Abstract
Prostatic acid phosphatase (PAP) has been investigated as the target of several antigen-specific anti-prostate tumor vaccines. The goal of antigen-specific active immunotherapies targeting PAP would ideally be to elicit PAP-specific CD8+ effector T cells. The identification of PAP-specific CD8+ T-cell epitopes should provide a means of monitoring the immunological efficacy of vaccines targeting PAP, and these epitopes might themselves be developed as vaccine antigens. In the current report, we hypothesized that PAP-specific epitopes might be identified by direct identification of pre-existing CD8+ T cells specific for HLA-A2-restricted peptides derived from PAP in the blood of HLA-A2-expressing individuals. 11 nonamer peptides derived from the amino acid sequence of PAP were used as stimulator antigens in functional ELISPOT assays with peripheral blood mononuclear cells from 20 HLA-A2+ patients with prostate cancer or ten healthy blood donors. Peptide-specific T cells were frequently identified in both groups for three of the peptides, p18–26, p112–120, and p135–143. CD8+ T-cell clones specific for three peptides, p18–26, p112–120, and p299–307, confirmed that these are HLA-A2-restricted T-cell epitopes. Moreover, HLA-A2 transgenic mice immunized with a DNA vaccine encoding PAP developed epitope-specific responses for one or more of these three peptide epitopes. We propose that this method to first identify epitopes for which there are pre-existing epitope-specific T cells could be used to prioritize MHC class I-specific epitopes for other antigens. In addition, we propose that the epitopes identified here could be used to monitor immune responses in HLA-A2+ patients receiving vaccines targeting PAP to identify potentially therapeutic immune responses.
Keywords: CTL, Prostatic acid phosphatase (PAP), HLA-A2, ELISPOT, Epitope
Introduction
Prostatic acid phosphatase (PAP) is a prostate cancer antigen, and has become a model antigen for active immunotherapies targeting prostate cancer. PAP was one of the first proteins detected in the serum of patients with cancer to be identified as a potential serum marker [1]. It was subsequently shown to have expression essentially restricted to normal and malignant prostate tissue, and immunohistochemical staining for PAP is still performed to establish a prostate origin of metastatic adenocarcinoma [2]. Detection of serum PAP has been essentially replaced by serum prostate-specific antigen (PSA) as a cancer biomarker, given the sensitivity of the latter marker for prostate cancer progression [3]. PSA and prostate-specific membrane antigen (PSMA) are two other proteins being investigated as prostate tumor antigens. However, PSMA expression has been identified in several normal non-prostate tissues, making it potentially a less specific target than PAP or PSA for anti-prostate tumor vaccines [4]. The presence of a rat homolog of PAP has provided an animal model for testing the efficacy of vaccines in preclinical models, making this a particularly attractive prostate-specific target antigen [5–9]. In addition, while there has been significant interest in PSA as an antigen in anti-tumor vaccines, targeting PAP has a potential advantage by permitting the measurement of serum PSA as a biomarker for clinical response without potentially confounding this analysis by also targeting it immunologically. Several vaccines targeting PAP have entered clinical testing [10–13]. Results from a trial targeting PAP by means of an antigen-presenting cell vaccine approach have suggested clinical benefit in terms of overall survival [14], and a confirmatory phase III randomized trial to test this approach in patients with advanced, metastatic prostate cancer has been recently completed with results confirming the earlier suggestion that patients receiving vaccine survive longer than patients not receiving vaccine [15].
CD8+ T lymphocytes are widely believed to be the most potent effector population in a successful adaptive anti-tumor immune response. The generation of antigen-specific CD8+ T cells would be expected to be critical for an effective anti-tumor immune response following vaccination against an intracellular or secreted antigen such as PAP. Because CD8+ T cells recognize target cells by peptide epitopes presented in the context of MHC class I molecules, there has been great interest over the last decade to identify MHC class I-restricted peptide epitopes for specific tumor antigens. The identification of CD8+ T-cell epitopes for PAP would provide peptide antigens that could be used for directly monitoring CD8+ T-cell responses resulting from vaccination, or alternatively could be used as peptide vaccines directly. To date, there have been several reports seeking to identify either HLA-A2 or HLA-A24 restricted CD8+ T-cell epitopes for PAP [16–19].
In the current report, we sought to identify in a comprehensive fashion HLA-A2-restricted CD8+ T-cell epitopes specific for PAP. We hypothesized that naturally processed epitopes specific for PAP should be identifiable within the T-cell repertoire of HLA-A2+ patients with prostate cancer. Moreover, because the ultimate goal in identifying MHC class I restricted epitopes is to use these as possible vaccine antigens or as means of detecting immune responses to PAP, we sought to prioritize those epitopes for which there was evidence that peptide-specific T cells can already exist in vivo. We used ELISPOT as a means to first prioritize peptides from 11 potential peptides fitting the consensus motif for binding HLA-A2, determining whether peptide-specific T cells could be detected in the peripheral blood of 20 HLA-A2-expressing patients with prostate cancer compared with healthy donors. Three HLA-A2-binding peptides were identified for which peptide-specific T cells could be detected in vivo in multiple individuals. These peptides, plus an additional epitope that had been identified by Peshwa et al. [16], were then tested in traditional in vitro culture studies. These studies confirmed that patients with prostate cancer can have pre-existing CD8+ T cells specific for these peptides. Furthermore, the characterization of peptide-specific T-cell lines and clones demonstrated that three of these four PAP-derived peptides are naturally processed HLA-A2-restricted epitopes and CD8+ T cells specific for these epitopes could lyse PAP-expressing prostate tumor cells. Finally, we show that immunization of HLA-A2 transgenic mice with a DNA vaccine encoding PAP elicited epitope-specific T cells recognizing one or more of these peptides, demonstrating that these epitopes could be used for monitoring the induction of PAP-specific CD8+ T-cell responses following immunization.
Materials and methods
Subjects
With informed consent, peripheral blood or leukapheresis products were obtained from HLA-A2-expressing male subjects (with or without a diagnosis of prostate cancer) at the University of Washington Medical Center between 1999 and 2001 [20], and at the University of Wisconsin between 2001 and 2008. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque centrifugation (Pharmacia AB, Uppsala, Sweden) and either used immediately or cryopreserved in liquid nitrogen. HLA-A2 expression of individual subjects was confirmed serologically from PBMC samples (monoclonal antibody clone BB7.2, BD Biosciences, San Jose, CA, USA), and/or by genotype PCR analysis of genomic DNA isolated from PBMC using HLA-A*0201-specific oligonucleotide primers (5′ CGTCCCCAGGCTCTCACTCCAT, 3′ TCACTTTCCGTGTCTCCCC) specific for HLA-A*0201, as previously described [21].
