Abstract
The research was carried out in the spruce forests of Barania Góra (Silesian Beskids, Poland) affected by pandemic dying of trees. Twenty-seven samples were collected from the O layer in two plots: 17 in a cut down forest infested with insect pests (bark beetle) and ten in a 120-year-old healthy forest. The analyses covered basic parameters (pHH2O, pHKCl, worg, Ctot, Ntot, CEC) and the concentrations of aluminium in the fractions leached with 0.1 M BaCl2 (Alexch), 0.5 M CuCl2 and 0.1 M Na4P2O7 (Albio) solutions. The total aluminium concentration in the soil was assayed digesting samples with hydrofluoric acid. The effect of pH and organic matter content on the amount of exchangeable (Alexch) and bioavailable (Alexch) aluminium in the soil was analysed. It has been found that the soils in both plots are strongly acidic and contain 550 to 1,700 mg kg−1 of exchangeable aluminium and 1,200 to 4,800 mg kg−1 of bioavailable aluminium. The lack of disease symptoms in the spruce trees in plot 2 can be explained by the higher content of organic matter in the soil. Unfortunately, one might expect that the high concentration of exchangeable aluminium will also cause the trees in the area to wither.
Keywords: Forest soil, Mobile aluminium, Speciation analysis
Introduction
Barania Góra (1,220 m above sea level) is the second highest mountain in the Silesian Beskids range, part of the Western Carpathians. It stretches mainly south and northward and is a watershed between the Vistula valley from the west and the Soła valley from the east. The rock body of its substantial part is composed of Godula sandstone and the massif itself is made up of Istebna sandstone. The climate in the Silesian Beskids is characterized by relatively heavy rainfalls (800–1,200 mm year−1) and snowfalls. The weather is under the influence of maritime polar air masses. The average annual temperature at the ridge reaches 5.4°C. The climate is significantly affected by strong winds, and those blowing from the west and north-west predominate the peak parts of Barania Góra.
The area has been exposed to acid rain for years. Barania Góra lies adjacent to the Trzyniec smelting plant in the Czech Republic and is in close proximity to Katowice conurbation and large industrial plants, including coal-fired power stations (Rybnik Industrial Region in Poland and Ostrava and Karvina Region in the Czech Republic). It is also attractive in terms of tourism, which is the reason of the heavy traffic. The annual average concentrations of ions in the precipitation are as follows: NO3 = 2.0–2.1 mg dm−3 and SO4 = 3.45–4.21 mg dm−3 (Staszewski 2004). The forests of Barania Góra are dominated by spruce monocultures started in the nineteenth century. Since the seeds were imported from the Alpine regions (others as well), the local and foreign spruce species intermingled.
Recently, the area of Barania Góra and the entire Silesian Beskids has been experiencing a deterioration in spruce stand. The youngest sprouts are turning yellow and shedding needles, crowns are getting thinner and in extreme cases, the trees wither. The area has been suffering an increasing plague of pests, e.g., bark beetle, armillaria and the like. In order to save healthy stands, large stretches of infested forest are cut down. Other parts of the world have experienced similar problems (USA, Canada, China, France, Germany, Scandinavia), including Poland—the Izera Mountains in the second half of the twentieth century and Opole Region in the 1990s.
Investigations into the pandemic dying of spruce forests are carried out in a number of countries. A series of works published by Czech researchers over 1999–2007 period (Borůvka et al. 2005a, b, c; Mládková et al. 2004, 2006; Musil and Pavliček 2002) was of special interest to us since they focused on the mountainous areas located in close proximity to the Żywiec and Silesian Beskids. In the Czech Republic, the degradation of spruce forest occurred in various regions of the country: the Jizerske Mountains, Beskids, Ore Mountains, Krusne Mountains. The authors point to the release of aluminium ions, toxic to coniferous trees in particular, as the main cause of forest degradation.
In Poland, the most extensive research in this field until now was carried out in the 1990s and covered the determination of different aluminium fractions in river and lake waters (Kotowski et al. 1994, 1995) and the soil solution itself (Smal and Misztal 1996). This work is aimed at assessing the quantity and forms of aluminium occurring in the soils of the Barania Góra range.
Experimental
The acidity of the soil in the area is caused primarily by anthropogenic factors the surface layer of the soil is particularly vulnerable to. The changes in the mineral layer are controlled mainly by the processes of soil formation (Borůvka et al. 2005a). Therefore, the soil samples were collected in the O layers in spruce forests and mixed spruce–beech forests, spruce being predominant.
