Abstract
The differentiation and survival of heterozygous Lurcher (+/Lc) Purkinje cells in vitro was examined as a model system for studying how chronic ionic stress affects neuronal differentiation and survival. The Lurcher mutation in the δ2 glutamate receptor (GluRδ2) converts an orphan receptor into a membrane channel that constitutively passes an inward cation current. In the GluRδ2+/Lc mutant, Purkinje cell dendritic differentiation is disrupted and the cells degenerate following the first week of postnatal development. To determine if the GluRδ2+/Lc Purkinje cell phenotype is recapitulated in vitro, +/+ and +/Lc Purkinje cells from postnatal day 0 pups were grown in either isolated cell or cerebellar slice cultures. GluRδ2+/+ and GluRδ2+/Lc Purkinje cells appeared to develop normally through the first 7 days in vitro (DIV), but by 11 DIV GluRδ2+/Lc Purkinje cells exhibited a significantly higher cation leak current. By 14 DIV, GluRδ2+/Lc Purkinje cell dendrites were stunted and the number of surviving GluRδ2+/Lc Purkinje cells was reduced by 75% compared to controls. However, treatment of +/Lc cerebellar cultures with 1-naphthyl acetyl spermine (NASP) increased +/Lc Purkinje cell survival to wild type levels. These results support the conclusion that the Lurcher mutation in GluRδ2 induces cell autonomous defects in differentiation and survival. The establishment of a tissue culture system for studying cell injury and death mechanisms in a relatively simple system like GluRδ2+/Lc Purkinje cells will provide a valuable model for studying how the induction of a chronic inward cation current in a single cell type affects neuronal differentiation and survival.
Keywords: δ2 glutamate receptor, excitotoxicity, organotypic cultures, dendritic differentiation, ionic stress
INTRODUCTION
The Lurcher mutant (gene symbol, Lc) has been studied for over 40 years as a model for neuronal cell death in cerebellar neurons (Vogel et al., 2007). Homozygous Lurcher mutants die around birth following the extensive loss of hindbrain neurons (Cheng and Heintz, 1997), while the phenotype of heterozygote Lurcher mutants includes the postnatal loss of virtually all cerebellar Purkinje cells, 90% of the granule cells and 75% of the olivary neurons (Caddy and Biscoe, 1979). Studies of +/Lc ⇔ wild type chimeras showed that +/Lc Purkinje cells are a primary site of the mutant gene action, while granule cell and olivary neuron cell loss is secondary to the death of their postsynaptic targets (Wetts and Herrup, 1982; Wetts and Herrup, 1982). In 1997, the Lurcher mutation was identified as a gain of function alteration in the orphan delta 2 glutamate receptor (GluRδ2) that turns the receptor into a constitutively open cation membrane channel that chronically depolarizes GluRδ2+/Lc Purkinje cells (Zuo et al., 1997). GluRδ2 receptors do not bind glutamate or glutamate agonists and the leak current in GluRδ2+/Lc channels is independent of synaptic transmission (Araki et al., 1993; Lomeli et al., 1993; Mayat et al., 1995; Zuo et al., 1997). The GluRδ2 receptor is preferentially expressed in cerebellar Purkinje cells (Araki et al., 1993; Lomeli et al., 1993; Takayama et al., 1996) and in GluRδ2+/Lc Purkinje cells the chronic cation leak starts during the first postnatal week of development (Selimi et al., 2003). GluRδ2+/Lc Purkinje cell death begins around the same time and continues until virtually all GluRδ2+/Lc Purkinje cells have died by the end of the second postnatal month. As such, the GluRδ2+/Lc Purkinje cell is an excellent model to study the effects of membrane depolarization on cell survival and death mechanisms because the Lurcher mutation in GluRδ2 provides a well characterized cation leak current that specifically targets one cell type during an important phase of neuronal development.
However, despite the identification of the mutant receptor in Lurcher Purkinje cells, the mechanisms responsible for their death have remained elusive. Both apoptotic and autophagic pathways have been implicated in GluRδ2+/Lc Purkinje cell death (Norman et al., 1995; Selimi et al., 2000; Yue et al., 2002; Wang et al., 2006): overexpression or deletion of the apoptosis related genes Bcl-2 or Bax, respectively, in GluRδ2+/Lc Purkinje cells will delay, but not prevent, their degeneration (Zanjani et al., 1998; Doughty et al., 2000; Selimi et al., 2000). The GluRδ2 receptor is linked by n-PIST to the autophagy related protein, Beclin, and it has been suggested that the Lurcher mutation in GluRδ2 may constitutively activate Beclin, leading to an autophagic cell death pathway (Orr, 2002; Yue et al., 2002). However, Beclin also interacts with Bcl-2 (Liang et al., 1998; Pattingre et al., 2005), so it is not clear how apoptotic and autophagic pathways interact in GluRδ2+/Lc Purkinje cell death.
The search for the mechanisms that trigger and execute cell death pathways in GluRδ2+/Lc Purkinje cells is hampered, in part, by the lack of an in vitro system where pharmacological, molecular, genetic, and electrophysiological manipulations can be used to elucidate the pathways of GluRδ2+/Lc Purkinje cell death. Only one previous study has examined GluRδ2+/Lc Purkinje cell survival in cerebellar slice cultures (Doughty et al., 1995) and the authors found that wild type and GluRδ2+/Lc Purkinje cell survival in vitro was equivalent. They concluded that olivary neuron innervation was needed to stimulate increased GluRδ2+/Lc Purkinje cell death. However, this study was conducted before the Lurcher mutation had been identified and the overall rate of Purkinje cell survival was relatively low in both wild type and GluRδ2+/Lc cultures. In light of new information about the Lurcher mutation and improved methods for culturing Purkinje cells, we have re-examined GluRδ2+/Lc Purkinje cell survival in vitro with the goal of studying how chronic ionic stress affects neuronal differentiation and survival.. The results of our study indicate that GluRδ2+/Lc Purkinje cell survival is reduced in vitro compared with wild type Purkinje cells, and that the timing and phenotype of GluRδ2+/Lc Purkinje cell degeneration, including chronic depolarization, are similar in vitro and in vivo. Furthermore, treatment of +/Lc cerebellar cultures with the AMPA, kainate and GluRδ2Lc channel antagonist, 1-naphthyl acetyl spermine (NASP; Koike et al., 1997; Kohda et al., 2000), significantly increases the survival of GluRδ2+/Lc Purkinje cells, suggesting that the leak current is responsible for the increased death of GluRδ2+/Lc Purkinje cells in vitro.
METHODS
Animals
GluRδ2+/Lc mutant and wild type (GluRδ2+/+) pups were generated by mating B6CBACa Aw-J/A-Grid2Lc/J males (NB: Grid2 is an alternative abbreviation for the GluRδ2 receptor) with wild type females (C57BL/6J or B6CBA), from Jackson or Janvier Laboratories. For electrophysiology experiments, GluRδ2+/Lc mutants were mated with mice carrying the eGFP transgene under control of the L7 promoter (Tomomura et al., 2001); thus, the Purkinje cells in GluRδ2+/Lc and GluRδ2+/+ offspring expressed GFP, which aided identification during electrophysiological recording. Males were harem mated with one male to two or three females and the females were checked for copulatory plugs the day after initially placing the females with the male and every day the mice remained together. The day of finding the copulatory plug was considered embryonic day 0.5 (E0.5) and the day of birth was counted as postnatal day 0 (P0). All animals were housed in standard conditions (14 hours light, 10 hours dark) in animal facilities either at the Maryland Psychiatric Research Center or at the Universite P. et M. Curie and provided with food and water ad libitum. The animal facilities at the MPRC are fully accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC) and the animal facilities at the UPMC are fully accredited by the French Research and superior education ministry. The studies were conducted in accordance with the Guide for Care and Use of Laboratory Animals provided by the NIH and by the guidelines established by “le comité national d’éthique pour les sciences de la vie et de la santé.”
The GluRδ2+/Lc or GluRδ2+/+ genotype of P0 pups was identified by PCR and single-stranded conformation polymorphism (SSCP) as described previously (Selimi et al., 2003). GluRδ2 S1 and AS primers were used to amplify a stretch of DNA that spans the single base pair change in the GluRδ2Lc mutation (Zuo et al., 1997).