Peptides
Peptides were synthesized, purified to >80% by HPLC, and the identity and purity confirmed by mass spectrum analysis (United Biochemical Research, Inc., Seattle, WA, USA). Purified peptides were reconstituted in sterile phosphate-buffered saline (PBS) or dimethylsulfoxide (DMSO), sterile filtered, and stored in aliquots at –20°C. Control peptides included the HLA-A2 9-mer epitopes from the influenza A virus matrix protein (FILGFVFTL [22]) and the Epstein-Barr virus latent membrane protein (LMP-1) (YLLEMLWRL [23]).
T2 in vitro HLA-A2 binding assay
The HLA-A2 expressing, TAP-1 deficient human T-cell line T2 was used as an assay of HLA-A2 peptide binding efficiency, in similar fashion to what has been described previously [16]. Specifically, T2 cells were cultured for 24 h in serum-free RPMI media. Cells were then washed and resuspended in serum-free RPMI media and plated to triplicate wells of a 96-well U-bottom microtiter plate (Corning, Acton, MA, USA) at 105 cells/well. Human ß2-microglobulin (Sigma, St. Louis, MO, USA) was added to 5 μg/ml, and nonamer peptides were added to 50 μg/ml. Cells were then incubated overnight at 37°C/5% CO2, washed with PBS + 2% bovine serum albumin (BSA), and probed with one of two different mouse HLA-A2-specific monoclonal antibodies [0131HA (Ab 1) or 0791HA (Ab 2), One Lambda, Canoga Park, CA, USA] for 1 h on ice. Cells were then washed with PBS + 2% BSA, and probed with a secondary PE-conjugated goat anti-mouse IgG antibody (Sigma) for 1 h on ice. Cells were then washed with PBS + 2% BSA and fixed with PBS + 1% paraformaldehyde prior to flow cytometric analysis (FACSCAN, Becton Dickinson). Results are reported as a relative mean fluorescence index (MFI), calculated as the MFI of peptide-pulsed T2 cells compared with the MFI of unpulsed T2 cells. Assays were performed in triplicate, with the standard deviation for replicate analyses shown.
Enzyme-linked immunosorbent spot (ELISPOT) assay
A 10-day ELISPOT assay was used to determine precursor frequencies of peptide-specific CD8+ T lymphocytes as previously described [24]. Briefly, on day 1, PBMC were plated into 96-well plates in 6-well replicates in 200 μl of RPMI-1640 medium containing l-glutamine, penicillin, streptomycin and 10% human AB serum (T-cell medium) in the presence or absence of 10 μg/ml peptide antigen or 0.5 U/ml tetanus toxoid protein. The cells were incubated at 37°C/5% CO2. On day 5, recombinant human IL-2 was added to 10 U/ml. On day 8, 2.5 × 105/well irradiated (3,000 cGy) autologous PBMC and 10 μg/ml peptide antigens were added. In addition, nitrocellulose-backed 96-well plates (NC-plates, Millipore Corporation, Bedford, MA, USA) were coated with 10 μg/ml anti-IFNγ monoclonal antibody (1-D1 K, Mabtech, Nacka, Sweden) in PBS at 50 μl/well. On day 9 of culture, the NC-plate was washed with PBS, blocked for 2 h with PBS containing 2% BSA, and washed again with PBS. The cultured cells were then gently resuspended, pooled, centrifuged, resuspended in fresh T-cell medium, and then transferred into the NC-plate in a volume of 100 μl/well. The NC-plate was incubated at 37°C/5% CO2 for a further 20–24 h, and then washed with PBS containing 0.05% Tween-20 and incubated for 2.5 h at room temperature with 50 μl/well PBS containing 5 μg/ml biotinylated anti-IFNγ antibody (7 B6-1, Mabtech, Nacka, Sweden). After incubation, wells were washed with PBS, and further incubated with 100 μl/well streptavidin-labeled alkaline phosphatase (BioRad, Hercules, CA, USA) at a dilution of 1:1,000 in PBS for 2 h at room temperature. After incubation, wells were again washed with PBS, and then developed with 100 μl/well BCIP/NBT colorimetric substrate (BioRad) for 20–30 min. The colorimetric reaction was stopped by rinsing the plates under cool tap water, and wells were allowed to dry completely before spots were enumerated. Results are shown as either the number of spots per well or by T-cell frequencies, calculated by subtracting the mean number of spots obtained from the no antigen control wells from the mean number obtained in the experimental wells. Comparison of experimental wells with control, no antigen, wells was performed using a Student’s t test, with P < 0.05 defined as a significant T-cell response. Similar studies and analyses were performed with murine splenocytes using a commercially available kit according to the manufacturer’s instructions (R & D Systems, Minneapolis, MN, USA).
Generation of peptide-specific T-cell lines
Peptide-specific T-cell lines were generated by repetitive weekly in vitro stimulations with peptide-loaded autologous dendritic cells. Briefly, PBMC from HLA-A2-expressing individuals were plated at 108 cells/flask in T150 tissue culture flasks for 2 h at 37°C. Non-adherent cells were then removed, and the adherent cells were cultured for 6 days in the presence of 20 ng/ml rhGM-CSF (R & D Systems) and 10 ng/ml rhIL-4 (R & D Systems). The resulting cells were morphologically and phenotypically characterized as predominantly (>80%) dendritic cells (DC) by flow cytometry as CD14−, CD11c+, HLA-DR+. DC were then “matured” for 24–48 h by culture in the presence of 150 ng/mL IL-6, 10 ng/mL IL-1ß, 10 ng/mL TNFα, and 1 μg/mL prostaglandin E2. Autologous DC were then pulsed with 10 μg/ml peptide for 2 h, and cultured with autologous T cells (T cell negative isolation kit, Dynal, Carlsbad, CA, USA) in RPMI medium + 10% human AB serum. 20 U/ml rhIL-2 (R & D Systems) and 10 U/ml rhIL-7 (R & D Systems) were added after 24 h. Cells were restimulated at weekly intervals with irradiated (3,000 cGy) peptide-loaded antigen-presenting cells (autologous DC or B-cell lines), in similar fashion, and T-cell lines were characterized for cell surface phenotype and cytolytic function after 2–4 weeks. After 2–5 restimulations, T-cell lines were cloned by limiting dilution. Specifically, cells were diluted to limiting concentrations in 96-well culture plates, and cultured for 12–14 days with 5 × 104 irradiated (5,000 cGy) autologous PBMC, 50 U/ml IL-2, and 30 ng/ml anti-CD3 as previously described [25]. Individual clonal lines were tested for cytotoxicity, and peptide-specific lines were further expanded by incubating with 30 ng/mL anti-CD3 (BD Biosciences) along with a 100:1 ratio of feeder lymphoblastoid cell lines (LCL) to effector cells and a 500:1 ratio of autologous, irradiated PBMC to effector cells. Cultures were given 30 U/mL IL-2 24 h later, and cultures continued 12–14 days with fresh media and IL-2 added every 3–4 days as needed, until suitable numbers of cells were obtained for further analysis.