Overall, twenty-seven soil samples were collected. Seventeen were taken along the traverse of the mountain (Istebna Forest District), in the area of a cut down spruce forest infested with insect pests (bark beetles). Three soil samples were collected at the same height in a 300-m strip of land (at 100-m intervals). The sampling height ranged from 690 to 850 m. Ten samples were collected in a healthy 120-year-old spruce forest in the Bukowiec Forest District—also along the traverse of the mountain at 645–720 m. Three reference samples were collected, one in a 60-year-old spruce forest in Koszęcin and two in Sądecki Beskid—a spruce enclave in a mixed forest at 750 m.
Known techniques (Drábek et al. 2003; Ostrowska et al. 1991; Polish Standard PN-ISO 11260:1999) were used to determine: pH in water pHH20 and 1 M KCl solution pHKCl; organic weight w org [% w]; total carbon Ctot [% w] and total nitrogen Ntot [% w]; cation exchange capacity CEC [cmolc kg−1]; contents of calcium Caexch [mg kg−1], magnesium Mgexch [mg kg−1], potassium Kexch [mg kg−1], iron Feexch [mg kg−1] and manganese Mnexch [mg kg−1] in the exchangeable fraction; total content of calcium Catot [mg kg−1] and magnesium Mgtot [mg kg−1]; aluminium in fractions leached with barium chloride AlBaCl2 [mg kg−1], copper chloride(II) AlCuCl2 [mg kg−1] and sodium pyrophosphate AlNa4P207 [mg kg−1] and finally total aluminium Altot [mg kg−1] after the samples had been digested in hydrofluoric and hydrochloric acids.
The following aluminium forms described by (Drábek et al. 2003; Walna et al. 2005) were assumed: exchangeable (Alexch), weakly organically bound (AlorgI), organically bound (Alorg), bioavailable (Albio) and total (Altot), which were then assayed by speciation analysis (Fig. 1). The notation was as follows:
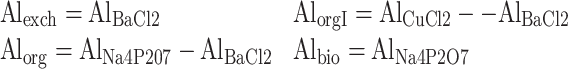 |
Each assay was repeated at least three times. The results are given in Table 1.
Fig. 1.
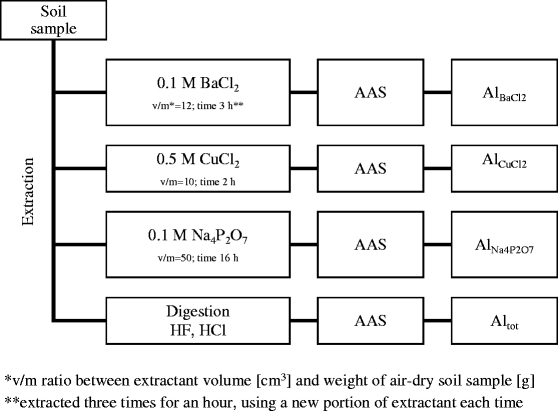
Schematic of assays of selected aluminium forms in soil. Single asterisk refers to v/m ratio between extractant volume [cm3] and weight of air-dry soil sample [g]. Double asterisks represent extraction of three times for an hour, using a new portion of extractant each time
Table 1.
Range, average and median of parameters measured in the soil of Barania Góra range
| Parameter | Istebna | Bukowiec | SD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample no. | Range | Average | Median | Sample no. | Range | Average | Median | ||
| pHH2O | 17 | 3.39–3.91 | 3.66 | 3.65 | 10 | 3.35–3.69 | 3.57 | 3.59 | 0.02 |
| pHKCl | 2.79–3.39 | 3.02 | 3.12 | 2.57–2.91 | 2.80 | 2.80 | 0.02 | ||
| w org (%) | 11.9–63.4 | 36.7 | 31.6 | 17.4–84.8 | 44.14 | 41.45 | 1 | ||
| Ctot (%) | 5.02–32.4 | 17.8 | 14.2 | 9.71–44 | 22.85 | 21.4 | 0.05 | ||
| Ntot (%) | 0.18–1.46 | 0.78 | 0.68 | 0.32–1.32 | 0.83 | 0.84 | 0.04 | ||
| CEC cmolc (kg−1) | 9.48–22.41 | 16.13 | 15.61 | 9.67–20.71 | 15.60 | 15.78 | 1.5 | ||
| Caexch (mg kg−1) | 14 | 22–563 | 161 | 110 | 9 | 28–703 | 265 | 224 | |
| Mgexch (mg kg−1) | 17 | 13–140 | 61 | 52 | 10 | 16–68 | 40 | 38 | |
| Kexch (mg kg−1) | 37–251 | 148 | 143 | 70–193 | 131 | 126 | |||
| Feexch (mg kg−1) | 68–269 | 144 | 128 | 79–176 | 112 | 102 | |||
| Mnexch (mg kg−1) | 2.69–28.84 | 12.11 | 7.73 | 0.87–33.2 | 16.35 | 14.35 | |||
| Catot (mg kg−1) | 125–1,797 | 581 | 337 | 216–771 | 527 | 536 | |||
| Mgtot (mg kg−1) | 949–5,840 | 2,481 | 1,784 | 513–1,330 | 1,028 | 1,062 | |||
| Alexch (mg kg−1) | 639–1,716 | 1,101 | 1,003 | 548–1,062 | 800 | 786 | 25 | ||
| AlCuCl2 (mg kg−1) | 893–1,969 | 1,435 | 1,328 | 868–1,711 | 1,188 | 1,156 | 62 | ||
| AlNa4P2O4 (Albio, mg kg−1) | 1,804–4,805 | 2,731 | 2,359 | 1,177–2,843 | 1,782 | 1,734 | 93 | ||
| AlorgI (mg kg−1) | 0–584 | 338 | 402 | 219–648 | 360 | 388 | |||
| Alorg (mg kg−1) | 846–3,342 | 1,630 | 1,459 | 528–1,781 | 982 | 883 | |||
| Altot, (g kg−1) | 23.1–82.2 | 50.4 | 44.8 | 33.5–85.4 | 23.2 | 24.4 | 0.5 | ||
| Height(m) above sea level | 690–850 | 645–720 | |||||||