Isolated tissue culture
Standard techniques (Hatten et al., 1988; Furuya et al., 1998) were used to culture Purkinje cells from GluRδ2+/Lc and GluRδ2+/+ control pups. Isolated cells from individual P0 cerebellum were plated onto cultures of granule cells that had been established 2 to 7 days earlier from the cerebella of P4 to P7 pups. Individual pups were euthanized by decapitation at P0.5 for isolation of Purkinje cells and at P4.5 to 7.5 for isolation of granule cells. The cerebellum from each pup was quickly dissected in ice cold Ca++/Mg++ free Hank's BSS solution. A tail sample was taken from each P0 pup to identify its genotype by PCR and SSCP. Cells were dissociated by digestion with 0.1% trypsin at 33−35 °C for 10 min followed by trituration with a Pasteur pipette in DNase solution. The isolated cells were cultured in a DMEM/F-12 medium (Gibco BRL Life Technologies) containing fetal calf serum (1%), N3 supplement (1x; see Furuya et al, 1998), T3 (0.5 ng/ml), and AraC (4μM). Cultures were plated at a density of 3.3 ×105 cells/culture on cover slips precoated with poly-L-lysine and laminin. In most experiments, sufficient cells were obtained to plate two coverslips per cerebellum. Half of the culture medium (1/2 ml) was changed every 2−3 days and cultures were fixed at 7, 14, and 28 days in vitro.
Organotypic tissue slice cultures
Cerebellar slices were prepared from GluRδ2+/Lc mutant and GluRδ2+/+ pups at P0. Pups were decapitated and the brain removed in ice cold Gey's balanced salt solution with 5 mg/mL glucose. The cerebellum was separated from the rest of the brain with forceps after removing the choroids plexus and dura. The entire cerebellum was then sliced into 350 μm sagittal sections with a Mc Ilwain Tissue Chopper and the slices were placed on the membrane of Millicell CM inserts (Millipore, MA). All of the sections from one cerebellum were arranged on one culture insert. Slices were maintained at the interface between the air and the culture media consisting of 50% Basal Medium Eagle (BME), 25% Hank's Balanced Salt Solution (HBSS), 25% heat inactivated horse serum, 1 mM L-glutamine, and 5 mg/ml D-glucose in a humidified chamber with 5% CO2 (pH 7.3) at 35° C. The medium was changed every 2−3 days and cultures were fixed at 7 and 14 days in vitro. NASP (Sigma-Aldrich) was added to the slice culture media at 100μM final concentration from DIV 0.
Immunohistochemistry
Slice cultures were fixed in 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4) for 30 minutes at room temperature followed by multiple washes with 10 mM phosphate buffered saline (0.9% NaCl, PBS). Slices were then incubated for 1 hour in PBS containing 0.2 % Triton X-100, 0.2% gelatin, 0.1M lysine before immunostaining with a mouse monoclonal antibody against calbindin (dilution 1:5000, Swant, Bellinzona, Switzerland) overnight at 4° C. Antibody binding was revealed with CY3- conjugated Donkey anti-mouse antibody (1:500 dilutions, Jackson Immuno Research Laboratories, Inc, West Grove, PA, USA). After 2 hours incubation in buffer containing the secondary antibody at room temperature, the slices were washed several times with PBS and counterstained with the DNA fluorochrome Hoechst 33258 (diluted 1/50000, Sigma-Aldrich, St. Louis, MO) and mounted in Mowiol (Calbiochem, La Jolla, CA, USA).
Isolated cultures were fixed with 4% paraformaldehyde in 0.1M phosphate buffer for half an hour and then rinsed several times in PBS. Cultures were prepared for fluorescence immunohistochemistry by incubating the cultures in two changes of 0.1M glycine (in 10 mM PBS, pH 7.4) for 5 min each. Endogenous fluorescence was reduced by incubation in 50 mM ammonium chloride (in 10mM PBS, pH 6.8 − 7.1) for 1 hour. The cultures were then rinsed three times in PBS and then incubated for an hour in blocking solution containing 3% normal goat serum and 0.3%Triton X-100. Cultures were then incubated in primary antibodies overnight at 4°C. All cultures were labeled with antibodies to calbindin to label Purkinje cells (either mouse monoclonal, Sigma-Aldrich: 1/10,000 or rabbit polyclonal, Calbiochem: 1/1000). A sampling of cultures were double labeled for calbindin and GluRδ2 (rabbit-anti-GluRδ1/2; Chemicon now Millipore: www.millipore.com). After removing the primary antibody, sections were rinsed 3 times in PBS and then incubated for 2 hours with fluorescent labeled secondary antibodies (anti-mouse or anti-rabbit Alexa 594 and Alexa 488: Molecular Probes (now Invitrogen: www.invitrogen.com): 1/200). After a 2 hour incubation at room temperature, sections were rinsed 3 times in 10 mM PBS, once in distilled water, mounted on standard microscope slides, and then coverslipped with GEL/MOUNT (Biomedia, Foster City, USA). In some cases, the cultures were counterstained with DAPI (300nM in phosphate buffer, Invitrogen) for 30 minutes before rinsing the cultures and mounting on slides. The finished slides were then photographed using either an Olympus BH-2 fluorescence microscope with a Pixera digital camera, a Zeiss Axioplan microscope with an Olympus DP70 digital camera, or a Nikon E800 microscope. The photographs for publication were prepared by focusing through the microscope field and taking a digital image every few microns in the z-axis. The resulting stack of photographs was then collapsed into a single image using Helicon Focus (www.heliconsoft.com) with the goal of maximizing the depth of focus in the photograph. The color photographs were rendered in gray scale and adjusted for contrast using Adobe Photoshop (www.Adobe.com) before being assembled in a photoplate.
Labeling experiments with and without the 1° antibody to control for non-specific immunolabeling were conducted on fixed cyrostat-sectioned cerebellar slices mounted on slides so that all of the tissue culture experiments could be used for Purkinje cell counts.
Immunolabeling with anti-calbindin antibodies are a standard technique for identifying Purkinje cells (e.g. (Tano et al., 1992; Armstrong et al., 2005) and the specificity of the GluRδ1/2 antibody from Chemicon was established in a previous publication (Selimi et al., 2003). The mouse monoclonal antibody against calbindin from Swant was routinely used for slice cultures in experiments at the Univ. M. et P. Cuire by Dr. Zanjani. The mouse monoclonal anti-calbindin antibody from Sigma-Aldrich or the rabbit polyclonal anti-calbindin antibody from Calbiochem was used for staining slice and isolated cell cultures at the University of Maryland School of Medicine depending on whether the cultures were co-labeled with rabbit-anti-GluRδ1/2 or mouse monoclonal anti-nitrotyrosine (Upstate: data not shown). All three anti-calbindin antibodies provided quantitatively similar results.
Purkinje cell counts and area measurements
The total number of Purkinje cells per culture was directly determined by systematically scanning the entire culture area and counting all calbindin fluorescent labeled Purkinje cells, either in the isolated cell cultures or the slice cultures. The cell counts were conducted with either a 20x or 40x objective. An eyepiece graticule was used to systematically sweep across the culture and to keep track of counted and uncounted areas. In a subsample of cerebellar slice cultures the total area of the slices per culture was calculated by photographing each cultured slice and then measuring the area of the slice with NIH image. The average number and density of Purkinje cells is reported as the mean ± standard error of the mean. Slice and isolated cell cultures were counted independently by one or two of four of the authors (H.Z., R.M., A.B., and M.W.V.) and the results averaged in cases where cultures were counted by two authors. In all cases, the genotype and treatment of the culture was masked from the counter.
The number of Purkinje cell primary dendrites and the area covered by the dendrites and somas was determined by taking digital images of calbindin-labeled Purkinje cells with a 40x objective and then measuring the area of the cell with ImageJ (http://rsb.info.nih.gov/ij/). Each image was calibrated with a standard stage micrometer. The number of primary dendrites leaving the cell body was counted for each cell. The area of the soma was measured by tracing around the outline of the cell body. tThe threshold function was then used to select the pixels that contain the total dendritic field and cell body of the Purkinje cell. The add and subtract function or the drawing tools were used to add areas that could not be selected with the threshold function or to delete interior pixels that did not represent a Purkinje cell surface. Purkinje cell dendritic area was then calculated by subtracting the soma area from the total cell area. For isolated cell cultures, an average of 26.6 ± 3 (mean ± s.e.) Purkinje cells per culture were randomly selected for analysis and only cells that were completely isolated from other cells were measured. In slice cultures it was more difficult to find Purkinje cells that were completely isolated so an average of 5.5 ± 1 Purkinje cells per culture were analyzed for dendritic and soma area measurements.
