylated by PKC at Thr38 and is also reported to be phosphorylated by Rho-kinase at the same site [Kitazawa et al., 2000] [Koyama et al., 2000]. Several studies using permeabilized smooth muscle and selective inhibitors have shown that Rho-kinase activity is involved in the modulation of smooth muscle tone, especially during aberrant vascular contractions, such as coronary vasospasm, cerebral vasospasm after subarachnoid hemorrhage, and pulmonary hypertension [Shimokawa and Rashid, 2007]. Recently, we found that Rho-kinase phosphorylates p190RhoGAP, one of the major negative regulators of Rho, and presumably inhibits its GAP activity in smooth muscle cells [Mori et al., 2009], which may form a positive feedback loop that accounts for the hyperactivation of Rho at spastic sites.
Stress fiber formation
Stress fibers and focal adhesions confer contractility on a cell and are mediated by Rho/Rho-kinase signaling pathway [Amano et al., 1997]. MLC phosphorylation which is necessary for the formation and maintenance of stress fibers and focal adhesions, is regulated by Rho-kinase and MLC phosphatase downstream of Rho, in cooperation with the Rho effector mDia [Watanabe et al., 1999]. LIM kinases 1 and 2 are phosphorylated by Rho-kinase at Thr508 and Thr505, respectively, resulting in increased cofilin phosphorylation at Ser3 [Maekawa et al., 1999]. Cofilin is an actin-depolymerizing factor and regulates actin dynamics, and its activity is inhibited by phosphorylation. Rho-kinase seems to induce and maintain stress fibers by increasing contractility via MLC phosphorylation and by stabilizing actin filaments through LIMK activation which results in cofilin phosphorylation. Isolated contractile stress fibers from fibroblasts contain Rho, Rho-kinase and MYPT1, and their contraction is sensitive to Rho-kinase inhibitors [Katoh et al., 2001], suggesting that Rho, Rho-kinase and MLC phosphatase associate with microfilaments to form a modulatory apparatus for acto-myosin contraction.
Cell migration
Mode of cell migration
Multiple events are coordinately regulated during cell migration: polarization in the direction of movement, reorganization of the cytoskeleton, turnover of membranes, and remodeling of cell adhesions. Cell-to-cell and cell-to-substratum interactions are important for cell migration. Proper cell movement is essential for development, wound healing and inflammation. Aberrant cell migration may lead to increased tumor cell invasion and arteriosclerosis. Rho family GTPases play critical roles in regulation of cell migration through their specific effectors [Etienne-Manneville and Hall, 2002; Fukata et al., 2003].
The roles of Rho/Rho-kinase in cell migration depend on the cell type and mode of migration [Nakayama et al., 2005]. Inhibition of Rho or Rho-kinase results in the inhibition of migration through Boyden chambers, but not attachment, of monocytes [Worthylake et al., 2001], neutrophils, HL-60 [Hauert et al., 2002], eosinophils [Alblas et al., 2001], and smooth muscle cells [Ai et al., 2001; Nishiguchi et al., 2003]. Monocytes treated with Y-27632 or C3 exoenzyme (an inhibitor of Rho) have elongated tails, and cause a defect in tail detachment, and a mislocalizated integrins, the latter of which is not observed in cells treated with myosin inhibitors [Worthylake et al., 2001]. This suggests that not only myosin-mediated contraction but also integrin reorganization is necessary for the Rho/Rho-kinase-mediated tail retraction. Rho or Rho-kinase inhibitors also affect the chemokinesis of neutrophils and HL-60 cells. fNLPNTL-induced neutrophil polarization is inhibited to some extent by treatment with Y-27632, whereas the number of non-polar cells with ruffles is increased [Hauert et al., 2002]. Similar results have been shown for HL-60 cells stimulated with fMLP - the number of cells with flattened morphologies and multiple pseudopods is increased by the inhibition of Rho, Rho-kinase or myosin II [Xu et al., 2003]. In Y-27632-treated HL-60 cells, the level of activated Rac is increased after stimulation with fMLP, suggesting that the multiple pseudopods observed after the inhibition of Rho/Rho-kinase are the consequence of aberrant activation of Rac. The migration of fibroblasts during wound healing or random migration is not inhibited, but rather enhanced, by inhibition of Rho or Rho-kinase [Nobes and Hall, 1999; Magdalena et al., 2003; Totsukawa et al., 2004]. In contrast to neutrorphils and HL-60 cells, Rho-kinase inhibition by Y-27632 leads to faster and straighter migration with a single leading edge and without Rac activation, whereas MLCK inhibition results in the formation of multiple protrusions in gerbil fibroma cells [Totsukawa et al., 2004].