Results and Discussion
The soils were strongly acidic. The pHH2O and pHKCl in the O layer changed from 3.55 to 3.91 and from 2.57 to 3.39, respectively. The higher the content of humus (which has strong sorptive properties) in the soil, the higher the difference ΔpH = pHH2O − pHKCl. The soils collected in the Bukowiec Forest District are more acidic than in Istebna. The difference between average pHKCl values in the samples taken in Istebna and Bukowiec was statistically significant (ΔpHKCl = 0.22 F = 3.45 < F α=0.01 = 4.92, t = 3.776 > t α=0.01 = 2.787). The area did not reveal any marked effects of the sampling height on the changes in soil pH. The concentrations of organic matter w org, total carbon Ctot and nitrogen Ntot correlated strongly, which indirectly indicated a low carbonate content in the soils. The high variability in organic matter content in the soil samples (from 10% to 90%) and Ctot/Ntot [g/g] ratio is worth mentioning. In Bukowiec, on average, the soil contained more organic matter and was characterized by a higher Ctot/Ntot ratio. A weak negative correlation between Ctot/Ntot and the sampling height was found (Smoliński et al. 2009). The cation exchange capacity (CEC) changed over 9.48–22.41. Obviously, the higher organic matter content implies the higher CEC. All the samples showed that aluminium content in the cation exchange complex (Alexch/(Caexch + Mgexch + Feexch + Mnexch + Kexch + Alexch) [(cmolc kg−1)/(cmolc kg−1)]) was higher than 50%, and it exceeded 80% in a number of samples, especially those collected in Istebna.
Exchangeable Aluminium
The soils contained over 100 mg kg−1 of exchangeable aluminium Alexch, a toxic dose to coniferous trees (Motowicka-Terelak and Stuczyński 1993). Its concentrations in soil are primarily affected by its acidity (Driscoll and Schecher 1988; Illmer et al. 2003; Simonsson 2000; van Hees et al. 2001), which was confirmed by our results (Fig. 2). An increase in soil acidity brought an exponential increase in aluminium concentration. It is interesting that the amount of exchangeable aluminium in the forest soil at the same pH was smaller in the samples collected in Bukowiec than Istebna. The difference is better seen for Alexch = f(pHKCl). It seems that in the soils collected in Bukowiec, the negative effect of acidity was reduced by the sorptive properties of organic matter or the formation of aluminium ion complexes by organic matter (organic acids; Kotowski et al. 1995).
Fig. 2.
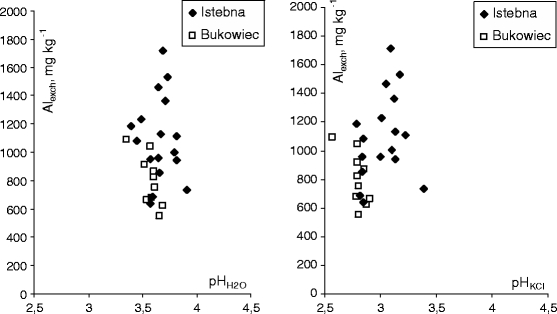
Effect of pHH2O and pHKCl on Alexch concentrations in O layer
Among other authors, the effect of organic matter on exchangeable aluminium concentrations in soil has been emphasized by: Thomas (1976); Bloom et al. (1979); Lundstrom (1993); Wesselink and Mulder (1995); de Wit et al. (1999); Berggren and Muller (1995) and Mulder et al. (2001). Since it was impossible to select soil samples of different pH and constant humus content, an additional series of tests was carried out. Five soil samples which markedly differed in organic matter concentrations were collected from different layers of the soil profile. After they had been dried at room temperature, the samples were sieved through a 2-mm sieve and pHKCl, pHH2O and weight percentage of organic matter w org [%] were determined in each one. Then, each sample was divided into five to nine 200-g sub-samples and placed in quartz beakers, adding either 90 ml of 0.1 ÷ 0.5 M nitric(V) acid solution (alternatively 0.1 ÷ 0.5 M sulfuric(VI) acid), 0.1 ÷ 1.0 M potassium hydroxide or distilled water. The beakers were covered with watch glasses and stored in the dark at room temperature. The content of the beakers was occasionally wetted with distilled water and mixed. After 3 months, the sub-samples were dried at room temperature, sieved and then pHHCl, pHH2O and exchangeable aluminium concentrations Alexch were assayed again. Each assay was repeated three times. The averaged results are given in Table 2 and Figs. 3 and 4.