Electrophysiology
Slice cultures were prepared as described above, except that the P0 pups were derived from crosses between +/Lc mutants and L7-GFP mice (see Animals). The cerebellar slices were used for electrophysiological recordings at 11−14 DIV (+/Lc slices were matched for age with wild type slices). The slice recording chamber was continuously superfused with artifical cerebral spinal fluid (ACSF) containing (mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2, 1 MgCl2, 25 glucose, continuously bubbled with 95% O2, 5% CO2. Whole-cell patch-clamp recordings were made from Purkinje cells using an Axopatch 200 amplifier (Axon Instruments, Foster City, CA, U.S.A). Patch pipettes were filled with a solution containing (mM) 6 KCl, 140 K D-gluconate, 10 HEPES, 1 EGTA, 0.1 CaCl2, 5 MgCl2, 4 Na2ATP, 0.4 NaGTP; pH 7.3, 290−300 mOsm. Purkinje cells expressing GFP were visually identified and voltage clamped at −70 mV while recording the holding current. Following baseline voltage clamp recordings, the superfusion buffer was replaced by a solution in which NaCl was substituted by 125 mM N-methyl-d-glucamine (NMDG) chloride (Zuo et al., 1997). NMDG is too large to pass through cation channels so this control was used to determine if the leak current was indeed due to an inward Na+ current carried by an ion channel (presumably the GluRδ2Lc channel) and not to poor patch clamp quality. The values reported here were derived only from those Purkinje cells for which the post-NMDG holding currents were more positive than −500 pA, indicating that the patch clamp on the neuron was adequate.
Statistical analysis
Purkinje cell survival in GluRδ2+/Lc and GluRδ2+/+ isolated or slice cultures was separately analyzed using two-way analysis of variance (ANOVA) including genotype and days in vitro (DIV) as co-variants (Statview 5.0, Cary, North Carolina). A log transformation of the number of Purkinje cells per culture was used to stabilize the variance for the ANOVA. Since there were significant interactions between genotype and DIV in both isolated and slice culture data sets, the data was split by DIV to conduct separate one-way ANOVAs with genotype as the variable to determine if there were significant differences between the number of +/+ and +/Lc Purkinje cells at each timepoint (7, 14, and 28 DIV). Overall comparisons between GluRd2+/+ and GluRd2+/Lc Purkinje cell survival following NASP treatment was also analyzed using two-way ANOVA with genotype and NASP treatment as co-variants. Separate one-way ANOVAs were conducted to compare the effect of NASP treatment within GluRd2+/+ and GluRd2+/Lc cultures.
For statistical analysis of Purkinje cell areas, the mean Purkinje cell soma and dendritic area for each culture was calculated from an average of 26.6 ± 3 Purkinje cells per isolated cell culture and 5 ± 1 Purkinje cells per slice culture. The mean area measurements per culture were combined from both cerebellar slice and isolated cell cultures at 14 DIV and the differences between GluRδ2+/Lc and GluRδ2+/+ Purkinje soma and dendritic areas were separately analyzed by one-way ANOVA with genotype as the variable. For the statistical analysis of mean leak currents measured electrophysiologically, the variance in average leak currents in GluRδ2+/Lc and GluRδ2+/+ Purkinje cells (without or with NMDG) differed more than two fold. Therefore the non-parametric Mann-Whitney U test was used to compare the differences between leak currents in GluRδ2+/Lc and GluRδ2+/+ Purkinje cells.
RESULTS
GluRδ2+/Lc Purkinje cell survival in vitro
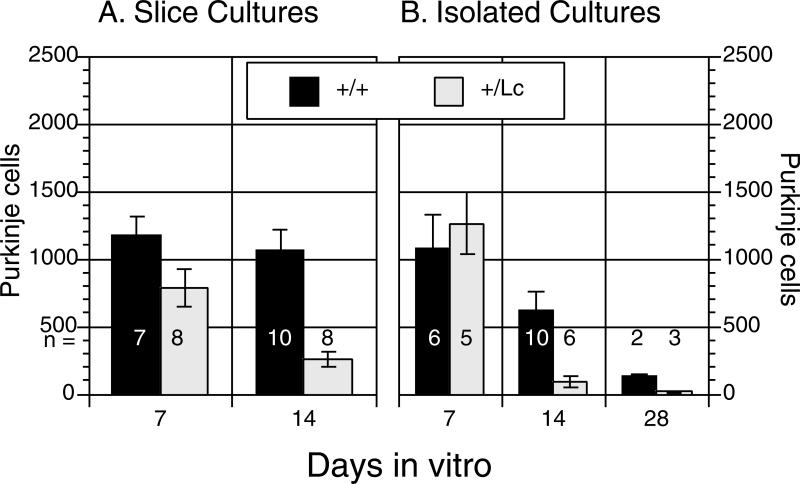
GluRδ2+/+ wild type and GluRδ2+/Lc mutant cerebella were dissected at P0 and Purkinje cell survival was analyzed in both isolated cell and cerebellar slice cultures at 7 and 14 days in vitro (7 and 14 DIV), plus 28 DIV for isolated cultures (Fig. 1). In both culture systems, two-way analysis of variance of the counts of the total number of Purkinje cells per culture (with a log transformation) showed that there are significant effects of genotype (Isolated culture: ANOVA, F1, 26 = 18.4, p < .0005; Slice cultures, ANOVA, F1,29 = 30.3, p < .0001) and length of time in culture (DIV: Isolated culture: ANOVA, F2, 26 = 28.5, p < .0001; Slice cultures, ANOVA, F1,29 = 12.5, p < .002). Furthermore, there are significant genotype by DIV interactions (Isolated culture: ANOVA, F2, 26 = 7.7, p < .003; Slice cultures, ANOVA, F1,29 = 7.3, p < .02). At 7 DIV, there is no significant difference between the number of surviving GluRδ2+/+ and GluRδ2+/Lc Purkinje cells in either the isolated (one-way ANOVA, F1,9 = 0.276, p > 0.5) or slice cultures (one-way ANOVA, F1,13 = 3.78, p > 0.05). However, by 14 DIV there is a significant reduction in the survival of GluRδ2+/Lc Purkinje cells compared to controls (Isolated cultures: one-way ANOVA, F1,14 = 8.63, p < 0.015; Slice cultures, one-way ANOVA, F1,16 = 21.4, p < 0.001). In the isolated cell cultures, although overall Purkinje cell survival declines with age, in both genotypes there are still significantly fewer surviving GluRδ2+/Lc Purkinje cells compared to controls by 28 DIV (one-way ANOVA, F1,3 = 35.6, p < 0.01).
Figure 1.
GluRδ2+/+ wild type (+/+) and GluRδ2+/Lc mutant (+/Lc) Purkinje survival in organotypic (A) and isolated cultures (B). The sample size for each genotype and time point is given in a row below the 500 Purkinje cell line. The results demonstrate that GluRδ2+/Lc Purkinje cells die more rapidly in vitro compared with control Purkinje cell cultures.
We have chosen to report Purkinje cell survival data in terms of the total numbers of Purkinje cells per culture. We believe that it was more accurate to carefully count all of the Purkinje cells in each culture rather than estimate the density of Purkinje cells based on a random sampling system. However, in the study by Doughty et al. (1995), Purkinje cell survival was reported in terms of the density of Purkinje cells. In order to compare our results with the previous study, the area of the cultured slices in a subsample of 5 GluRδ2+/Lc and 4 GluRδ2+/+ cultures at 14 DIV was measured from low magnification micrographs using NIH image and the density of Purkinje cells calculated. Purkinje cell density ranged from a low of 5.46 Purkinje cells per mm2 in a GluRδ2+/Lc slice culture to a high of 50.5 Purkinje cells per mm2 in a GluRδ2+/+ slice culture. The mean density of Purkinje cells in GluRδ2+/Lc slice cultures (13.1 ± 2.5 PCs/mm2) was significantly lower than in GluRδ2+/+ cultures (40.4 ± 3.4; ANOVA, F1,7 = 43.1, p < 0.0005).