Furthermore, it has been shown that tumor cells use at least two types of migration in a three-dimensional matrix: Rho/Rho-kinase-dependent and Rac-dependent migration [Sahai and Marshall, 2003]. Several tumor cell lines, such as A375 melanoma cell lines, can migrate through a 3D matrix in a Rac-dependent mesenchymal fashion or in a Rho/Rho-kinase-dependent amoeboid fashion. Inhibition of Rho-kinase converts their morphology from amoeboid to mesenchymal in a DOCK3 (RacGEF)/Rac/WAVE2-dependent manner, whereas silencing of DOCK3 or Rac1 converts cells from the mesenchymal morphology to amoeboid one in a Rho-kinase/ARHGAP22 (RacGAP)-dependent manner, suggesting that these two types of migration are interconvertible and antagonistic [Sanz-Moreno et al., 2008].
Front-rear polarity
The establishment of front-rear polarity is another important process for efficient cell migration. Various molecules and/or activation states of proteins are distributed asymmetrically in the polarized cell. Rho-kinase inhibitors impair cell polarization, leading to random protrusions and multiple leading edges in certain cell types. This suggests that Rho-kinase is necessary for breaking symmetry and/or maintaining the polarized imbalance, by suppressing extension of membrane except at the front leading edge. In neutrophils, PTEN is localized at the rear of cell and suppresses the production of PI(3,4,5)P3; the distribution of PTEN is under the control of Rho/Rho-kinase, most likely by the phosphorylation of PTEN by Rho-kinase [Li et al., 2005]. We recently showed that Rho-kinase also phosphorylates PAR-3, a component of the PAR polarity complex, which leads to dissociation of PAR-3 from the PAR-6/aPKC complex [Nakayama et al., 2008]. Because PAR-3 binds to the Rac GEF Tiam1/Tiam2 (STEF) and aPKC stimulates Rac GEF activity, Rho-kinase indirectly abrogates Rac activation by phosphorylating PAR-3. PAR-3 phosphorylation by Rho-kinase is observed not only at the rear of the cell but also at the leading edge, which may reflect the role of Rho-kinase in modulating the extent of protrusions by counteracting Rac activity.
Neurite elongation and neuronal architecture
Growth cone collapse
Rho and Rho-kinase are highly expressed in the nervous system, implying important roles for these proteins in neuron and/or glial cells. Several neuron-specific substrates for Rho-kinase have been identified. Collapsin response mediator protein 2 (CRMP-2) is abundant in brain tissue, and is implicated in growth cone collapse and neuronal polarization through its interaction with tubulin heterodimers, numb, kinesin-1, and Sra-1. CRMP-2 is phosphorylated by Rho-kinase at Thr555, which impairs its ability to bind to tubulin and numb [Arimura et al., 2005]. Phosphorylation of CRMP-2 is increased by stimulation with LPA and ephrin-A5 in DRG and hippocampal neurons, respectively, and is implicated in growth cone collapse induced by these repulsive cues [Arimura et al., 2000; Arimura et al., 2005]. MAP2, Tau, and neurofilament are also neuron-specific substrates for Rho-kinase, and their microtubule polymerizing activity and neurofilament assembly are inhibited by phosphorylation by Rho-kinase [Hashimoto et al., 1998; Amano et al., 2003]. Phosphorylation of MAP2/Tau and neurofilament may prevent neurite elongation and shorten neurites by destabilizing microtubules and intermediate filaments. The control of cellular contraction through MYPT1/MLC phosphorylation by Rho-kinase is also thought to participate in neurite retraction and inhibition of neurite outgrowth [Amano et al., 1998; Hirose et al., 1998]. LIMK1/cofilin has also been implicated in regulation of growth cone morphology in chick DRG neurons [Endo et al., 2003], potentially downstream of Rho-kinase.
Neuronal architecture and neurite elongation
Pharmacological studies have revealed many roles for Rho-kinase in the nervous system. Treatment of cultured cerebellar granule neurons with Y-27632 triggered immediate neurite outgrowth at an early stage, though it was less effective at later stages, suggesting that Rho-kinase controls the initiation of axonogenesis [Bito et al., 2000]. At the neuronal maturation stage, dendritic branching and spine formation are markedly inhibited when constitutively activated RhoA is expressed in hippocampal slice cultures; this effect can be abrogated by the administration of Y-27632 [Nakayama et al., 2000], implying that Rho-kinase plays a role in dendrite formation.