Table 2.
Effect of pH on exchangeable aluminium concentration in soil (soil samples 1–5)
| Soil sample no. | Initial sample | Samples after acidity change | ||||||
|---|---|---|---|---|---|---|---|---|
| Level | pHH2O | pHHCl | w org (%) | pHH2O range | pHKCl range | Alexch (mg kg−1) | Number of samples | |
| 1 | Mineral | 5.08 | 4.38 | 1.00 | 3.28 ÷ 9.13 | 3.52 ÷ 7.48 | 1,248 ÷ 34 | 6 |
| 2 | Mineral | 4.69 | 4.09 | 3.64 | 3.40 ÷ 6.85 | 3.56 ÷ 5.13 | 684 ÷ 19 | 5 |
| 3 | Mineral | 6.04 | 4.06 | 5.60 | 1.86 ÷ 6.71 | 2.00 ÷ 5.35 | 1,020 ÷ 43 | 9 |
| 4 | Organic | 3.94 | 2.95 | 11.73 | 2.63 ÷ 7.92 | 2.38 ÷ 5.74 | 522 ÷ 31 | 8 |
| 5 | Organic | 4.01 | 2.93 | 38.20 | 2.49 ÷ 5.06 | 2.60 ÷ 3.28 | 1,349 ÷ 472 | 9 |
Fig. 3.
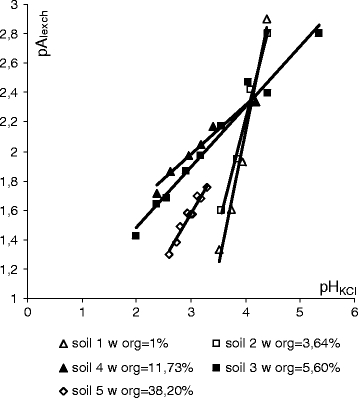
Effect of pHKCl on exchangeable aluminium concentration in soil
Fig. 4.
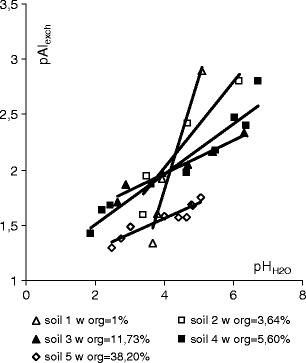
Effect of pHH2O on exchangeable aluminium concentration in soil
Figure 3 shows pAlexch = f(pHKCl) trend lines for all five soil samples (exchangeable aluminium concentration was expressed in mmol g−1) and their correlation coefficients R 2 are shown in Table 3. A group of trend lines formed crossed at pHKCl ≈ 4.10, Alexch ≈ 110 mg kg−1. The inclination coefficient of the α trend lines for the first four soil samples decreased—the amount of exchangeable aluminium was smaller in the soils of the same acidity and higher content of humus. Humus reduced the negative effects of soil acidity. In sample number five, which was characteristic of the highest organic matter content, the α coefficient of the trend line was higher than in samples 3 and 4.
Table 3.
pAlexch = −log(Alexch) = f(pH) trends (soil samples 1–5)
| Soil sample no. | w org (%) | Equation of a trend line pAlexch = −log(Alexch) = αpHKCl + β | Equation of a trend line pAlexch = −log(Alexch) = ApH2O + B | ||||
|---|---|---|---|---|---|---|---|
| α | β | R 2 | A | B | R 2 | ||
| 1 | 1.00 | 1.8805 | −5.2666 | 0.9831 | 1.0194 | −2.2460 | 0.9631 |
| 2 | 3.64 | 1.4708 | −3.6566 | 0.9909 | 0.3908 | 0.4555 | 0.9138 |
| 3 | 5.60 | 0.4116 | 0.6566 | 0.9770 | 0.2279 | 1.0483 | 0.9302 |
| 4 | 11.73 | 0.3375 | 0.9648 | 0.9656 | 0.1501 | 1.3700 | 0.9676 |
| 5 | 38.20 | 0.6574 | −0.3890 | 0.9572 | 0.1418 | 0.9921 | 0.8911 |
If soil acidity is expressed as pHH2O measured in water, the position of trend lines pAlexch = f(pHH2O) is slightly different (Fig. 4). The inclination coefficient of A trend lines decreased for the samples with increasing humus content in the soil and the A coefficient for sample 5 was the lowest (Table 3). However, compared to other trends, this one was shifted towards the higher exchangeable aluminium concentrations in the soil.