Cytotoxicity assays
Cytolytic activity was measured by LDH release from target cell lines (Cytotox 96 Assay kit, Promega, Madison, WI, USA). In brief, effector cell lines were plated in 96-well plates at various effector-to-target (E:T) cell ratios. Targets used were either T2 cells pulsed with peptide, a human HLA-A2-expressing B cell line (TK6) stably transduced with lentivirus to express either human PAP or green-fluorescent protein (GPF), or human prostate cancer cell lines (LNCaP or DU145) stably transduced with lentivirus to express HLA-A2. After 4–6 h at 37°C, plates were centrifuged, and 50 μl of culture supernatant was assessed for LDH concentration spectrophotometrically, according to the manufacturer’s instructions. Controls included wells with effector cells only, wells with media only, target cells only (minimum release), and target cells with 1% Triton X-100 (maximum release). To confirm HLA-A2-restricted response, PAP-expressing cell lines were pre-incubated with an HLA-A2 specific antibody (BD Pharmingen, San Jose, CA, USA) or non-specific control murine IgG. The optical density (OD) signal contributed by the media alone was subtracted from all values. The percent specific activity was then calculated as: [ODexperimental well – ODeffector only – ODtarget minimum release]/[ODtarget maximum release – ODtarget minimum release]. All sample conditions were evaluated in triplicate, with the standard deviation shown.
Animal studies
HHD-II transgenic mice, expressing the α1 and α2 chains of human HLA-A201 chimeric with the intracellular α3 chain of the H-2Db allele, and expressing HLA-DR1, with mouse MHC class I (H-2b) and II (I-Ab) knocked out, were graciously provided by Dr. François Lemonnier (Institut Pasteur, Paris, France). Animals were housed in a facility maintained by the Laboratory Animals Resources of the University of Wisconsin Medical School, and all treatments and euthanasia were conducted under an institutional animal care and use (IACUC) committee-approved protocol. Mice were immunized intradermally in the ear pinna at 14-day interval six times with 100 μg of plasmid DNA encoding PAP (pTVG-HP) or vector control (pTVG4), similar to that described previously [8]. Two weeks after the last immunization, animals were euthanized with collection of spleens. A cell suspension was prepared, and red blood cells were removed by osmotic lysis. The lymphocyte-enriched fraction was cultured for 1 week in RPMI medium in the presence of 2–4 μg/mL of individual peptides and 10 U/mL murine IL-2 prior to analysis by IFNγ ELISPOT (R & D Systems) similar to the methodology described above. Comparison of mean responses was made using a two-tailed, non-paired T test, with P < 0.05 considered as statistically significant.
Results
HLA-A2-binding peptides from PAP predicted by sequence analysis
The amino acid sequence of human PAP, including the secretory peptide from the immature polypeptide, was scanned for 9-amino acid polymers conforming to the HLA-A2 binding consensus motif (X-I/L-XXXXXX-V/L/M) previously identified [26]. A similar analysis was reported previously with identification of seven potential peptides [16]. Our analysis, however, identified ten peptides fitting this consensus motif. The sequence of the peptides identified, the SYFPEITHI-predicted binding score (http://www.syfpeithi.de/) [27], and the predicted t 1/2 of dissociation from HLA-A2 by the method of Parker [28], are shown in Table 1. As shown, the SYFPEITHI method predicted p6–14, p30–38, and p13–21 to be the peptides with highest binding affinity, and the Parker method predicted p18–26, p30–38, and p135–143 to be the peptides with highest binding affinity.
Table 1.
HLA-A2-binding peptides from PAP can be predicted by sequence analysis
| Peptide | Sequence | SYFPEITHI predicted binding score | Predicted t 1/2 dissociation (s) | FI (Ab 1) | FI (Ab 2) |
|---|---|---|---|---|---|
| p6–14 | LLLARAASL | 28 | 134 | 1.84 ± 0.12 | 2.57 ± 0.04 |
| p13–21 | SLSLGFLFL | 25 | 285 | 0.83 ± 0.02 | 1.34 ± 0.06 |
| p18–26 | FLFLLFFWL | 23 | 22,037 | 5.69 ± 0.26 | 6.06 ± 1.78 |
| p30–38 | VLAKELKFV | 26 | 1,496 | 3.64 ± 0.46 | 9.19 ± 1.15 |
| p33–41 | KELKFVTLV | 18 | 153 | 0.73 ± 0.13 | 0.94 ± 0.37 |
| p112–120 | TLMSAMTNL | 21 | 182 | 5.15 ± 0.19 | 6.47 ± 0.33 |
| p135–143 | ILLWQPIPV | 24 | 438 | 1.60 ± 0.17 | 2.36 ± 0.40 |
| p140–148 | PIPVHTVPL | 19 | 0.1 | 0.89 ± 0.05 | 1.26 ± 0.04 |
| p201–209 | DLFGIWSKV | 24 | 33 | 0.90 ± 0.04 | 1.38 ± 0.09 |
| p299–307 | ALDVYNGLL | 23 | 1.1 | 2.74 ± 1.38 | 2.14 ± 0.56 |
| p352–360 | PLERFAELV | 17 | 0.2 | 1.22 ± 0.12 | 1.25 ± 0.31 |
| pFlu | GILGFVFTL | 30 | 551 | 1.62 ± 0.10 | 1.41 ± 0.05 |
| pEBV | YLLEMLWRL | 26 | 11,180 | 1.32 ± 0.08 | 1.54 ± 0.09 |
Nonamer peptides from the amino acid sequence of PAP were chosen based on sequence conforming to the HLA-A2 binding motif (X-I/L-XXXXXX-V/L/M) [26], or based on predicted binding stability using the method of Parker [28]. The table shows the 11 peptides from PAP chosen, as well as the previously identified HLA-A2 epitopes from the influenza matrix protein and the EBV LMP-1 protein. Also shown in the table are the SYFPEITHI binding score (higher score, higher relative predicted binding affinity), predicted t 1/2 of dissociation (higher score, higher predicted binding affinity), and the MFI determined in in vitro T2 binding assays using two separate HLA-A2 monoclonal antibodies (Ab1 and Ab2), as described
In vitro HLA-A2 binding of the identified peptides
The binding to HLA-A2 of the 11 peptides identified above was evaluated in in vitro T2 binding assays, using the stabilization of cell surface expression of HLA-A2 on TAP-1-deficient T2 cells as a relative measure of peptide binding affinity. Two separate monoclonal antibodies specific for HLA-A2 were used to ensure that the peptides were specific for HLA-A2. These results are also depicted in Table 1. As shown, there was good concordance with the two separate monoclonal antibodies. This method identified peptides p6–14, p18–26, p112–120, p30–38, and p299–307 as the peptides with the highest binding affinity in vitro to HLA-A2.