GluRδ2+/Lc Purkinje cell differentiation in vitro
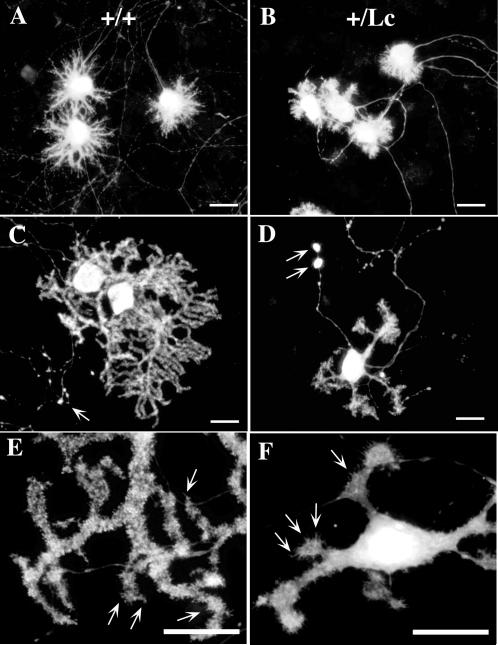
After 1 week of postnatal development in vitro, most GluRδ2+/Lc and GluRδ2+/+ Purkinje cells resemble Stage II type Purkinje cells as described by Armengol and Sotelo (Armengol and Sotelo, 1991) and Boukhtouche et al. (Boukhtouche et al., 2006) (Figure 2A, B, photos from slice cultures). The 7 DIV stage II Purkinje cells have many short processes extending from the soma, giving the Purkinje cells a stellate appearance. Each Purkinje cell has one apparent axon that exits from the cell body and grows extensively through the culture. In the cerebellar slice cultures, the axons appear to coalesce in one region of the slice as if they were appropriately innervating the deep cerebellar nuclei. As delineated by calbindin labeling, there did not appear to be any obvious qualitative differences between the appearance of GluRδ2+/Lc and GluRδ2+/+ Purkinje cells at this stage (Fig 2 A and B).
Figure 2.
Photomicrographs of immunolabeled GluRδ2+/+ wild type (A, C, E) and GluRδ2+/Lc mutant (B, D, F) Purkinje cells from slice cultures at 7 (A, B) and 14 (C, D) DIV and isolated cell cultures at 14 DIV (E,F). The Purkinje cells in A to D were immunostained with primary antibodies directed against calbindin while those in E and F were labeled with anti-GluRδ1/2. The white arrows in C and D indicate axonal blebs on both GluRδ2+/+ and GluRδ2+/Lc Purkinje cell axon-like processes. The white arrows in E and F indicate GluRδ2 immunolabeling of dendritic spines on Purkinje cell dendrites. Scale bars: 20 μm
By 14 DIV, both GluRδ2+/Lc and GluRδ2+/+ Purkinje cells have matured to the point that they have one or more primary dendrites that may give rise to complex side branches (Fig. 2 C, D, photos from slice cultures). The secondary and tertiary dendrites are often studded with dendritic-spine-like structures (Fig. 2 E, F, photos from isolated cell cultures). The Purkinje cells from slice cultures shown in Figure 2 C and D were immunostained for calbindin, while the isolated Purkinje cells in Figure 2 E and F were immunostained for GluRδ1/2. Most Purkinje cells in the 14DIV slices have matured to stage III or IV Purkinje cells. As in the 7DIV cultures, the Purkinje cells have long axonal processes with many branches at the distal extent of their axonal arborizations (data not shown). In isolated cultures, the axons of both GluRδ2+/Lc and GluRδ2+/+ Purkinje cell are studded with axonal varicosities (Fig. 2C and D) although they are more frequent in the GluRδ2+/Lc Purkinje cells. In slice cultures, Purkinje axonal varicosities are rarer in sections from wild type cerebella, but there are many in GluRδ2+/Lc Purkinje cell axons at 14 DIV and they appear to be larger than the varicosities along GluRδ2+/+ Purkinje cell axons.
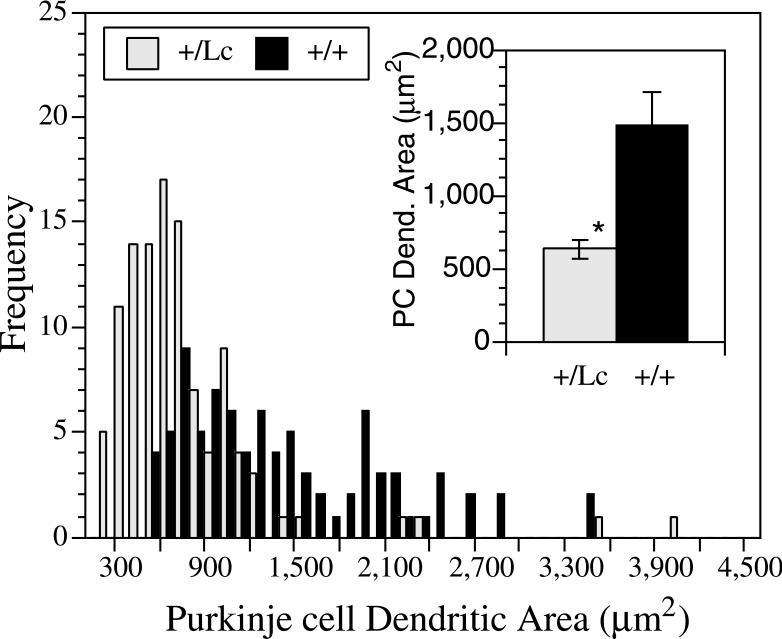
There is considerable variability in the appearance, size, and complexity of the dendritic trees at 14 DIV, depending in part on where the Purkinje cells lie with respect to other Purkinje cells and granule cells. However, by 14 DIV the surviving GluRδ2+/Lc Purkinje cells are recognizably smaller than GluRδ2+/+ Purkinje cells and their dendrites have the morphological characteristics reported for GluRδ2+/Lc Purkinje cell dendrites in vivo (Fig. 2 C-F; e.g. (Caddy and Biscoe, 1979; McFarland et al., 2007) : their dendrites are thickened and stubby and many have multiple primary dendrites exiting from the cell body. To quantify the differences in the growth of GluRδ2+/+ and GluRδ2+/Lc Purkinje cells by 14 DIV, the total soma and dendritic areas of 26.6 ± 3 Purkinje cells per culture from 3 GluRδ2+/+ and 3 GluRδ2+/Lc isolated cell cultures and 5.5 ± 1 Purkinje cells from 3 GluRδ2+/+ and 4 GluRδ2+/Lc cerebellar slice cultures were measured using ImageJ. The data from all of the measured Purkinje cells were combined to construct the frequency histogram shown in Figure 3. The distribution of dendritic sizes shows that there is considerable variation in Purkinje cell growth by 14 DIV, but in general the distribution of GluRδ2+/Lc Purkinje cell dendritic area is shifted towards smaller dendritic fields compared with wild type Purkinje cells. The mean Purkinje cell dendritic field and soma area for each GluRδ2+/+ and GluRδ2+/Lc isolated cell and slice culture was calculated and used to calculate the average Purkinje cell dendritic and soma area in the GluRδ2+/+ (n= 6) and GluRδ2+/Lc cultures (n=7; Fig. 3 insert). The mean area of GluRδ2+/Lc Purkinje cell dendritic trees are significantly reduced compared with controls from an average area of 1,486 ± 218 μm2 to 633 ± 65 μm2 (mean ± s.e.; ANOVA, F1,11 = 16.1, p < 0.005). The mean size of the soma of GluRδ2+/Lc Purkinje cells (241.7 ± 8.5 μm2) was also significantly reduced compared to controls (315.8 ± 30.5 μm2; ANOVA, F1,11 = 6.3, p < 0.03). There were no significant differences between the number of primary dendrites in GluRδ2+/Lc Purkinje cells (4.1 ± 0.5) compared to controls (3.6 ± 0.5; ANOVA, F1,11 = 0.44, p > 0.1).
Figure 3.
Frequency histogram of the area of individual Purkinje cell dendrites from anti-calbindin labeled wild type (WT; n = 88 cells from 6 cultures) and GluRδ2+/Lc mutant (+/Lc; n = 113 cells from 7 cultures) Purkinje cells from isolated cell and slice cultures at 14 DIV. The inset shows the average mean Purkinje cell size from all 6 GluRδ2+/+ and 7 GluRδ2+/Lc isolated cell and cerebellar slice cultures. The mean cell size is significantly reduced in GluRδ2+/Lc Purkinje cells compared with wild type Purkinje cells at 14 DIV (ANOVA, F1,11 = 16.1, p < 0.005).
To verify that wild type and mutant Purkinje cells in culture express the GluRδ2 receptor, a subsample of isolated cell cultures at 14 DIV (n= 3 GluRδ2+/+ and n= 3 GluRδ2+/Lc) were labeled with antibodies to GluRδ1/2. As shown in Figure 2 E and F, both GluRδ2+/+ and GluRδ2+/Lc Purkinje cells express the GluRδ2 receptor at 14 DIV. There is diffuse immunolabeling throughout the dendrites and cell bodies, but there are also areas of higher intensity labeling on the dendrites where the receptors may be clustering and there are many dendritic spine-like objects projecting from the distal dendrites. The white arrows in Figure 2 E and F indicate a few of the many dendritic spine-like projections that are immunolabeled for GluRδ2.