This indicates that another additional factor decides about the concentration of exchangeable aluminium in the soil in this case. It might be the presence of cations which compete with aluminium in the formation of organic complexes in the soil. Therefore, the extraction of the five soil samples with 10% nitric acid was carried out, followed by assays of potassium, calcium, magnesium, manganese, zinc and iron in the extract (10% nitric acid is used for extracting soil in order to estimate the number of metal forms that can enter the food chain (Ostrowska et al. 1991)). It has been found that the soils differed in the amount of extracted iron, manganese, potassium, calcium and magnesium (Table 4). Only zinc concentrations were similar in samples 1–4 and considerably higher in sample 5 characterized by the highest organic matter content. Potassium, calcium and magnesium concentrations correlated positively with organic matter content. Iron concentration did not correlate with the humus content. It seems that, apart from the content of organic matter, the α coefficient of trend lines pAlech = f(pHKCl) was also affected by exchangeable iron and calcium concentrations in soils samples 1–5. The conclusion needs to be confirmed by further research.
Table 4.
Extraction of soil samples 1–5 with 10% HNO3
| Soil sample | worg,% | K, mg kg−1 | Ca, mg kg−1 | Mg, mg kg−1 | Fe, mg kg−1 | Mn, mg kg−1 | Zn, mg kg−1 |
|---|---|---|---|---|---|---|---|
| 1 | 1.00 | 14 | 40 | 6.1 | 499 | 5.1 | 5.6 |
| 2 | 3.64 | 16 | 49 | 4.8 | 1,236 | 1.0 | 5.4 |
| 3 | 5.60 | 961 | 75 | 7.4 | 81 | 0.4 | 6.4 |
| 4 | 11.73 | 911 | 119 | 13 | 879 | 1.1 | 9.6 |
| 5 | 38.20 | 2,446 | 195 | 44 | 934 | 3.9 | 17.5 |
Other parameters that greatly affected aluminium Alexch content in the soils in Istebna and Bukowiec include exchangeable calcium and magnesium concentrations. The larger the amount of calcium Caexch and magnesium Mgexch, the smaller the amount of exchangeable aluminium in the surface layer of the soil. The correlation is indirectly connected with the effect of pH—the higher soil acidity, the smaller amount of exchangeable basic cations (Fig. 5). Ca/Al and Mg/Al molar ratios are used as an indicator of aluminium toxicity in soil (Urlich et al. 1980).
Fig. 5.
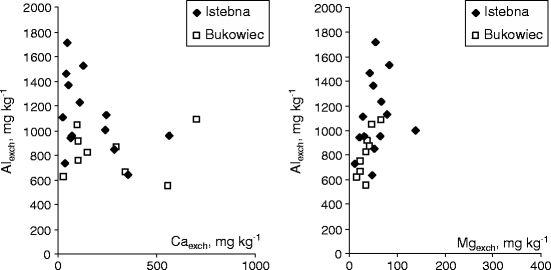
Effect of exchangeable calcium and magnesium on exchangeable aluminium content in the surface O layer of soil
Weakly Organically Bound and Bioavailable Aluminium
Copper displaces aluminium in organic complexes; therefore, the assays of AlorgI fraction for soil extraction make use of 0.5 M solution of its chloride. The fraction is a sum of the exchangeable and weakly organically bound forms. The effect of pH on aluminium AlCuCl2 concentration is very similar to the effect of acidity on Alexch. The influence of the other parameters is ambiguous. The concentration of weakly organically bound aluminium ranges from several to 650 mg kg−1. Average values for the samples collected in Istebna and Bukowiec were similar (Table 1). The wide scatter of the results may be caused by the fact that the amount of aluminium AlorgI is calculated from the difference of experimentally measured values of AlCuCl2 and Alexch. Figure 6 demonstrates the effect of organic matter on the percentage of weakly organically bound AlorgI in the total amount of aluminium extracted with copper(II) chloride (AlorgI/AlCuCl2). The ratio of AlorgI/AlCuCl2 fell within 0.2–0.4. It was lower than 0.1 for three points. As it can be seen, the ratio AlorgI/AlCuCl2 considerably increased with increasing humus content in the soil.
Fig. 6.