PAP-derived peptide-specific T cells identified in HLA-A2-expressing individuals by ELISPOT
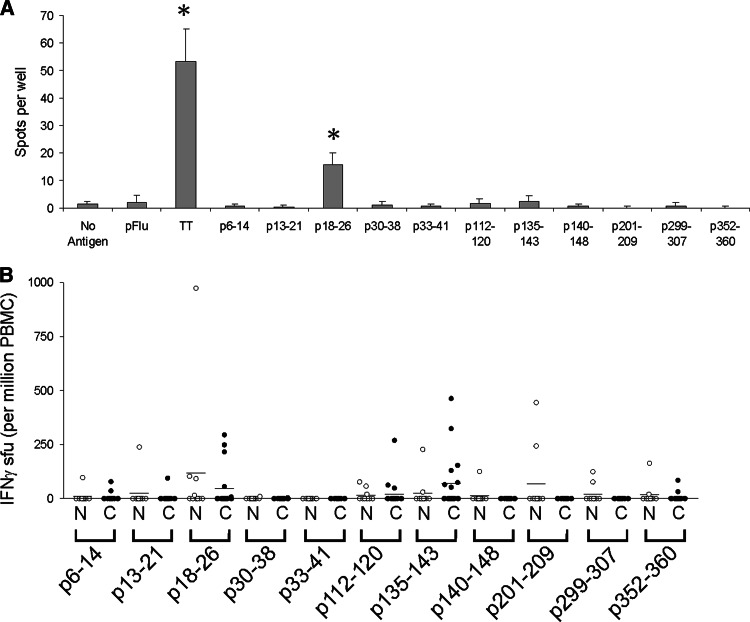
Peripheral blood mononuclear cells (PBMC) were obtained from 20 HLA-A2-expressing patients with various stages of recurrent prostate cancer [20] and ten HLA-A2-expressing men without prostate cancer. PBMC were assessed for the presence of PAP-derived peptide-specific T cells by ELISPOT. An example of the method is depicted in Fig. 1a, in which PBMC from a 49 year-old HLA-A2-expressing patient with stage D2 prostate cancer were assessed for peptide-specific T-cell responses to each of the individual peptides. After one in vitro stimulation, an average of 57 spot-forming units (sfu) per 106 cells could be detected in response to p18–26 peptide stimulation (P < 0.001), whereas no significant response was detected following stimulation with the other peptides. A summary of similar findings from all subjects is shown in Fig. 1b. As shown in this figure, ELISPOT responses were most commonly detected to p135–143, p18–26, and p112–120. There were no significant differences in response frequency to individual peptides between the control and patient populations. Of note, no significant responses were detected to p30–38, one of the peptides found to have the highest binding in vitro to HLA-A2. And as expected, no significant responses were detected to p33–41, a peptide found to not bind HLA-A2.
Fig. 1.
PAP-derived peptide-specific T cells identified in HLA-A2-expressing individuals by ELISPOT. a Representative ELISPOT assay using peripheral blood mononuclear cells (PBMC) from a 49-year-old patient with stage D2 prostate cancer. PBMC were stimulated once in vitro with 10 μg/ml of the indicated peptides or tetanus toxoid protein (1 LFU/ml, as a positive control), and assayed for peptide-specific IFNγ release upon restimulation with peptide, as described. In this assay, IFNγ spots in response to tetanus toxoid stimulation and PAP peptide p18–26 were highly significant (P < 0.001) compared with media only (no antigen). b ELISPOT assays were performed in identical fashion using PBMC from multiple individuals. T-cell frequencies were calculated as spot-forming units (sfu) per 106 PBMC. Open circles (N) show data for the ten volunteer blood donors (n = 10), and closed circles (C) show data for the patients with prostate cancer (n = 20)
Peptides p18–26, p112–120, and p299–307 represent PAP-specific HLA-A2-restricted epitopes
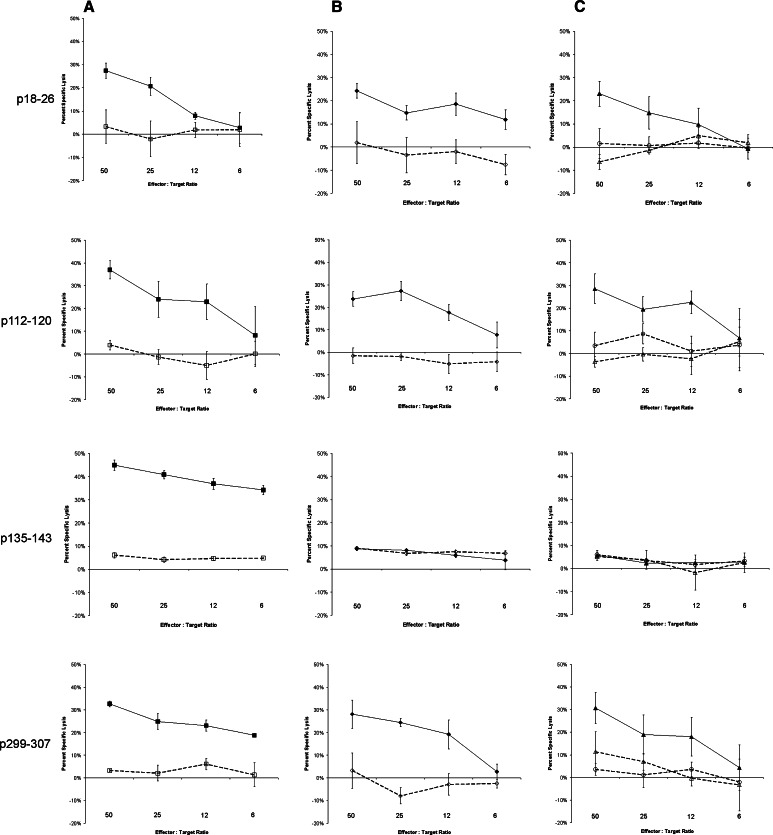
The three peptides prioritized above, as well as p299–307 which had been previously identified by another group [16], were then further investigated to determine whether they represented naturally processed PAP-specific HLA-A2-restricted epitopes and whether cytolytic T cells (CTL) could be cultured specific for these epitopes. Peripheral blood mononuclear cells from 10–16 separate HLA-A2-expressing patients with prostate cancer, all with biochemically recurrent disease, were stimulated weekly with peptide-pulsed antigen-presenting cells using these peptides or the influenza A matrix HLA-A2-specific peptide epitope (FILGFVFTL) and assessed for peptide-specific cytolytic activity after 2–5 weekly in vitro stimulations. As shown in Table 2, peptide-specific CTL for all four PAP peptides could be cultured from the peripheral blood of multiple patients. Of note, peptide-specific CTL could most frequently be cultured from individuals specific for p18–26, p112–120, and p135–143, as expected from our ELISPOT findings. Peptide-specific T-cell lines were then cloned from individual cultures by limiting dilution for further characterization. Peptide-specific T-cell clones were all CD8+, and secreted IFNγ and TNFα in response to peptide stimulation (data not shown). All clones could also specifically lyse T2 cells pulsed with the corresponding peptide (Fig. 2a), and HLA-A2 blocking studies confirmed that this lysis was MHC class I-restricted (data not shown). Characterization of these clones for lysis of PAP-expressing cell lines, however, demonstrated that only T-cell clones specific for peptides p18–26, p112–120, and p299–307 could lyse an HLA-A2+ cell line expressing PAP (Fig. 2b). These cells could also lyse an HLA-A2-expressing LNCaP prostate cancer cell lines expressing PAP (Fig. 2c) but not an HLA-A2-expressing prostate cancer cell line that does not express PAP (DU145). This lysis could be blocked with the addition of an anti-HLA-A2 antibody, again confirming their MHC restriction (Fig. 2c). These peptides, consequently, represent naturally expressed HLA-A2-restricted epitopes.