Purkinje cell leak currents in GluRδ2+/Lc and GluRδ2+/+ slice cultures
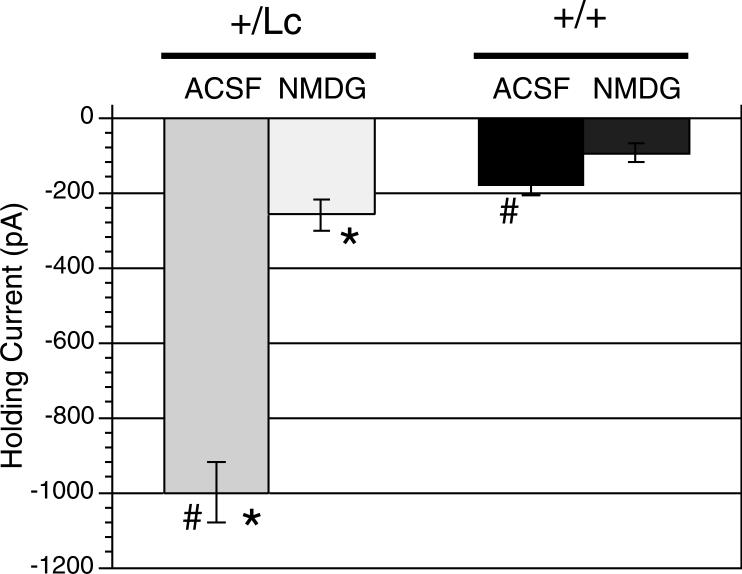
Previous studies have shown that GluRδ2+/Lc Purkinje cells demonstrate a large inward leak current beginning around P5 (Selimi et al., 2003). Because the timing of Purkinje cell death seems to be similar in vivo and in cell cultures (slice or dissociated), we wanted to test whether the leak current also was similar in slice cultures. Patch-clamp recordings (voltage-clamped at −70mV) were made from Purkinje cells in slice cultures from 11 to 14 DIV (Fig. 4). 11 DIV was the earliest time when L7-GFP was intense enough to reliably identify Purkinje cells in the slice cultures. Purkinje cells from GluRδ2+/Lc cultures had significantly larger leak currents in normal ACSF (−999.5 ± 78 pA; n = 19 cells from 14 animals) compared with wild type Purkinje cells (−178.0 ± 30 pA; n = 21 cells from 11 animals; Mann-Whitney U, p < 0.0001). The magnitude of the leak current in +/Lc Purkinje cells can not be attributed to poor patch clamp recordings since the holding currents were significantly reduced (almost 4-fold) in amplitude when NaCl in the normal external solution was replaced with NMDG (−258.5 ± 42 pA; Mann-Whitney U, p < 0.0001). Replacing NaCl with NMDG had a more moderate effect on the holding current of GluRδ2+/+ Purkinje cells (−178 ± 30 vs. −94.8 ± 24 pA; Mann-Whitney U, p < 0.039). While in NMDG treated slices, the leak current in +/Lc Purkinje cells was still significantly higher than in wild type Purkinje cells (−258.5 ± 42 vs −94.8 ± 24 pA; Mann-Whitney U, p < 0.0016), which could be due to a sustained Ca++ leak current in the GluRδ2Lc channel (Wollmuth et al., 2000). These results are consistent with the hypothesis that the mutant GluRδ2+/Lc receptor conveys a large cation leak current in +/Lc Purkinje cells and that the receptor is expressed in a similar manner in slice culture as it is in vivo.
Figure 4.
Average value of the holding current in Purkinje cells from wild-type (n= 21 cells from 11 animals) and +/Lc mice (n=19 cells from 14 animals) in slice cultures at 11−14 DIV. The mean holding current (± s.e.) is shown in ACSF and with NaCl replaced with NMDG. The magnitude of the mean holding current is significantly increased in GluRδ2+/Lc Purkinje cells in ACSF compared with wild type Purkinje cells (# comparison; Mann-Whitney U, p < 0.0001). However, the leak current is significantly reduced when NaCl is replaced by NMDG, indicating that the current was primarily a Na+ influx through an ion channel (* comparison; p < 0.0001, Mann-Whitney U test).
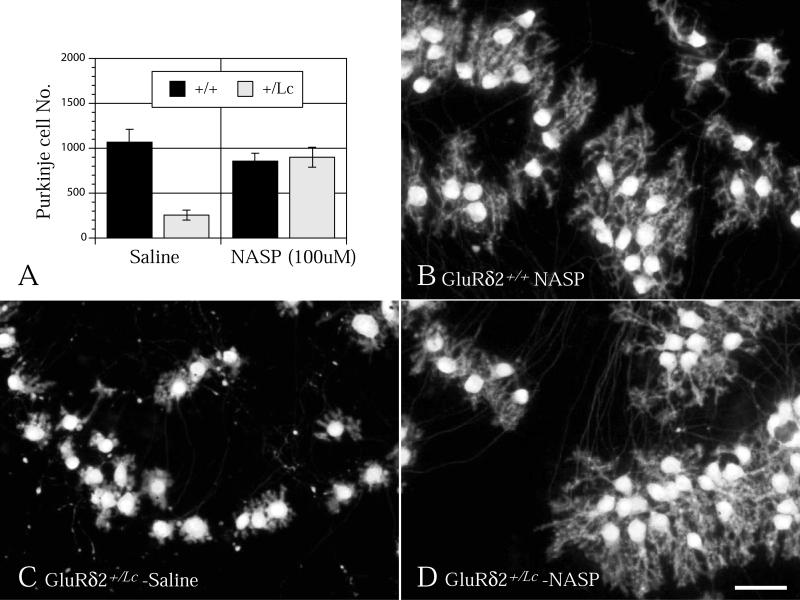
Increased GluRδ2+/Lc Purkinje cell survival following treatment with NASP
One prevailing hypothesis for the degeneration of GluRδ2+/Lc Purkinje cells is that the depolarizing leak current initiates excitotoxic pathways of cell death. To test this hypothesis, wild type and GluRδ2+/Lc slice cultures were treated continuously with NASP (100 μM) from the 1st to 14th day in vitro. NASP, a polyamine analog of Jora spider toxin, is an open-channel blocker of AMPA and kainate receptors (Koike et al., 1997) and it is one of the few glutamate channel antagonists that will significantly reduce the GluRδ2Lc leak current (Kohda et al., 2000). As shown in Figure 5, NASP treatment has significant genotype (two-way ANOVA, F1, 29 = 21.2, p < 0.0001) and drug treatment effects (two-way ANOVA, F1, 29 = 15.0, p < 0.001) as well as a significant interaction between genotype and NASP treatment (two-way ANOVA, F1, 29 = 24.8, p < 0.0001). Separate one-way ANOVAs (split by genotype) show that NASP treatment does not significantly affect the survival of GluRd2+/+ Purkinje cells (ANOVA F1, 17 = 0.8, p > 0.5), while GluRδ2+/Lc Purkinje cell survival (ANOVA, F1,12 = 29.4 , p < 0.0005) is significantly increased to match the number of surviving GluRd2+/+ Purkinje cells. In addition to promoting GluRδ2+/Lc Purkinje cell survival, qualitative assessments of Purkinje cell dendritic morphology in wild type and GluRδ2+/Lc Purkinje cell cultures suggest that chronic treatment with 100 μM NASP promotes GluRδ2+/Lc Purkinje cell dendritic differentiation. As shown in Figure 5, the dendrites of GluRδ2+/Lc Purkinje cells treated with NASP (Fig. 5D) for all two weeks in vitro closely resemble the more robust dendritic trees observed in cultured wild type Purkinje cells (Fig. 5B). Most of the NASP treated GluRδ2+/Lc Purkinje cells have one or two primary dendrites and a well developed field of secondary and tertiary dendrites. In contrast, many saline-treated GluRδ2+/Lc Purkinje cells at this stage are multipolar with short, stubby dendritic branches that fill substantially less area than wild type or NASP-treated Purkinje cells.
Figure 5.
A) Chronic treatment of cerebellar slice cultures with NASP (100 μM) from 1 to 14 DIV significantly increases GluRδ2+/Lc Purkinje cell survival (* ANOVA, F1,12 = 29.4 , p < 0.0005), but does not affect the survival of GluRδ2+/+ Purkinje cells. In addition, NASP treatment promotes dendritic differentiation in the mutant Purkinje cells (D) compared with untreated GluRδ2+/Lc Purkinje cells (C). The NASP treated GluRδ2+/Lc Purkinje cells appear similar to NASP treated wild type Purkinje cells (B). Scale bar 50 μM.