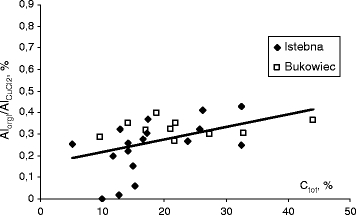
Effect of total carbon Ctot on the weakly organically bound aluminium fraction AlorgI in the total amount of aluminium leached with copper(II) chloride solution
The fraction of bioavailable aluminium Albio is a sum of exchangeable Alexch and organic Alorg aluminium. The assays of the bioavailable fraction pose problems, since sodium diphosphate(V) solution can leach part of amorphous aluminium as well (Walna et al. 2005). Bertsch and Bloom (1996) emphasize that extraction should be carried out with a solution of constant pH and the final results are affected by the time of aluminium assays in eluates.
The percentages of exchangeable and organic aluminium in the total content of bioavailable aluminium were calculated in the soil samples collected in Bukowiec, where humus content ranged widely (Table 1). The increasing concentration of organic matter in the soil brought about an increase in aluminium content in the organic fraction, less dangerous to plants, and its decrease in the phytotoxic exchangeable fraction (Fig. 7).
Fig. 7.
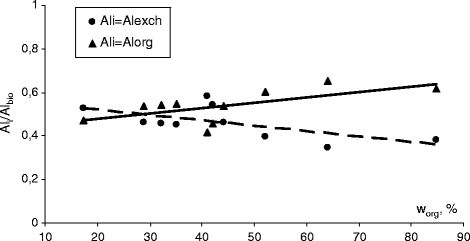
Effect of organic matter on the exchangeable and organically bound aluminium fractions in the total amount of mobile aluminium (Bukowiec series)
Total aluminium was also assayed in the soil samples, digesting them with hydrofluoric acid. Total aluminium concentration ranged widely (Table 1) and correlated with organic matter concentration. The metal, being one of the basic components of soil, is bound primarily in sparingly soluble aluminosilicates. The more organic matter and less mineral fractions in the soil, the more aluminium assayed in the samples.
The amount of the bioavailable Albio/Altot and exchangeable aluminium Alexch/Altot fractions in the total aluminium content in the soil were on average 0.09 and 0.03, respectively. Increasing pH decreased the content of exchangeable and bioavailable aluminium in the soil (Fig. 8).
Fig. 8.
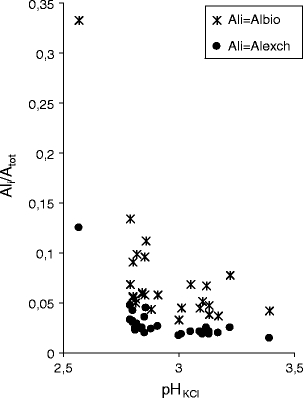
Effect of pHKCl on the amount of bioavailable and exchangeable aluminium in the total concentration of aluminium in soil
Assays of Soil Samples Collected in the Areas Free of Degradation
Three reference samples were taken for comparison: two in the Poprad Landscape Park, the Sądecki Beskids (the Western Carpathians) and one in Koszęcin (Silesia). The areas do not experience the pandemic dying of spruce trees.
The Sądecki Beskids are part of the Western Carpathians composed of flysch of Magura nappe. They are densely covered with spruce and beech trees. Koszęcin is located in the so called White Silesia, Lubliniec Land, in the north-west part of Silesian-Kraków Upland. The ground is composed of limestone and sandstone.
The assays focused on the selected parameters only. The results are given in Table 5 and show that the concentration of exchangeable aluminium in the reference soil was much lower. The good condition of the forests in the Sądecki Beskids is particularly satisfying. Both samples revealed much lower acidity than that in the soil collected in the Silesian Beskids. The exchangeable complex is predominant with calcium and magnesium (approximately 80% of CEC), and aluminium content reached 20% of CEC. Slightly worse parameters were found in the O soil layer collected in Koszęcin. The higher acidity and lower Caexch and Mgexch content may be also caused by the proximity of the zinc plant in Miasteczko Śląskie.
Table 5.