Rho-kinase inhibitors have also been shown to be effective for treatment of some neurological disorders, including spinal cord injury and Alzheimer's disease [Mueller et al., 2005]. Several myelin-derived proteins are known to prevent axon regeneration after axon damage in the central nervous system. Myelin-associated inhibitors, such as Myelin-associated glycoprotein (MAG), Nogo, and oligodendrocyte myelin glycoprotein (OMgp) activate Rho through the p75 receptor in complex with co-receptors, and Rho-kinase inhibitors abolish myelin-associated inhibitor-induced repellant effects in both cultured neuron and animal models [Mueller et al., 2005]. One of the potential downstream effectors of Rho-kinase in these signaling pathways is CRMP-2. CRMP-2 is phosphorylated at Thr555 following treatment with MAG or Nogo, and overexpression of CRMP-2 causes neurite extension in the presence of MAG [Mimura et al., 2006].
Rho-kinase inhibitors also show a potential protective effects in Alzheimer's disease [Tang and Liou, 2007]. Alzheimer's disease is characterized by extracellular amyloid aggregates of toxic 40- or 42-amino-acid long amyloid-β (Aβ40 or Aβ42) peptides, which are generated from amyloid precursor protein (APP) that is abnormally cleaved by α- and γ-secretases. Treatment with Y-27632 decreased the production of Aβ42 in SH-SY5Y cells expressing mutant APP (APPswe) and in model mice [Zhou et al., 2003]. Shedding of APP, which determines the alternative forms, soluble APP (sAPP) and toxic Aβ42, was also found to be modulated by Rho-kinase in APPswe-expressing N2a cells [Pedrini et al., 2005].
Cytokinesis
Drastic reorganization of the cytoskeleton occurs during cell division. During cytokinesis, cells are divided into two daughter cells via an actomyosin-based contractile apparatus known as the contractile ring to which Rho and Rho-kinase are recruited. In addition to Rho, Plk1 has also been found to associate with and activate Rho-kinase synergistically with Rho during cytokinesis [Lowery et al., 2007]. Rho and Rho-kinase are involved in both the progression of the cleavage furrow formation and the disassembly of intermediate filaments beneath cleavage furrow [Matsumura, 2005] [Izawa and Inagaki, 2006]. Rho-kinase regulates MLC phosphorylation through MLC phosphatase, and possibly by direct phosphorylation, at the contractile ring. At the same time, Rho-kinase phosphorylates the head domain of intermediate filaments, such as vimentin, leading to the disassembly of filaments that ensures furrow completion. Inhibition of Rho-kinase by Y-27632 does not completely arrest cytokinesis in cultured cells, though it does induce some abnormalites and delays in cleavage [Kosako et al., 2000]. Various other kinases, such as Aurora-B and Citron-kinase, seem to play redundant roles in cytokinesis.
Other functions
Ezrin/radixin/moesin (ERM) proteins were originally identified as Rho-kinase substrates that are phosphorylated at Thr567, Thr564, and Thr558, respectively; phosphorylation of ERM proteins by Rho-kinase maintains their activity by crosslinking transmembrane proteins to F-actin [Fukata et al., 1998; Matsui et al., 1998]. PKC and MRCK were also demonstrated to phosphorylate ERM at these sites, and the contribution of Rho-kinase to ERM phosphorylation is thought to depend on the cell type and situation, such as in smooth muscle cells upon static pressure [Onoue et al., 2008], in hippocampal neurons upon glutamate stimulation [Jeon et al., 2002], in T cells from systemic lupus erythematosus patients [Li et al., 2007], and in Jurkat cells upon Fas ligand stimulation [Hebert et al., 2008].
Rho-kinase is also localized in the nucleus and interacts with p300 acetyltransferase, which forms a large nuclear protein complex [Tanaka et al., 2006]. Rho-kinase phosphorylates p300 and activates its acetyltransferase activity, in vitro and in vivo, resulting in the enhancement of p300-dependent transcription in U205 cells [Tanaka et al., 2006].
Many genetic and pharmacological studies have implicated Rho-kinase in various cellular functions in which the key substrates remain obscure. Rho/Rho-kinase has been demonstrated to be involved in planar polarity establishment downstream of the Wnt/Frizzled/Dishevelled pathway in Drosophila eye and wing development [Winter et al., 2001] and in zebrafish gastrulation convergence and extension [Marlow et al., 2002]. Nucleophosmin (NPM)/B23 is a centrosomal protein that is reported to associate with Rho-kinase at the centrosome [Ma et al., 2006]. NPM activates Rho-kinase in a phosphorylation state-dependent manner by CDK2/cyclin E, which is involved in preventing centrosome reduplication [Ma et al., 2006]. Several lines of evidence suggest that the Rho-kinase signaling pathway also modulates cell survival and apoptosis, as Rho-kinase is known to be involved in morphological changes that occur during apoptosis [Coleman et al., 2001; Sebbagh et al., 2001; Sebbagh et al., 2005]. Recently, Rho-kinase inhibitors such as Y-27632 have been shown to have protective effects on human embryonic stem cells. Administration of Y-27632 prevents apoptosis and enhances survival of human embryonic stem cells at low culture density [Watanabe et al., 2007], possibly through the regulation of MLC phosphorylation and cell-cell interactions [Harb et al., 2008]. However, inhibition of Rho-kinase activity can promote apoptosis in certain situations [Shibata et al., 2003; Svoboda et al., 2004; Moore et al., 2004] and enhances cytotoxic polyglutamate (polyQ) aggregates of huntingtin, androgen receptor, ataxin-2, and atropin-1 [Shao et al., 2008; Bauer et al., 2009].