Table 2.
PAP-derived peptide-specific T cells can be cultured from HLA-A2-expressing patients with prostate cancer
| Peptide | Sequence | Number of HLA-A2 expressing patients from whom peptide-specific CTL detected following 2–5 in vitro stimulations (%) |
|---|---|---|
| p18–26 | FLFLLFFWL | 6/16 (38) |
| p112–120 | TLMSAMTNL | 7/15 (47) |
| p135–143 | ILLWQPIPV | 6/15 (40) |
| p299–307 | ALDVYNGLL | 2/15 (13) |
| pFlu | GILGFVFTL | 7/10 (70) |
PBMC from multiple HLA-A2-expressing patients with prostate cancer were cultured with individual peptides, and re-stimulated weekly for up to 5 weeks. Cultures were assayed weekly after the second in vitro stimulation for peptide-specific lysis of T2 target cells pulsed with specific versus non-specific peptide. The table demonstrates the number of cultures which demonstrated peptide-specific lysis at least once during the 5 weeks of culture
Fig. 2.
Peptide-specific T-cell clones specific for p18–26, p112–120, and p299–307, but not p135–143, can lyse PAP-expressing cells in an HLA-A2-restricted fashion. Clonal T-cell lines specific for p18–26, p112–120, p135–143, or p299–307 were assessed for their ability to lyse peptide-pulsed T2 target cell lines (column a), HLA-A2-expressing TK6 cell lines expressing PAP (TK6-PAP) versus control TK6 cells expressing GFP (TK6-GFP, column b), or prostate cancer cell lines (column c). Cytotoxicity was measured as LDH release after 4-h co-culture of effector cells with target cells. Assays were performed in triplicate at multiple effector-to-target ratios as indicated. Shown is the mean and standard deviation of percent specific lysis at each effector-to-target ratio, determined from triplicate assessments, of each clonal line and target cell line. column a: solid squares represent T2 cells pulsed with the relevant peptide, and open squares represent T2 target cells pulsed with an irrelevant HLA-A2-binding peptide. column b: solid diamonds represent TK6-PAP target cells, and open diamonds represent TK6-GFP target cells. column c: closed triangles represent HLA-A2-transduced LNCaP target cells (expressing PAP), and open triangles represent HLA-A2-transduced LNCaP target cells in the presence of an HLA-A2 blocking antibody. Open circles represent HLA-A2-transduced DU145 target cells (not expressing PAP). Results are representative of multiple independent experiments
DNA immunization with a plasmid DNA encoding PAP can elicit peptide-specific CTL
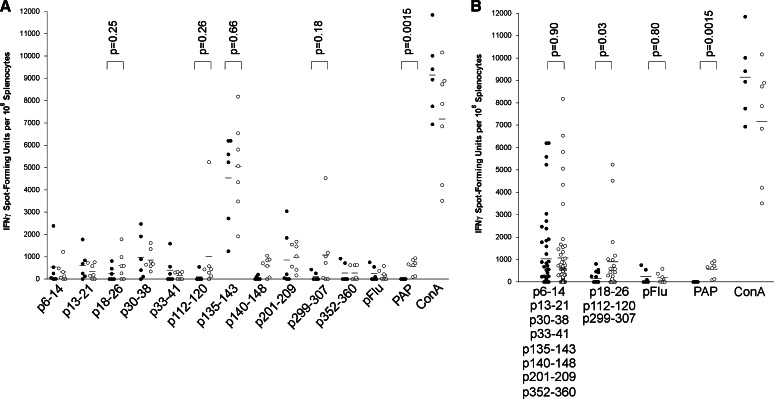
To further demonstrate whether the peptides identified represent naturally processed PAP-specific MHC class I epitopes, and to also determine whether they might be useful as monitoring tools following immunization, HLA-A2-expressing HHD-II mice were immunized six times at 2-week intervals with a DNA vaccine encoding PAP, or immunized with a control vector. Two weeks after the last immunization, splenocytes were collected and assessed for peptide-specific T cells by IFNγ ELISPOT. As demonstrated in Fig. 3, PAP-specific IFNγ-secreting T cells could be detected in mice immunized with the DNA vaccine encoding PAP compared with controls after multiple immunizations. p135–143-specific T cells could be detected in all animals irrespective of immunization, further suggesting this while this is a highly immunogenic peptide, it is not a naturally processed epitope specific for PAP. T cells specific for p18–26, p112–120, and p299–307 could be detected at higher frequencies in mice immunized with the DNA vaccine encoding PAP relative to control (Fig. 3a). However, while some animals had high responses to individual epitopes, not all animals had responses to one single epitope. In fact, it was observed that some animals had a response to one epitope but not another. As demonstrated in Fig. 3b, the evaluation of responses to any one or more of the three epitopes was superior in detecting CD8+ T-cell responses resulting from immunization than evaluation to single epitopes.