Characteristics of soil collected at reference sites
| Parameter | pHKCl | Corg | Caexch (mg kg−1) | Mgexch (mg kg−1) | Feexch (mg kg−1) | Mnexch (mg kg−1) | CEC cmolc (kg−1) | Alexch (mg kg−1) | AlorgI (mg kg−1) | Alorg (mg kg−1) | Albio (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Poprad 1 | 3.96 | 9.1 | 2,832 | 221 | 16 | 172 | 12.52 | 178 | 358 | 837 | 1,015 |
| Poprad 2 | 3.60 | 33.3 | 4,967 | 287 | 19 | 470 | 14.00 | 67 | 187 | 497 | 564 |
| Koszęcin | 3.19 | 7.7 | 404 | 32 | 88 | 8 | 8.42 | 326 | 93 | 1,374 | 1,700 |
Conclusions
The choice of the sampling site in the Barania Góra range resulted from the condition of the forest. The difference between the mean pHKCl values in the samples taken in Istebna (area of cut down spruce forest) and Bukowiec (area of healthy spruce forest) is statistically significant. The average content of exchangeable aluminium hazardous to coniferous trees was much higher than permissible standards in both areas, being approximately 1,090 mg kg−1 in Istebna (despite the lower acidity of the soil) and was about 300 mg kg−1 higher than in Bukowiec. It was probably caused by the lower content of organic matter and smaller amount of calcium in the exchangeable complex in the samples taken in Istebna. Those soils also revealed a higher content of bioavailable aluminium forms. The average CEC was similar for both. The exchangeable complex in the soil of the forest in Istebna contained 84% of aluminium and 9% of total calcium and magnesium on average. In Bukowiec, the percentage of aluminium in CEC was slightly lower and reached 78% while the value for total calcium and magnesium was 14%.
Various factors were taken into consideration as the causes of spruce stand deterioration in the Silesian Beskids: industrial contamination, diseases caused by fungi (armillaria), insect pests, genetically different origin of the stands (Conference Materials 2006). The results indicate that the chemical changes brought about by acid rain were the direct cause. The strongly acidic soils of the Silesian Beskids caused considerable solubilization of aluminium, its increase in the soil solution and finally resulting in its toxic properties. The significant decrease in calcium and magnesium content in the sorptive complex of the soil reduced the vitality of spruce trees vulnerable to aluminium effects and their general resistance to diseases. This means withering for young trees and for older ones, the thinning of the crown, brittle cellulose fibres, lack of resistance to abnormal weather conditions, invasions of insects or parasitic fungi, i.e., all the phenomena observed in the Silesian Beskids.
Unfortunately, there is a possibility that the areas where trees have not been withering so far might face the deterioration in the condition of spruce forests. This is indirectly indicated by our analysis of the soil in Bukowiec, the Barania Góra range. Despite the positive assessment of the forest condition made by foresters during the sampling period, the ongoing chemical changes in the soil affected adversely the condition of the trees. The following years the areas adjacent to the experimental plot experienced windthrow and recently, the situation deteriorated noticeably, which necessitated sanitation felling in some parts of the forest.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Berggren D, Muller J. The role of organic master in controlling aluminium solubility in acidic mineral horizons. Geochimica and Cosmochimica Acta. 1995;59:4167–4180. doi: 10.1016/0016-7037(95)94443-J. [DOI] [Google Scholar]
- Bertsch PM, Bloom PR. Aluminum. In: Bartels JM, editor. Methods of soil analysis. Soil science society of America and American society of agronomy. Part 3. Chemical methods. Madison, USA: Soil Science Society of America, Inc; 1996. pp. 517–550. [Google Scholar]
- Bloom PR, McBridge MB, Weaver RM. Aluminium organic matter in acid soil: buffering and solution aluminium activity. Soil Science Society of American Journal. 1979;43:488–493. doi: 10.2136/sssaj1979.03615995004300030012x. [DOI] [Google Scholar]
- Borůvka L, Mládkowá L, Drábek O. Factors controlling spatial distribution of soil acidification and Al forms in forest soils. Inorganic Biochemistry. 2005;99:1796–1806. doi: 10.1016/j.jinorgbio.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Borůvka L, Mládkowá L, Drábek O, Vašát R. Factors of spatial distribution of forest floor properties in the Jizerske Mountains. Plant Soil and Environment. 2005;51(10):447–455. [Google Scholar]
- Borůvka L, Podrázsky V, Mládková L, Keneš I, Drabek O. Some approaches to the research of forest soils affected by acidification in the Czech Republic. Soil Science & Plant Nutrition. 2005;51(5):745–749. doi: 10.1111/j.1747-0765.2005.tb00105.x. [DOI] [Google Scholar]
- de Wit HA, Kotowski M, Mulder J. Modelling aluminium and organic matter solubility in the forest floor using WHAM. Soil Science Society of American Journal. 1999;63:1141–1148. doi: 10.2136/sssaj1999.6351141x. [DOI] [Google Scholar]
- Drábek O, Borůvka L, Mládková L, Kocarek M. Possible method of aluminium speciation in forest soils. Journal of Inorganic Biochemistry. 2003;97:8–15. doi: 10.1016/S0162-0134(03)00259-9. [DOI] [PubMed] [Google Scholar]
- Driscoll CT, Schecher WD. Aluminum in environment. In: Sigel H, Sigel A, editors. Metal Jones in biological system, Vol. 24, Aluminum and its role in biology. New York: Marcel Dekker; 1988. pp. 59–122. [Google Scholar]
- “Hazards to the life of forests in the Beskids, causes and prevention” (in Polish) Ustroń–Jaszowiec, 23–24 November 2006, Conference Materials.