DISCUSSION
These in vitro studies demonstrate that the phenotype of GluRδ2+/Lc Purkinje cells is preserved when grown either in isolated or tissue slice cultures culture; their dendrites are stunted and they die significantly more quickly than +/+ Purkinje cells. Our qualitative and quantitative descriptions of GluRδ2+/Lc and GluRδ2+/+ Purkinje cell dendritic differentiation are consistent with the previous study by Doughty et al. (1995), but the cell count data from this study shows that the Lurcher mutation in the GluRδ2 receptor prematurely kill Purkinje cells even when grown in tissue culture. Furthermore, treatment with an GluRδ2Lc channel antagonist, NASP, significantly increases GluRδ2+/Lc Purkinje cell survival and appears to promote dendritic differentiation in the mutant Purkinje cells. It was previously argued that GluRδ2+/Lc Purkinje cell death may be due to changes in the developmental pattern of innervation by excitatory climbing fibers that leads to Purkinje cell death (Doughty et al., 1995). Thus, in slice cultures without innervation from olivary neurons, GluRδ2+/Lc Purkinje cell death will not be induced prematurely. However, the current findings for accelerated GluRδ2+/Lc Purkinje cell death in vitro compared with controls is more consistent with cell autonomous mechanisms of cell death that involve the molecular changes in the GluRδ2 receptor caused by the Lurcher mutation.
There are a number of reasons that may account for the differences in Purkinje cell survival observed between this study and the previous study by Doughty et al. (Doughty et al., 1995). In the previous paper Purkinje cell survival data was pooled from 10 to 20 DIV, but our results from the isolated cell cultures suggest that overall Purkinje cell survival decreases with time in culture. Thus, by pooling data from a 10-day period, the authors may have missed time-point dependent differences in GluRδ2+/+ and GluRδ2+/Lc survival, especially if overall survival rates were dramatically reduced in older cultures.
The disparity in results between the two studies may also be attributed to differences in culture conditions. The overall Purkinje cell survival rate was lower in the previous study; the mean density of both GluRδ2+/+ and GluRδ2+/Lc Purkinje cells was around 6 Purkinje cells per mm2 compared with a mean density of 13 to 40 Purkinje cells per mm2 in the GluRδ2+/Lc and GluRδ2+/+ cultures calculated in this study, respectively. Since the previous study was conducted before the mutant gene was identified, the authors had to rely on phenotypic markers closely linked to the Lurcher gene (microphthalmia and white coat color, Miwt) to independently identify the genotype of the GluRδ2+/+ and GluRδ2+/Lc pups. The phenotypic differences between genotypes are not apparent until 2 days after birth, so the Purkinje cell slice cultures were started from P2 pups as opposed to P0 pups in the current study. Recent studies of cerebellar slice cultures have shown that Purkinje cells are particularly sensitive to increased cell death when cultured from P1 through P5. Relatively few Purkinje cells survive when taken from P1 to P5 pups as opposed to embryos or P0 pups (Dusart et al., 1997; Ghoumari et al., 2000; Ghoumari et al., 2006). In addition, the cultures in Doughty et al. (Doughty et al., 1995) were grown in relatively high potassium concentrations (15 mM). While high potassium concentrations are known to promote granule cell survival (Gallo et al., 1987; Galli et al., 1995), Purkinje cells grown in vitro are usually susceptible to increased death in high potassium media (unpublished observations; M. Morrison, personal communication; (Chen et al., 2005)). There are some exceptions, however;. Cohen-Corey et al (Cohen-Cory et al., 1991) reported that the survival of rat Purkinje cells in vitro is promoted by high potassium. More recently, Ghoumari et al. (Ghoumari et al., 2006) found that high potassium levels and other depolarizing agents promote the survival of Purkinje cells in cerebellar slices from P3 rat pups. Purkinje cell differentiation and survival is strongly dependent on interactions with granule cells in vitro (Baptista et al., 1994) so it is not clear in any of these studies whether depolarization by high potassium levels is directly affecting granule cell or Purkinje cell differentiation and survival. With respect to the slice culture studies of GluRδ2+/Lc Purkinje cell survival by Doughty et al. (Doughty et al., 1995), it is possible that the combination of starting with cerebellar slices from P2 pups and growing the cultures in high potassium may have reduced overall Purkinje cell survival rates so it was not possible to distinguish between the survival rates of wild type and mutant Purkinje cells. Furthermore, since GluRδ2+/Lc Purkinje cell death has been linked with excitotoxicity due to their chronic inward current, chronically depolarizing both GluRδ2+/+ and GluRδ2+/Lc Purkinje cells with high potassium levels during development in culture may have obscured their separate capacities for survival.
In light of this re-examination of GluRδ2+/Lc Purkinje cell differentiation and death in vitro, the available evidence suggests that the Lurcher phenotype of stunted dendritic differentiation and cell death is cell autonomous and recapitulated in vitro. The Lurcher mutation in GluRδ2 is a base-pair substitution that changes an alanine to threonine in the highly conserved third hydrophobic segment of Grid2 and converts the receptor into a leaky membrane channel that constitutively opens an inward cation current (Zuo et al., 1997). In the adult mouse, wild type GluRδ2 receptors are preferentially expressed in Purkinje cells and they are concentrated at Purkinje cell:parallel fiber synapses in dendritic spines where they appear to regulate AMPA receptor density in relation to the induction of LTD (Kashiwabuchi et al., 1995; Lalouette et al., 2001; Hirai et al., 2003). Previous in vitro studies demonstrate that GluRδ2 receptors are expressed in cultured Purkinje cells (Hirai and Matsuda, 1999; Hirai, 2000; Hirai, 2001) and they are concentrated at Purkinje dendritic spines via an activity dependent mechanism (Hirai, 2001). A major consequence of the expression of the GluRδ2+/Lc channel is the chronic depolarization of mutant Purkinje cells followed by impaired differentiation and subsequent death (Caddy and Biscoe, 1979; Zuo et al., 1997). We have confirmed by patch-clamping recordings that this depolarization also occurs in +/Lc Purkinje cells in slice cultures. The cation leak current is almost 5 times as large in cultured +/Lc Purkinje cells as in wild type cells and it is substantially reduced by blocking Na+ influx with NMDG. In vivo, the timing of the GluRδ2 receptor translocation to the parallel-fiber synapse roughly correlates with the appearance of the chronic leak current and the initiation of +/Lc Purkinje cell abnormalities and death towards the end of the first week of postnatal development (Swisher and Wilson, 1977; Caddy and Biscoe, 1979; Selimi et al., 2003). The results of both this study and Doughty et al (Doughty et al., 1995) suggest that GluRδ2+/Lc Purkinje cells begin to develop normally in vitro. No differences in Purkinje cell survival and morphology were detected at 7DIV in this study and Doughty et al. (Doughty et al., 1995) only report detecting differences in GluRδ2+/+ and GluRδ2+/Lc Purkinje cell morphology after 10−11 days in vitro. By 14−15 DIV there are recognizable differences in the appearance of GluRδ2+/Lc Purkinje cell dendrites in both studies. Finally, as shown in this study, GluRδ2+/Lc Purkinje cells begin to degenerate after the first week of differentiation in vitro. It seems likely that the mechanisms of Purkinje cell death induced by the GluRδ2+/Lc receptor in vitro are similar to the in vivo mechanisms.