Fig. 3.
PAP-specific epitopes can be used for monitoring immune responses following DNA immunization. HHD-II mice were immunized six times at 14-day intervals with plasmid DNA encoding PAP (pTVG-HP, n = 7) or vector control (pTVG4, n = 6). Splenocytes obtained 2 weeks after the final immunization were cultured with all peptides in bulk and assessed after 7 days by IFNγ ELISPOT using individual peptides, an HLA-A2-specific influenza control peptide (pFlu), PAP protein (PAP), or concanavalin A (ConA) as stimulatory antigens. The frequency of IFNγ sfu per 106 is shown for each animal. Closed circles represent data from vector control-immunized mice, and open circles represent data from PAP DNA-immunized mice. Comparisons of means (shown as lines) between groups are made with an unpaired t test, and the P values are shown for the relevant comparisons. a Analysis of IFNγ ELISPOT responses by individual stimulatory peptide antigens. b The same data but pooled with respect to the PAP-derived non-epitope peptides or PAP-specific HLA-A2 epitopes
Discussion
Prostatic acid phosphatase has emerged as a target antigen for prostate cancer vaccines, in particular given clinical trials suggesting clinical benefit in patients with prostate cancer treated with vaccines targeting PAP by means of an antigen-presenting cell vaccine [10, 11, 14, 15, 29]. We report here the identification of three HLA-A2 epitopes derived from the amino acid sequence of prostatic acid phosphatase, p18–26, p112–120, and p299–307. These epitopes were identified by first searching for pre-existing T cells specific for these peptides among HLA-A2-expressing patients with prostate cancer and normal male blood donors, and then characterizing T-cell lines and clones specific for these epitopes. Of these epitopes, p299–307 and p112–120 have been previously identified by others [16, 17], and p18–26 had not. Direct immunization of HHD-II mice with a DNA vaccine encoding PAP elicited responses to several peptides suggests that there may not be one immunodominant HLA-A2 epitope. There is currently much interest in the identification of MHC class I-restricted epitopes from tumor antigens. Such epitopes might themselves be useful as peptide antigens for vaccines or adoptive immunotherapies [30, 31]. Alternatively, the identification of MHC-restricted peptides may be useful to develop immunological monitoring tools to track the development of CD8+ T-cell immune responses resulting from immunization by ELISPOT or multimer-type methodologies. This is particularly important for PAP, given that it is currently being targeted in antigen-presenting cell vaccines [14, 15, 29], and in earlier clinical trials using DNA vaccines [13]. The ability to detect effector CD8+ T cells may be relevant to determine whether patients achieved potentially therapeutic immune responses as a biomarker of response to immunization and/or might be relevant to the evaluation of future “booster” immunization schedules. Our results suggest it may be important to monitor immune responses to more than one single immunodominant epitope, at least in the case of PAP-specific HLA-A2-restricted epitopes, since even in an inbred murine strain responses were elicited to separate epitopes in individual animals.
There have been three previous reports of identification of HLA-A2 epitopes derived from PAP [16–18]. In the report of Peshwa et al. [16], the authors identified seven potential HLA-A2-restricted epitopes that conformed to the HLA-A2 binding consensus sequence. The seven epitopes were ranked with respect to HLA-A2 binding affinity in T2 assays, and T-cell lines were established for one of these peptides and tested for their ability to lyse HLA-A2-expressing, PAP-expressing cell lines. Curiously, these authors only report seven potential epitopes, whereas we identified ten potential epitopes using similar methods. The 11th potential epitope arose from use of the on-line algorithms. Three of the peptides not present in their prior analysis had some of the highest binding in vitro to HLA-A2. From the T-cell culture studies with the seven identified peptides conducted by that group the authors concluded that p299–307 was an HLA-A2 restricted epitope. Our results confirm these findings, and further identified two additional epitopes, p18–26 and p112–120. In a report by Harada et al. [17], the authors screened for IgG responses to a panel of 14 peptides derived from PAP and identified a high frequency of HLA-A2-expressing prostate cancer patients with IgG specific for p112–120, and then demonstrated that they could elicit p112–120 peptide-specific CTL. Again, our report confirms these findings, but more specifically demonstrates that a clonal line specific for this epitope can lyse PAP-expressing tumor cells in an HLA-A2-restricted fashion. In a report by Machlenkin et al. [18], HHD mice were immunized with peptides derived from PAP, with the identification of p135–143. The authors demonstrated that T-cell lines stimulated with this peptide could lyse LNCaP prostate cancer cells [18]. We cannot explain this discrepancy with our findings—our results demonstrate that this peptide is not a naturally processed epitope specific for PAP, but rather may potentially be recognized by naïve T cells to which a response was detectable in patient samples. Moreover, this peptide may be particularly immunogenic in the HHD mouse in which we were able to detect responses to this peptide in control immunized mice as well as mice immunized with a DNA vaccine encoding PAP. We believe our data are more definitive, as our conclusions are based on a more controlled animal study and from a clonal T-cell line specific for p135–143, as well as two other peptide-specific clonal lines from other individuals (data not shown), that do not demonstrate lysis of PAP-expressing HLA-A2+ cells.