Future Directions
Intensive studies of Rho and Rho-kinase have revealed their importance in diverse cellular processes and pathologies, paticularly in cultured cell lines, the cardiovascular system, and the nervous system. One of the critical roles of Rho-kinase is modulation of cellular contractility, and aberrant activation of Rho-kinase appears to be involved in some cardiovascular and neurological diseases. However, the functions of Rho-kinase in other tissues still remain largely unsolved, despite its nearly ubiquitous expression in the body. The generation and study of conditional knock-out and knock-in mice of two family members of Rho-kinase would provide important insight into their physioogical roles. Furthermore, a comprehensive identification of Rho-kinase substrates is necessary for better understanding of Rho-kinase signaling networks. We have recently identified more than 100 potential substrates of Rho-kinase by the interactome approach, using the catalytic domain of Rho-kinase, and have isolated novel substrates such as Doublecortin, AP180 and APP [Amano et al., 2010]. Rho-kinase contributes to complicated intracellular signaling networks in a wide range of situations, and further analysis will shed light on its biological mechanisms and potential therapeutics for the disease treatment.
References
- Ai S, Kuzuya M, Koike T, Asai T, Kanda S, Maeda K, Shibata T, Iguchi A. Rho-Rho kinase is involved in smooth muscle cell migration through myosin light chain phosphorylation-dependent and independent pathways. Atherosclerosis. 2001;155:321–327. doi: 10.1016/s0021-9150(00)00585-2. [DOI] [PubMed] [Google Scholar]
- Alblas J, Ulfman L, Hordijk P, Koenderman L. Activation of Rhoa and ROCK are essential for detachment of migrating leukocytes. Mol Biol Cell. 2001;12:2137–2145. doi: 10.1091/mbc.12.7.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, Ikebe M, Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3:177–188. doi: 10.1046/j.1365-2443.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Nakamura N, Kaneko T, Matsuura Y, Kaibuchi K. The COOH terminus of Rho-kinase negatively regulates rho-kinase activity. J Biol Chem. 1999;274:32418–32424. doi: 10.1074/jbc.274.45.32418. [DOI] [PubMed] [Google Scholar]
- Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- Amano M, Kaneko T, Maeda A, Nakayama M, Ito M, Yamauchi T, Goto H, Fukata Y, Oshiro N, Shinohara A, Kaibuchi K. Identification of Tau and MAP2 as novel substrates of Rho-kinase and myosin phosphatase. J Neurochem. 2003;87:780–790. doi: 10.1046/j.1471-4159.2003.02054.x. [DOI] [PubMed] [Google Scholar]
- Amano M, Tsumura Y, Taki K, Harada H, Mori K, Nishioka T, Kato K, Suzuki T, Nishioka Y, Iwamatsu A, Kaibuchi K. A proteomic approach for comprehensively screening substrates of protein kinases such as Rho-kinase. PloS One. 2010;5:e8704. doi: 10.1371/journal.pone.0008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki S, Ito M, Kureishi Y, Feng J, Machida H, Isaka N, Amano M, Kaibuchi K, Hartshorne DJ, Nakano T. Arachidonic acid-induced Ca2+ sensitization of smooth muscle contraction through activation of Rho-kinase. Pflugers Arch. 2001;441:596–603. doi: 10.1007/s004240000462. [DOI] [PubMed] [Google Scholar]
- Arimura N, Inagaki N, Chihara K, Menager C, Nakamura N, Amano M, Iwamatsu A, Goshima Y, Kaibuchi K. Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J Biol Chem. 2000;275:23973–23980. doi: 10.1074/jbc.M001032200. [DOI] [PubMed] [Google Scholar]
- Arimura N, Menager C, Kawano Y, Yoshimura T, Kawabata S, Hattori A, Fukata Y, Amano M, Goshima Y, Inagaki M, Morone N, Usukura J, Kaibuchi K. Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Mol Cell Biol. 2005;25:9973–9984. doi: 10.1128/MCB.25.22.9973-9984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PO, Wong HK, Oyama F, Goswami A, Okuno M, Kino Y, Miyazaki H, Nukina N. Inhibition of Rho kinases enhances the degradation of mutant huntingtin. J Biol Chem. 2009;284:13153–13164. doi: 10.1074/jbc.M809229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito H, Furuyashiki T, Ishihara H, Shibasaki Y, Ohashi K, Mizuno K, Maekawa M, Ishizaki T, Narumiya S. A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron. 2000;26:431–441. doi: 10.1016/s0896-6273(00)81175-7. [DOI] [PubMed] [Google Scholar]