Although the degeneration of GluRδ2+/Lc Purkinje cells would appear to be a simple model of cell death there are still many unresolved questions about the mechanisms of cell death and injury. The discovery that Lurcher is a single gene mutation in a membrane receptor that converts it into an open cation channel suggested that +/Lc Purkinje cells die by an excitotoxic mechanism that triggers apoptosis (Zuo et al., 1997). Apoptotic pathways have been implicated on the basis of TUNEL labeling (Norman et al., 1995; Wullner et al., 1995; Selimi et al., 2000), activated caspase expression (Selimi et al., 2000; Lu and Tsirka, 2002), and the ability of overexpression or deletion of Bcl-2 related proteins to alter the timing of Purkinje cell death (Zanjani et al., 1998; Zanjani et al., 1998; Doughty et al., 2000; Selimi et al., 2000; Bouillet et al., 2003). However, there is evidence that excess autophagy may also play a role based on the linkage of GluRδ2 receptors with beclin (Yue et al., 2002) and the accumulation of autophagosomes in GluRδ2+/Lc Purkinje cell axons (Wang et al., 2006). It is not clear if the increased autophagy observed in +/Lc Purkinje cell axons is due to the chronic depolarization of the cell body or is directly linked to release of beclin from the mutant GluRδ2Lc receptors. The association between the leak current and GluRδ2+/Lc Purkinje cell death has been questioned both on the basis of the association between the GluRδ2 receptor and beclin (Orr, 2002) and on the death of many Purkinje cells in the Lurcher-hotfoot double mutant before the appearance of the GluRδ2+/Lc leak current (Selimi et al., 2003). However, treatment of +/Lc Purkinje cell slice cultures with 100 μM NASP significantly increases Purkinje cell survival to levels comparable to wild type numbers. Although NASP may not completely block the GluRδ2+/Lc leak current (Kohda et al., 2000), the ability of NASP treatment to increase GluRδ2+/Lc Purkinje cell survival and promote dendritic differentiation supports the hypothesis that their dendritic abnormalities and premature death is associated with the GluRδ2+/Lc leak current.
The available evidence suggests that there are multiple interacting pathways of cell injury and death in this seemingly simple system (see review in (Vogel et al., 2007) and it will be a complex problem to resolve. However, the system is still relatively simple compared with other in vivo and in vitro models of cell death in that the Lurcher mutant is a single gene mutation that causes cell autonomous defects in differentiation and death in a single, well characterized cerebellar cell type. The establishment of a tissue culture system for studying cell injury and death mechanisms in a relatively simple system like GluRδ2+/Lc Purkinje cells will provide a valuable model for studying how the induction of a chronic inward cation current in a single cell type affects dendritic differentiation and neuronal survival.
Acknowledgements
This work was supported by NIH grant NS 34309 to M.W.V. and J.M. and by a grant from FRC (Federation de Recherche sur le Cerveau) to J.M..
Other Acknowledgments.
We would like to thank Dr. Mary Morrison for her helpful advice with Purkinje cell culture techniques and Ann Lohof for her encouragement and critical evaluation of the manuscript.
REFERENCES
- Araki K, Meguro H, Kushiya E, Takayama C, Inoue Y, Mishina M. Selective expression of the glutamate receptor channel d2 subunit in cerebellar Purkinje cells. Biochem Biophys Res Comm. 1993;197:1267–1276. doi: 10.1006/bbrc.1993.2614. [DOI] [PubMed] [Google Scholar]
- Armengol J-A, Sotelo C. Early dendritic development of Purkinje cells in the rat cerebellum. A light and electron microscopic study using axonal tracing in ‘in vitro’ slices. Developmental Brain Research. 1991;64:95–114. doi: 10.1016/0165-3806(91)90213-3. [DOI] [PubMed] [Google Scholar]
- Armstrong CL, Vogel MW, Hawkes R. Development of Hsp25 expression compartments is not constrained by Purkinje cell defects in the Lurcher mouse mutant. J Comp Neurol. 2005;491:69–78. doi: 10.1002/cne.20703. [DOI] [PubMed] [Google Scholar]
- Baptista CA, Hatten ME, Blazeski R, Mason CA. Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro. Neuron. 1994;12:243–260. doi: 10.1016/0896-6273(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Robati M, Adams JM, Strasser A. Loss of pro-apoptotic BH3-only Bcl-2 family member Bim does not protect mutant Lurcher mice from neurodegeneration. J Neurosci Res. 2003;74:777–781. doi: 10.1002/jnr.10805. [DOI] [PubMed] [Google Scholar]
- Boukhtouche F, Janmaat S, Vodjdani G, Gautheron V, Mallet J, Dusart I, Mariani J. Retinoid-related orphan receptor alpha controls the early steps of Purkinje cell dendritic differentiation. J Neurosci. 2006;26:1531–1538. doi: 10.1523/JNEUROSCI.4636-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddy KWT, Biscoe TJ. Structural and quantitative studies on the normal C3H and Lurcher mutant mouse. Phil. Trans. Roy. Soc. Lond. B. 1979;287:167–201. doi: 10.1098/rstb.1979.0055. [DOI] [PubMed] [Google Scholar]
- Chen S, Hirata K, Ren Y, Sugimori M, Llinas R, Hillman DE. Robust axonal sprouting and synaptogenesis in organotypic slice cultures of rat cerebellum exposed to increased potassium chloride. Brain Res. 2005;1057:88–97. doi: 10.1016/j.brainres.2005.07.039. [DOI] [PubMed] [Google Scholar]
- Cheng SSW, Heintz N. Massive loss of mid- and hindbrain neurons during embryonic development of homozygous Lurcher mice. J. Neurosci. 1997;17:2400–2407. doi: 10.1523/JNEUROSCI.17-07-02400.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, Dreyfus CF, Black IB. NGF and excitatory neurotransmitters regulate survival and morphogenesis of cultured cerebellar Purkinje cells. Journal of Neuroscience. 1991;11:462–471. doi: 10.1523/JNEUROSCI.11-02-00462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty ML, De Jager PL, Korsmeyer SJ, Heintz N. Neurodegeneration in Lurcher mice occurs via multiple cell death pathways. J Neurosci. 2000;20:3687–3694. doi: 10.1523/JNEUROSCI.20-10-03687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty ML, Patterson L, Caddy KWT. Cerebellar Purkinje cells from the lurcher mutant and wild-type mouse grown in vitro: a light and electron microscope study. J. Comp. Neurol. 1995;357:161–179. doi: 10.1002/cne.903570114. [DOI] [PubMed] [Google Scholar]
- Dusart I, Airaksinen MS, Sotelo C. Purkinje cell survival and axonal regeneration are age dependent: an in vitro study. J Neurosci. 1997;17:3710–3726. doi: 10.1523/JNEUROSCI.17-10-03710.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya S, Makino A, Hirabayashi Y. An improved method for culturing cerebellar Purkinje cells with differentiated dendrites under a mixed monolayer setting. Brain Res. Brain Res. Protoc. 1998;3:192–198. doi: 10.1016/s1385-299x(98)00040-3. [DOI] [PubMed] [Google Scholar]
- Galli C, Meucci O, Scorziello A, Werge TM, Calissano P, Schettini G. Apoptosis in cerebellar granule cells is blocked by high KCl, forskolin, and IGF-1 through distinct mechanisms of action: the involvement of intracellular calcium and RNA synthesis. J Neurosci. 1995;15:1172–1179. doi: 10.1523/JNEUROSCI.15-02-01172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Kingsbury A, Balazs R, Jorgensen OS. The role of depolarization in the survival and differentiation of cerebellar granule cells in culture. J Neurosci. 1987;7:2203–2213. doi: 10.1523/JNEUROSCI.07-07-02203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoumari AM, Piochon C, Tomkiewicz C, Eychenne B, Levenes C, Dusart I, Schumacher M, Baulieu EE. Neuroprotective effect of mifepristone involves neuron depolarization. Faseb J. 2006;20:1377–1386. doi: 10.1096/fj.05-5832com. [DOI] [PubMed] [Google Scholar]
- Ghoumari AM, Wehrle R, Bernard O, Sotelo C, Dusart I. Implication of Bcl-2 and Caspase-3 in age-related Purkinje cell death in murine organotypic culture: an in vitro model to study apoptosis. Eur J Neurosci. 2000;12:2935–2949. doi: 10.1046/j.1460-9568.2000.00186.x. [DOI] [PubMed] [Google Scholar]