Our data also suggest the importance of evaluating CTL activity to several different antigen-expressing cell lines. The LNCaP cell line, while used by many groups as a model HLA-A2-expressing prostate cancer cell line, expresses very low levels of HLA-A2 [32]. In our hands, we were unable to detect significant HLA-A2 expression on LNCaP cells even with IFNγ pretreatment (data not shown). For this reason, we used LNCaP cells transfected to express HLA-A2, but with native levels of PAP expression. Conversely, we also demonstrated that cells with native levels of HLA-A2, but transduced to express PAP, were also lysed by PAP-specific CTL. The downregulation of MHC class I in advanced prostate cancer has been previously described in human as well as mouse prostate cancer cells [33, 34]. These observations suggest that future studies could explore means to increase prostate tumor cell expression of MHC class I as a means to potentially increase the efficacy of CD8+ T-cell targeted therapies, particularly in advanced disease where this might be most relevant.
There are many reports identifying peptide epitopes for individual tumor antigens. In the typical application, peptides are first identified based on sequence, and then tested in in vitro culture for the ability to generate peptide-specific and tumor-specific CTL, similar to what we have shown. However, with this approach there is little prioritization, and for a large tumor antigen there could be many potential MHC-restricted epitopes. Ultimately, if the goal of such an approach is to identify epitopes that could elicit an immune response in patients, then there should ideally be some evidence that such T cells specific for these epitopes exist in vivo, which was our rationale for first prioritizing peptides based on whether there were peptide-specific responses detectable in the peripheral blood of patients with prostate cancer. Using ELISPOT, we were able to prioritize three of the 11 peptides identified. While ELISPOT has been used as a robust means of identifying peptide-specific effector cells, our results suggest that it can also identify non-specific immunogenic peptides, as in the case of p135–143. However, the ability to prioritize peptides and culture peptide-specific CTL lines from multiple HLA-A2-expressing patients with prostate cancer permitted the identification of PAP-specific epitopes against which CTL could be expanded from the peripheral blood of patients with prostate cancer. The use of ELISPOT, using peripheral blood mononuclear cells from MHC-restricted individuals, could serve as a general method to prioritize peptides for epitope identification for other tumor antigens.
The finding that individual mice from an inbred transgenic strain had responses to different epitopes suggests that there is likely not a single dominant PAP-specific HLA-A2 epitope, and underscores the importance of evaluating responses to multiple epitopes following PAP-specific immunization. The evaluation of PAP-specific CD8+ responses may be particularly relevant given the recent demonstrations that patients with advanced prostate cancer receiving the sipuleucel-T vaccine (Dendreon Corporation, Seattle, WA, USA) have an improved survival compared to placebo-treated patients, and FDA approval is being sought for this vaccine [15]. Because the sipuleucel-T vaccine targets PAP, and presumably works in part by augmenting PAP-specific CD8+ effector cells, it may be important to develop CD8+ monitoring techniques to identify immunological responders that might have benefited from this treatment and/or to identify optimal schedules of immunization, including whether subsequent immunizations should be considered in individuals with waning immunity. We chose this particular immunization strategy in HHD-II mice with a DNA vaccine to parallel human studies using this same DNA vaccine encoding PAP [13]. We plan to use these epitopes as a means of evaluating PAP-specific CD8+ T cells following DNA immunization of patients, as well as use this murine model to define more robust immunization schedules, including heterologous immunization strategies, targeting PAP.
Acknowledgments
This work was supported for BMO, TPF, LEJ and DGM by NIH (K23 RR16489) and the US Army Medical Research and Materiel Command (DAMD 17-03-1-0050 and W81XWH-07-1-0038). This work was supported for KLK and MLD by grants from the NIH (K24 CA85218 and R01 CA75163), and for LF by R01 CA136753.
References
- 1.Gutman AB, Gutman EB. An acid phosphatase in the serum of patients with metastasizing carcinoma of the prostate gland. J Clin Invest. 1938;17:473–479. doi: 10.1172/JCI100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jobsis AC, De Vries GP, Anholt RR, Sanders GT. Demonstration of the prostatic origin of metastases: an immunohistochemical method for formalin-fixed embedded tissue. Cancer. 1978;41:1788–1793. doi: 10.1002/1097-0142(197805)41:5<1788::AID-CNCR2820410521>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.Siddall JK, Cooper EH, Newling DWW, Robinson MRG, Whelan P. An evaluation of the immunochemical measurement of prostatic acid phosphatase and prostatic specific antigen in carcinoma of the prostate. Eur Urol. 1986;12:123–130. doi: 10.1159/000472596. [DOI] [PubMed] [Google Scholar]
- 4.O’Keefe DS, Bacich DJ, Heston WD. Comparative analysis of prostate-specific membrane antigen (PSMA) versus a prostate-specific membrane antigen-like gene. Prostate. 2004;58:200–210. doi: 10.1002/pros.10319. [DOI] [PubMed] [Google Scholar]
- 5.Laus R, Yang DM, Ruegg CL, Shapero MH, Slagle PH, Small E, Burch P, Valone FH. Dendritic cell immunotherapy of prostate cancer: preclinical models and early clinical experience. Canc Res Ther Control. 2001;11:1–10. [Google Scholar]
- 6.Fong L, Ruegg CL, Brockstedt D, Engleman EG, Laus R. Induction of tissue-specific autoimmune prostatitis with prostatic acid phosphatase immunization; implications for immunotherapy of prostate cancer. J Immunol. 1997;159:3113–3117. [PubMed] [Google Scholar]
- 7.Johnson LE, Frye TP, Arnot AR, Marquette C, Couture LA, Gendron-Fitzpatrick A, McNeel DG. Safety and immunological efficacy of a prostate cancer plasmid DNA vaccine encoding prostatic acid phosphatase (PAP) Vaccine. 2006;24:293–303. doi: 10.1016/j.vaccine.2005.07.074. [DOI] [PubMed] [Google Scholar]