- Boerma M, Fu Q, Wang J, Loose DS, Bartolozzi A, Ellis JL, McGonigle S, Paradise E, Sweetnam P, Fink LM, Vozenin-Brotons MC, Hauer-Jensen M. Comparative gene expression profiling in three primary human cell lines after treatment with a novel inhibitor of Rho kinase or atorvastatin. Blood Coagul Fibrinol. 2008;19:709–718. doi: 10.1097/MBC.0b013e32830b2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XQ, Tan I, Ng CH, Hall C, Lim L, Leung T. Characterization of RhoA-binding kinase ROKalpha implication of the pleckstrin homology domain in ROKalpha function using region-specific antibodies. J Biol Chem. 2002;277:12680–12688. doi: 10.1074/jbc.M109839200. [DOI] [PubMed] [Google Scholar]
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- Denker SP, Yan W, Barber DL. Effect of Rho GTPases on Na-H exchanger in mammalian cells. Methods Enzymol. 2000;325:334–348. doi: 10.1016/s0076-6879(00)25455-4. [DOI] [PubMed] [Google Scholar]
- Dvorsky R, Blumenstein L, Vetter IR, Ahmadian MR. Structural insights into the interaction of ROCKI with the switch regions of RhoA. J Biol Chem. 2004;279:7098–7104. doi: 10.1074/jbc.M311911200. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Endo M, Ohashi K, Sasaki Y, Goshima Y, Niwa R, Uemura T, Mizuno K. Control of growth cone motility and morphology by LIM kinase and Slingshot via phosphorylation and dephosphorylation of cofilin. J Neurosci. 2003;23:2527–2537. doi: 10.1523/JNEUROSCI.23-07-02527.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Kimura K, Oshiro N, Saya H, Matsuura Y, Kaibuchi K. Association of the myosin-binding subunit of myosin phosphatase and moesdual regulation of moesin phosphorylation by Rho-associated kinase and myosin phosphatase. J Cell Biol. 1998;141:409–418. doi: 10.1083/jcb.141.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Oshiro N, Kinoshita N, Kawano Y, Matsuoka Y, Bennett V, Matsuura Y, Kaibuchi K. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J Cell Biol. 1999;145:347–361. doi: 10.1083/jcb.145.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, Kim JK, Lee SW, Kim YB. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab. 2005;2:119–129. doi: 10.1016/j.cmet.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Goto H, Kosako H, Tanabe K, Yanagida M, Sakurai M, Amano M, Kaibuchi K, Inagaki M. Phosphorylation of vimentin by Rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J Biol Chem. 1998;273:11728–11736. doi: 10.1074/jbc.273.19.11728. [DOI] [PubMed] [Google Scholar]
- Hahmann C, Schroeter T. Rho-kinase inhibitors as therapeutics: from pan inhibition to isoform selectivity. Cell Mol Life Sci. 2010;67:171–177. doi: 10.1007/s00018-009-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Harb N, Archer TK, Sato N. The Rho-Rock-Myosin signaling axis determines cell-cell integrity of self-renewing pluripotent stem cells. PloS One. 2008;3:e3001. doi: 10.1371/journal.pone.0003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Nakamura Y, Goto H, Wada Y, Sakoda S, Kaibuchi K, Inagaki M, Takeda M. Domain- and site-specific phosphorylation of bovine NF-L by Rho-associated kinase. Biochem Biophys Res Commun. 1998;245:407–411. doi: 10.1006/bbrc.1998.8446. [DOI] [PubMed] [Google Scholar]
- Hauert AB, Martinelli S, Marone C, Niggli V. Differentiated HL-60 cells are a valid model system for the analysis of human neutrophil migration and chemotaxis. Int J Biochem Cell Biol. 2002;34:838–854. doi: 10.1016/s1357-2725(02)00010-9. [DOI] [PubMed] [Google Scholar]
- Hebert M, Potin S, Sebbagh M, Bertoglio J, Breard J, Hamelin J. Rho-ROCK-dependent ezrin-radixin-moesin phosphorylation regulates Fas-mediated apoptosis in Jurkat cells. J Immunol. 2008;181:5963–5973. doi: 10.4049/jimmunol.181.9.5963. [DOI] [PubMed] [Google Scholar]
- Hirose M, Ishizaki T, Watanabe N, Uehata M, Kranenburg O, Moolenaar WH, Matsumura F, Maekawa M, Bito H, Narumiya S. Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J Cell Biol. 1998;141:1625–1636. doi: 10.1083/jcb.141.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Ito M, Nakano T, Erdodi F, Hartshorne DJ. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem. 2004;259:197–209. doi: 10.1023/b:mcbi.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- Izawa I, Inagaki M. Regulatory mechanisms and functions of intermediate filaments: a study using site- and phosphorylation state-specific antibodies. Cancer Sci. 2006;97:167–174. doi: 10.1111/j.1349-7006.2006.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa T, Fukata Y, Kimura T, Iwamatsu A, Dohi K, Kaibuchi K. Elongation factor-1 alpha is a novel substrate of rho-associated kinase. Biochem Biophys Res Commun. 2000;278:72–78. doi: 10.1006/bbrc.2000.3772. [DOI] [PubMed] [Google Scholar]