- Hatten ME, Gao W-Q, Morrison ME, Mason CA. The Cerebellum: Purification and coculture of identified cell populations. In: Banker G, Goslin K, Banker G, Goslin Ks, editors. Culturing Nerve Cells. MIT Press; Boston: 1988. [Google Scholar]
- Hirai H. Clustering of delta glutamate receptors is regulated by the actin cytoskeleton in the dendritic spines of cultured rat Purkinje cells. Eur J Neurosci. 2000;12:563–570. doi: 10.1046/j.1460-9568.2000.00938.x. [DOI] [PubMed] [Google Scholar]
- Hirai H. Ca2+-dependent regulation of synaptic delta2 glutamate receptor density in cultured rat Purkinje neurons. Eur J Neurosci. 2001;14:73–82. doi: 10.1046/j.0953-816x.2001.01630.x. [DOI] [PubMed] [Google Scholar]
- Hirai H, Launey T, Mikawa S, Torashima T, Yanagihara D, Kasaura T, Miyamoto A, Yuzaki M. New role of delta2-glutamate receptors in AMPA receptor trafficking and cerebellar function. Nat Neurosci. 2003;6:869–876. doi: 10.1038/nn1086. [DOI] [PubMed] [Google Scholar]
- Hirai H, Matsuda S. Interaction of the C-terminal domain of delta glutamate receptor with spectrin in the dendritic spines of cultured Purkinje cells. Neurosci Res. 1999;34:281–287. doi: 10.1016/s0168-0102(99)00061-9. [DOI] [PubMed] [Google Scholar]
- Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Yagi T, Kang Y. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. al. e. [DOI] [PubMed] [Google Scholar]
- Kohda K, Wang Y, Yuzaki M. Mutation of a glutamate receptor motif reveals its role in gating and delta2 receptor channel properties. Nat Neurosci. 2000;3:315–322. doi: 10.1038/73877. [DOI] [PubMed] [Google Scholar]
- Koike M, Iino M, Ozawa S. Blocking effect of 1-naphthyl acetyl spermine on Ca(2+)-permeable AMPA receptors in cultured rat hippocampal neurons. Neurosci Res. 1997;29:27–36. doi: 10.1016/s0168-0102(97)00067-9. [DOI] [PubMed] [Google Scholar]
- Lalouette A, Lohof A, Sotelo C, Guenet J, Mariani J. Neurobiological effects of a null mutation depend on genetic context: comparison between two hotfoot alleles of the delta-2 ionotropic glutamate receptor. Neuroscience. 2001;105:443–455. doi: 10.1016/s0306-4522(01)00193-2. [DOI] [PubMed] [Google Scholar]
- Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomeli H, Sprengel R, Lauris DJ, Kohr G, Herb A, Seeburg PH, Wisden W. The rat delta-1 and delta-2 subunits extend the excitatory amino acid receptor family. FEBS Lett. 1993;315:318–322. doi: 10.1016/0014-5793(93)81186-4. [DOI] [PubMed] [Google Scholar]
- Lu W, Tsirka SE. Patial rescue of neural apoptosis in the Lurcher mutant mouse through elimination of tissue plasminogen activator. Development. 2002;129:2043–2050. doi: 10.1242/dev.129.8.2043. [DOI] [PubMed] [Google Scholar]
- Mayat E, Petralia RS, Wang Y-X, Wenthold RJ. Immunoprecipitation, immunoblotting, and immunocytochemistry studies suggest that glutamate receptor d subunits form novel postsynaptic receptor complexes. J. Neurosci. 1995;15:2533–2546. doi: 10.1523/JNEUROSCI.15-03-02533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland R, Blokhin A, Sydnor J, Mariani J, Vogel MW. Oxidative stress, nitric oxide, and the mechanisms of cell death in Lurcher Purkinje cells. Devel. Neurobio. 2007;67:1032–1046. doi: 10.1002/dneu.20391. [DOI] [PubMed] [Google Scholar]
- Norman DJ, Feng L, Cheng SS, Gubbay J, Chan E, Heintz N. The lurcher gene induces apoptotic death in cerebellar Purkinje cells. Development. 1995;121:1183–1193. doi: 10.1242/dev.121.4.1183. [DOI] [PubMed] [Google Scholar]
- Orr HT. Lurcher, nPIST, and autophagy. Neuron. 2002;35:813–814. doi: 10.1016/s0896-6273(02)00871-1. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Selimi F, Doughty M, Delhaye-Bouchaud N, Mariani J. Target-related and intrinsic neuronal death in Lurcher mutant mice are both mediated by caspase-3 activation. J. Neurosci. 2000;20:992–1000. doi: 10.1523/JNEUROSCI.20-03-00992.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selimi F, Lohof AM, Heitz S, Lalouette A, Jarvis CI, Bailly Y, Mariani J. Lurcher GRID2-Induced Death and Depolarization Can Be Dissociated in Cerebellar Purkinje Cells. Neuron. 2003;37:813–819. doi: 10.1016/s0896-6273(03)00093-x. [DOI] [PubMed] [Google Scholar]
- Selimi F, Vogel MW, Mariani J. Bax inactivation in Lurcher mutants rescues cerebellar granule cells but not Purkinje cells or inferior olivary neurons. J Neurosci. 2000;20:5339–5345. doi: 10.1523/JNEUROSCI.20-14-05339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher DA, Wilson DB. Cerebellar histogenesis in the Lurcher (Lc ) mutant mouse. J. Comp. Neurol. 1977;173:205–217. doi: 10.1002/cne.901730112. [DOI] [PubMed] [Google Scholar]
- Takayama C, Nakagawa S, Watanabe M, Mishina M, Inoue Y. Developmental changes in expression and distribution of the glutamate receptor channel d2 subunit according to the Purkinje cell maturation. Devel. Brain Res. 1996;92:147–155. doi: 10.1016/0165-3806(95)00212-x. [DOI] [PubMed] [Google Scholar]
- Tano D, Napieralski JA, Eisenman LM, Messer A, Plummer J, Hawkes R. Novel developmental boundary in the cerebellum revealed by Zebrin expression in the lurcher (Lc/+) mutant mouse. Journal of Comparative Neurology. 1992;323:128–136. doi: 10.1002/cne.903230111. [DOI] [PubMed] [Google Scholar]
- Tomomura M, Rice DS, Morgan JI, Yuzaki M. Purification of Purkinje cells by fluorescence-activated cell sorting from transgenic mice that express green fluorescent protein. Eur J Neurosci. 2001;14:57–63. doi: 10.1046/j.0953-816x.2001.01624.x. [DOI] [PubMed] [Google Scholar]
- Vogel MW, Caston J, Yuzaki M, Mariani J. The Lurcher mouse: Fresh insights from an old mutant. Brain Res. 2007;1140:4–18. doi: 10.1016/j.brainres.2005.11.086. [DOI] [PubMed] [Google Scholar]
- Wang QJ, Ding Y, Kohtz DS, Mizushima N, Cristea IM, Rout MP, Chait BT, Zhong Y, Heintz N, Yue Z. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci. 2006;26:8057–8068. doi: 10.1523/JNEUROSCI.2261-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetts R, Herrup K. Interaction of granule, Purkinje and inferior olivary neurons in lurcher chimeric mice. I. Qualitative studies. J. Embryol. Exp. Morphol. 1982;68:87–98. [PubMed] [Google Scholar]
- Wetts R, Herrup K. Interaction of granule, Purkinje and inferior olivary neurons in lurcher chimeric mice. II. Granule cell death. Brain Res. 1982;250:358–363. doi: 10.1016/0006-8993(82)90431-0. [DOI] [PubMed] [Google Scholar]
- Wollmuth LP, Kuner T, Jatzke C, Seeburg PH, Heintz N, Zuo J. The Lurcher mutation identifies delta 2 as an AMPA/kainate receptor-like channel that is potentiated by Ca(2+). J Neurosci. 2000;20:5973–5980. doi: 10.1523/JNEUROSCI.20-16-05973.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullner U, Loschmann P-A, Weller M, Klockgether T. Apoptotic cell death in the cerebellum of mutant weaver and lurcher mice. Neurosci. Lett. 1995;200:109–112. doi: 10.1016/0304-3940(95)12090-q. [DOI] [PubMed] [Google Scholar]
- Yue Z, Horton A, Bravin M, DeJager PL, Selimi F, Heintz N. A novel protein complex linking the delta 2 glutamate receptor and autophagy: implications for neurodegeneration in lurcher mice. Neuron. 2002;35:921–933. doi: 10.1016/s0896-6273(02)00861-9. [DOI] [PubMed] [Google Scholar]
- Zanjani HS, Rondi-Reig L, Vogel MW, Martinou JC, Delhaye-Bouchaud N, Mariani J. Overexpression of a Hu-Bcl-2 gene in Lurcher mutant mice delays Purkinje cell death. Comptes Rendus Acad. Sci. 1998;321:633–640. doi: 10.1016/s0764-4469(98)80002-4. [DOI] [PubMed] [Google Scholar]
- Zanjani HS, Vogel MW, Martinou JC, Delhaye-Bouchaud N, Mariani J. Postnatal expression of Hu-Bcl-2 gene in Lurcher mutant mice fails to rescue Purkinje cells, but protects inferior olivary neurons from target related cell death. J. Neurosci. 1998;18:319–327. doi: 10.1523/JNEUROSCI.18-01-00319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, De Jager PL, Takahashi KA, Jiang W, Linden DJ, Heintz N. Neurodegeneration in Lurcher mice caused by mutation in δ2 glutamate receptor. Nature. 1997;388:769–773. doi: 10.1038/42009. [DOI] [PubMed] [Google Scholar]