- 8.Johnson LE, Frye TP, Chinnasamy N, Chinnasamy D, McNeel DG. Plasmid DNA vaccine encoding prostatic acid phosphatase is effective in eliciting autologous antigen-specific CD8+ T cells. Cancer Immunol Immunother. 2007;56:885–895. doi: 10.1007/s00262-006-0241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNeel DG. Prostate cancer antigens and vaccines, preclinical developments. In: Giaccone G, Schilsky R, Sondel P, editors. Cancer chemotherapy and biological response modifiers, annual 22. Amsterdam: Elsevier; 2005. pp. 247–261. [DOI] [PubMed] [Google Scholar]
- 10.Burch PA, Breen JK, Buckner JC, Gastineau DA, Kaur JA, Laus RL, Padley DJ, Peshwa MV, Pitot HC, Richardson RL, Smits BJ, Sopapan P, Strang G, Valone FH, Vuk-Pavlovic S. Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res. 2000;6:2175–2182. [PubMed] [Google Scholar]
- 11.Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, Valone FH. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 12.Fong L, Brockstedt D, Benike C, Breen JK, Strang G, Ruegg CL, Engleman EG. Dendritic cell-based xenoantigen vaccination for prostate cancer immunotherapy. J Immunol. 2001;167:7150–7156. doi: 10.4049/jimmunol.167.12.7150. [DOI] [PubMed] [Google Scholar]
- 13.McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, Horvath DL, Straus J, Alberti D, Marnocha R, Liu G, Eickhoff JC, Wilding G. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. 2009;27:4047–4054. doi: 10.1200/JCO.2008.19.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 15.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 16.Peshwa MV, Shi JD, Ruegg C, Laus R, van Schooten WC. Induction of prostate tumor-specific CD8+ cytotoxic T-lymphocytes in vitro using antigen-presenting cells pulsed with prostatic acid phosphatase peptide. Prostate. 1998;36:129–138. doi: 10.1002/(SICI)1097-0045(19980701)36:2<129::AID-PROS8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Harada M, Matsueda S, Yao A, Ogata R, Noguchi M, Itoh K. Prostate-related antigen-derived new peptides having the capacity of inducing prostate cancer-reactive CTLs in HLA-A2+ prostate cancer patients. Oncol Rep. 2004;12:601–607. [PubMed] [Google Scholar]
- 18.Machlenkin A, Paz A, Bar Haim E, Goldberger O, Finkel E, Tirosh B, Volovitz I, Vadai E, Lugassy G, Cytron S, Lemonnier F, Tzehoval E, Eisenbach L. Human CTL epitopes prostatic acid phosphatase-3 and six-transmembrane epithelial antigen of prostate-3 as candidates for prostate cancer immunotherapy. Cancer Res. 2005;65:6435–6442. doi: 10.1158/0008-5472.CAN-05-0133. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Harada M, Yano H, Ogasawara S, Takedatsu H, Arima Y, Matsueda S, Yamada A, Itoh K. Prostatic acid phosphatase as a target molecule in specific immunotherapy for patients with nonprostate adenocarcinoma. J Immunother. 2005;28:535–541. doi: 10.1097/01.cji.0000175490.26937.22. [DOI] [PubMed] [Google Scholar]
- 20.McNeel DG, Knutson KL, Schiffman K, Davis DR, Caron D, Disis ML. Pilot study of an HLA-A2 peptide vaccine using flt3 ligand as a systemic vaccine adjuvant. J Clin Immunol. 2003;23:62–72. doi: 10.1023/A:1021904432489. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Vina MA, Falco M, Sun Y, Stastny P. DNA typing for HLA class I alleles: I. Subsets of HLA-A2 and of -A28. Hum Immunol. 1992;33:163–173. doi: 10.1016/0198-8859(92)90068-X. [DOI] [PubMed] [Google Scholar]
- 22.Sauma SY, Gammon MC, Bednarek MA, Cunningham B, Biddison WE, Hermes JD, Porter G, Tamhankar S, Hawkins JC, Bush BL, et al. Recognition by HLA-A2-restricted cytotoxic T lymphocytes of endogenously generated and exogenously provided synthetic peptide analogues of the influenza A virus matrix protein. Hum Immunol. 1993;37:252–258. doi: 10.1016/0198-8859(93)90508-X. [DOI] [PubMed] [Google Scholar]
- 23.Khanna R, Burrows SR, Nicholls J, Poulsen LM. Identification of cytotoxic T cell epitopes within Epstein-Barr virus (EBV) oncogene latent membrane protein 1 (LMP1): evidence for HLA A2 supertype-restricted immune recognition of EBV-infected cells by LMP1-specific cytotoxic T lymphocytes. Eur J Immunol. 1998;28:451–458. doi: 10.1002/(SICI)1521-4141(199802)28:02<451::AID-IMMU451>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 24.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest. 2001;107:477–484. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rammensee HG, Falk K, Rotzschke O. Peptides naturally presented by MHC class I molecules. Annu Rev Immunol. 1993;11:213–244. doi: 10.1146/annurev.iy.11.040193.001241. [DOI] [PubMed] [Google Scholar]
- 27.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 28.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 29.Small EJ, Rini B, Higano C, Redfern C, Nemunaitis J, Valone F, Kylstra J, Schellhammer PF. A randomized, placebo-controlled phase III trial of APC8015 in patients with androgen-independent prostate cancer (AiPCa) Proc Am Soc Clin Oncol. 2003;22:1534. [Google Scholar]
- 30.Berzofsky JA, Oh S, Terabe M. Peptide vaccines against cancer. Cancer Treat Res. 2005;123:115–136. doi: 10.1007/0-387-27545-2_5. [DOI] [PubMed] [Google Scholar]
- 31.Disis ML, McNeel DG, Rinn K, Schiffman KA, Knutson KL. Peptide-based tumor vaccines. Curr Opin Oncol Endo Met Invest Drugs. 1999;1:253–259. [Google Scholar]
- 32.Kouiavskaia DV, Berard CA, Datena E, Hussain A, Dawson N, Klyushnenkova EN, Alexander RB. Vaccination with agonist peptide PSA: 154-163 (155L) derived from prostate specific antigen induced CD8 T-cell response to the native peptide PSA: 154-163 but failed to induce the reactivity against tumor targets expressing PSA: a phase 2 study in patients with recurrent prostate cancer. J Immunother. 2009;32:655–666. doi: 10.1097/CJI.0b013e3181a80e0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanda MG, Restifo NP, Walsh JC, Kawakami Y, Nelson WG, Pardoll DM, Simons JW. Molecular characterization of defective antigen processing in human prostate cancer. J Natl Cancer Inst. 1995;87:280–285. doi: 10.1093/jnci/87.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HM, Timme TL, Thompson TC. Resistance to lysis by cytotoxic T cells: a dominant effect in metastatic mouse prostate cancer cells. Cancer Res. 2000;60:1927–1933. [PubMed] [Google Scholar]