- Jacobs M, Hayakawa K, Swenson L, Bellon S, Fleming M, Taslimi P, Doran J. The structure of dimeric ROCK I reveals the mechanism for ligand selectivity. J Biol Chem. 2006;281:260–268. doi: 10.1074/jbc.M508847200. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Ann Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jeon S, Kim S, Park JB, Suh PG, Kim YS, Bae CD, Park J. RhoA and Rho kinase-dependent phosphorylation of moesin at Thr-558 in hippocampal neuronal cells by glutamate. J Biol Chem. 2002;277:16576–16584. doi: 10.1074/jbc.M110380200. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Ann Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Amano M, Maeda A, Goto H, Takahashi K, Ito M, Kaibuchi K. Identification of calponin as a novel substrate of Rho-kinase. Biochem Biophys Res Commun. 2000;273:110–116. doi: 10.1006/bbrc.2000.2901. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Maeda A, Takefuji M, Aoyama H, Nakayama M, Kawabata S, Kawano Y, Iwamatsu A, Amano M, Kaibuchi K. Rho mediates endocytosis of epidermal growth factor receptor through phosphorylation of endophilin A1 by Rho-kinase. Genes Cells. 2005;10:973–987. doi: 10.1111/j.1365-2443.2005.00895.x. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Amano M, Onishi H, Kaibuchi K, Fujiwara K. Rho-kinase—mediated contraction of isolated stress fibers. J Cell Biol. 2001;153:569–584. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Sci. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Eto M, Woodsome TP, Brautigan DL. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem. 2000;275:9897–9900. doi: 10.1074/jbc.275.14.9897. [DOI] [PubMed] [Google Scholar]
- Komander D, Garg R, Wan PT, Ridley AJ, Barford D. Mechanism of multi-site phosphorylation from a ROCK-I:RhoE complex structure. EMBO J. 2008;27:3175–3185. doi: 10.1038/emboj.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosako H, Yoshida T, Matsumura F, Ishizaki T, Narumiya S, Inagaki M. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene. 2000;19:6059–6064. doi: 10.1038/sj.onc.1203987. [DOI] [PubMed] [Google Scholar]
- Koyama M, Ito M, Feng J, Seko T, Shiraki K, Takase K, Hartshorne DJ, Nakano T. Phosphorylation of CPI-17, an inhibitory phosphoprotein of smooth muscle myosin phosphatase, by Rho-kinase. FEBS Lett. 2000;475:197–200. doi: 10.1016/s0014-5793(00)01654-9. [DOI] [PubMed] [Google Scholar]
- Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;270:29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, Wu D. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- Li Y, Harada T, Juang YT, Kyttaris VC, Wang Y, Zidanic M, Tung K, Tsokos GC. Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. J Immunol. 2007;178:1938–1947. doi: 10.4049/jimmunol.178.3.1938. [DOI] [PubMed] [Google Scholar]
- Lowery DM, Clauser KR, Hjerrild M, Lim D, Alexander J, Kishi K, Ong SE, Gammeltoft S, Carr SA, Yaffe MB. Proteomic screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1 substrate. EMBO J. 2007;26:2262–2273. doi: 10.1038/sj.emboj.7601683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Kanai M, Kawamura K, Kaibuchi K, Ye K, Fukasawa K. Interaction between ROCK II and nucleophosmin/B23 in the regulation of centrosome duplication. Mol Cell Biol. 2006;26:9016–9034. doi: 10.1128/MCB.01383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Magdalena J, Millard TH, Machesky LM. Microtubule involvement in NIH 3T3 Golgi and MTOC polarity establishment. J Cell Sci. 2003;116:743–756. doi: 10.1242/jcs.00288. [DOI] [PubMed] [Google Scholar]
- Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–884. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005;15:371–377. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Mimura F, Yamagishi S, Arimura N, Fujitani M, Kubo T, Kaibuchi K, Yamashita T. Myelin-associated glycoprotein inhibits microtubule assembly by a Rho-kinase-dependent mechanism. J Biol Chem. 2006;281:15970–15979. doi: 10.1074/jbc.M510934200. [DOI] [PubMed] [Google Scholar]