- Illmer P, Obertegger U, Schinner F. Microbiological properties in acidic forest soils with special cosideration of KCl extractable Al. Water, Air and Soil Pollution. 2003;148:3–14. doi: 10.1023/A:1025422229468. [DOI] [Google Scholar]
- Kotowski, M., Wieteska, E., Pawłowski, L. & Kozak, Z. (1994). Characteristics of different aluminium forms occurrence in selected environmental elements in Poland (in Polish) Państwowa Inspekcja Ochrony Środowiska, Warszawa.
- Kotowski, M., Pawłowski, L., & Zhu, X. (1995). Aluminium in the environment (in Polish) Wydawnictwo Politechniki Lubelskiej, Lublin.
- Lundstrom US. The role of organic acids in the soil solution chemistry of a podzolized soil. Journal of Soil Science. 1993;44:121–133. doi: 10.1111/j.1365-2389.1993.tb00439.x. [DOI] [Google Scholar]
- Mládková L, Borůvka L, Drábek O, Vasat R. Factors influencing distribution of different Al forms in forest soils of the Jizerskè hory Mts. Journal of Forest Science. 2006;52(Special Issue):87–92. [Google Scholar]
- Mládkowá L, Borůvka L, Drábek O. Distribution of labile aluminium forms in soils of Jizera Mountains region. Plant Soil and Environment. 2004;50:46–351. [Google Scholar]
- Motowicka-Terelak, T. & Stuczyński, T. (1993). Effect chemical contaminants on aluminium in soils. Chromium, nickel and aluminium in the environment—ecological and methodological problems. (in Polish). Ossolineum.
- Mulder J, de Wit HA, Boonen HWJ, Bakken LR. Increased levels of aluminium in forest soils: effects on the stores of soil organic carbon. Water Air and Soil Pollution. 2001;130:989–994. doi: 10.1023/A:1013987607826. [DOI] [Google Scholar]
- Musil I, Pavliček V. Liming of forest soils: effectiveness of particle size fractions. Journal of Forest Science. 2002;48:121–129. [Google Scholar]
- Ostrowska, A., Gawliński, St., & Szczubiałka, Z. (1991). Methods for analyzing and assessing the properties of soil and plants. (in Polish) Instytut Ochrony Środowiska, Warszawa.
- Polish Standard PN-ISO 11260:1999 Soil quality—determination of effective cation exchange capacity and base saturation degree, using barium chloride solution. (in Polish).
- Simonsson M. Interactions of aluminium and fulvic acid in moderately acid solutions: stoichiometry of the H+/Al3+ exchange. European Journal of Soil Science. 2000;51:655–666. [Google Scholar]
- Smal H, Misztal M. Soil solution chemistry in the profiles of forest and arable light textured soils, SE Poland. Applied Geochemistry. 1996;11(1):81–85. doi: 10.1016/0883-2927(95)00095-X. [DOI] [Google Scholar]
- Smoliński A, Zołotajkin M, Ciba J, Dydo P, Kluczka J. PLS-EP algorithm to predict aluminium content in soils of Beskid Mountains region. Chemosphere. 2009;76:565–571. doi: 10.1016/j.chemosphere.2009.02.057. [DOI] [PubMed] [Google Scholar]
- Staszewski, T. (2004). Reaction of spruce stands to the deposition of air contaminants. (in Polish). Wydawnictwo Uniwersytetu Śląskiego, Katowice.
- Thomas GW. The relationship between organic matter content and exchangeable aluminium in acid soils. Soil Science Society of American Journal. 1976;39:591. doi: 10.2136/sssaj1975.03615995003900030056x. [DOI] [Google Scholar]
- Urlich BR, Mayer C, Khanna PK. Chemical changes to acid precipitation in loess derived soil in central Europe. Soil Science. 1980;130:193–199. doi: 10.1097/00010694-198010000-00005. [DOI] [Google Scholar]
- van Hees P, Lundstöm U, Danielsson R, Nyberg L. Controlling mechanisms of aluminium in soil solution—an evaluation of 180 podzolic forest soils. Chemosphere. 2001;45:1091–1101. doi: 10.1016/S0045-6535(00)00515-4. [DOI] [PubMed] [Google Scholar]
- Walna B, Spychalski W, Siepak J. Assessment of potentially reactive pools of aluminium in poor forest soils using two methods of fractionation analysis. Journal of Inorganic Biochemistry. 2005;99:1807–1816. doi: 10.1016/j.jinorgbio.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Wesselink L, Mulder J. Modelling Al-solubility controls in an acid forest soil, Solling, Germany. Ecological Modelling. 1995;83:109–117. doi: 10.1016/0304-3800(95)00090-I. [DOI] [Google Scholar]