- Moore M, Marroquin BA, Gugliotta W, Tse R, White SR. Rho kinase inhibition initiates apoptosis in human airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30:379–387. doi: 10.1165/rcmb.2003-0019OC. [DOI] [PubMed] [Google Scholar]
- Mori K, Amano M, Takefuji M, Kato K, Morita Y, Nishioka T, Matsuura Y, Murohara T, Kaibuchi K. Rho-kinase contributes to sustained RhoA activation through phosphorylation of p190A RhoGAP. J Biol Chem. 2009;284:5067–5076. doi: 10.1074/jbc.M806853200. [DOI] [PubMed] [Google Scholar]
- Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev. 2005;4:387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- Nagumo H, Ikenoya M, Sakurada K, Furuya K, Ikuhara T, Hiraoka H, Sasaki Y. Rho-associated kinase phosphorylates MARCKS in human neuronal cells. Biochem Biophys Res Commun. 2001;280:605–609. doi: 10.1006/bbrc.2000.4179. [DOI] [PubMed] [Google Scholar]
- Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Amano M, Katsumi A, Kaneko T, Kawabata S, Takefuji M, Kaibuchi K. Rho-kinase and myosin II activities are required for cell type and environment specific migration. Genes Cells. 2005;10:107–117. doi: 10.1111/j.1365-2443.2005.00823.x. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Goto TM, Sugimoto M, Nishimura T, Shinagawa T, Ohno S, Amano M, Kaibuchi K. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell. 2008;14:205–215. doi: 10.1016/j.devcel.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- Nishiguchi F, Fukui R, Hoshiga M, Negoro N, Ii M, Nakakohji T, Kohbayashi E, Ishihara T, Hanafusa T. Different migratory and proliferative properties of smooth muscle cells of coronary and femoral artery. Atherosclerosis. 2003;171:39–47. doi: 10.1016/j.atherosclerosis.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8:803–814. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- Onoue N, Nawata J, Tada T, Zhulanqiqige D, Wang H, Sugimura K, Fukumoto Y, Shirato K, Shimokawa H. Increased static pressure promotes migration of vascular smooth muscle cells: involvement of the Rho-kinase pathway. J Cardiovasc Pharmacol. 2008;51:55–61. doi: 10.1097/FJC.0b013e31815b9d26. [DOI] [PubMed] [Google Scholar]
- Oshiro N, Fukata Y, Kaibuchi K. Phosphorylation of moesin by rho-associated kinase (Rho-kinase) plays a crucial role in the formation of microvilli-like structures. J Biol Chem. 1998;273:34663–34666. doi: 10.1074/jbc.273.52.34663. [DOI] [PubMed] [Google Scholar]
- Pedrini S, Carter TL, Prendergast G, Petanceska S, Ehrlich ME, Gandy S. Modulation of statin-activated shedding of Alzheimer APP ectodomain by ROCK. PLoS Med. 2005;2:e18. doi: 10.1371/journal.pmed.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinner S, Sahai E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol. 2008;10:127–137. doi: 10.1038/ncb1675. [DOI] [PubMed] [Google Scholar]
- Riento K, Guasch RM, Garg R, Jin B, Ridley AJ. RhoE binds to ROCK I and inhibits downstream signaling. Mol Cell Biol. 2003;23:4219–4229. doi: 10.1128/MCB.23.12.4219-4229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- Sebbagh M, Hamelin J, Bertoglio J, Solary E, Breard J. Direct cleavage of ROCK II by granzyme B induces target cell membrane blebbing in a caspase-independent manner. J Exp Med. 2005;201:465–471. doi: 10.1084/jem.20031877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Welch WJ, Diprospero NA, Diamond MI. Phosphorylation of profilin by ROCK1 regulates polyglutamine aggregation. Mol Cell Biol. 2008;28:5196–5208. doi: 10.1128/MCB.00079-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Kai H, Seki Y, Kusaba K, Takemiya K, Koga M, Jalalidin A, Tokuda K, Tahara N, Niiyama H, Nagata T, Kuwahara F, Imaizumi T. Rho-kinase inhibition reduces neointima formation after vascular injury by enhancing Bax expression and apoptosis. J Cardiovasc Pharmacol. 2003;42(Suppl 1):S43–S47. doi: 10.1097/00005344-200312001-00011. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Ihara K, Maesaki R, Amano M, Kaibuchi K, Hakoshima T. Parallel coiled-coil association of the RhoA-binding domain in Rho-kinase. J Biol Chem. 2003;278:46046–46051. doi: 10.1074/jbc.M306458200. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo MM, Narumiya S. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–953. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa H, Rashid M. Development of Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol Sci. 2007;28:296–302. doi: 10.1016/j.tips.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Nakayama M, Goto TM, Amano M, Komori K, Kaibuchi K. Rho-kinase phosphorylates eNOS at threonine 495 in endothelial cells. Biochemi Biophys Res Commun. 2007;361:462–467. doi: 10.1016/j.bbrc.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Svoboda KK, Moessner P, Field T, Acevedo J. ROCK inhibitor (Y27632) increases apoptosis and disrupts the actin cortical mat in embryonic avian corneal epithelium. Dev Dyn. 2004;229:579–590. doi: 10.1002/dvdy.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takefuji M, Mori K, Morita Y, Arimura N, Nishimura T, Nakayama M, Hoshino M, Iwamatsu A, Murohara T, Kaibuchi K, Amano M. Rho-kinase modulates the function of STEF, a Rac GEF, through its phosphorylation. Biochem Biophys Res Commun. 2007;355:788–794. doi: 10.1016/j.bbrc.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Nishimura D, Wu RC, Amano M, Iso T, Kedes L, Nishida H, Kaibuchi K, Hamamori Y. Nuclear Rho kinase, ROCK2, targets p300 acetyltransferase. J Biol Chem. 2006;281:15320–15329. doi: 10.1074/jbc.M510954200. [DOI] [PubMed] [Google Scholar]
- Tang BL, Liou YC. Novel modulators of amyloid-beta precursor protein processing. J Neurochem. 2007;100:314–323. doi: 10.1111/j.1471-4159.2006.04215.x. [DOI] [PubMed] [Google Scholar]
- Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo MM, Narumiya S. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003;23:5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumkeo D, Shimizu Y, Sakamoto S, Yamada S, Narumiya S. ROCK-I and ROCK-II cooperatively regulate closure of eyelid and ventral body wall in mouse embryo. Genes Cells. 2005;10:825–834. doi: 10.1111/j.1365-2443.2005.00882.x. [DOI] [PubMed] [Google Scholar]
- Tominaga T, Barber DL. Na-H exchange acts downstream of RhoA to regulate integrin-induced cell adhesion and spreading. Mol Biol Cell. 1998;9:2287–2303. doi: 10.1091/mbc.9.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsukawa G, Wu Y, Sasaki Y, Hartshorne DJ, Yamakita Y, Yamashiro S, Matsumura F. Distinct roles of MLCK and ROCK in the regulation of membrane protrusions and focal adhesion dynamics during cell migration of fibroblasts. J Cell Biol. 2004;164:427–439. doi: 10.1083/jcb.200306172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Vahebi S, Kobayashi T, Warren CM, de Tombe PP, Solaro RJ. Functional effects of rho-kinase-dependent phosphorylation of specific sites on cardiac troponin. Circ Res. 2005;96:740–747. doi: 10.1161/01.RES.0000162457.56568.7d. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X, Surks HK. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res. 2009;104:531–540. doi: 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward Y, Yap SF, Ravichandran V, Matsumura F, Ito M, Spinelli B, Kelly K. The GTP binding proteins Gem and Rad are negative regulators of the Rho-Rho kinase pathway. J Cell Biol. 2002;157:291–302. doi: 10.1083/jcb.200111026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1:136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154:147–160. doi: 10.1083/jcb.200103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Kasa M, Amano M, Kaibuchi K, Hakoshima T. Molecular mechanism for the regulation of rho-kinase by dimerization and its inhibition by fasudil. Structure. 2006a;14:589–600. doi: 10.1016/j.str.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Miwa Y, Kasa M, Kitano K, Amano M, Kaibuchi K, Hakoshima T. Structural basis for induced-fit binding of Rho-kinase to the inhibitor Y-27632. J Biochem. 2006b;140:305–311. doi: 10.1093/jb/mvj172. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Amano M, Nagata K, Inagaki N, Nakamura H, Saya H, Kaibuchi K, Inagaki M. Roles of Rho-associated kinase in cytokinesis; mutations in Rho-associated kinase phosphorylation sites impair cytokinetic segregation of glial filaments. J Cell Biol. 1998;143:1249–1258. doi: 10.1083/jcb.143.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda A, Multhaupt HA, Couchman JR. The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol. 2005;170:443–453. doi: 10.1083/jcb.200412043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Su Y, Li B, Liu F, Ryder JW, Wu X, Gonzalez-DeWhitt PA, Gelfanova V, Hale JE, May PC, Paul SM, Ni B. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science. 2003;302:1215–1217. doi: 10.1126/science.1090154. [DOI] [PubMed] [Google Scholar]


