Abstract
We proposed a new approach to improve the control of prosthetic arm rotation in amputees. Arm rotation is sensed by implanting a small permanent magnet into the distal end of the residual bone, which produces a magnetic field. The position of the bone rotation can be derived from magnetic field distribution detected with magnetic sensors on the arm surface, and then conveyed to the prosthesis controller to manipulate the rotation of the prosthesis. Proprioception remains intact for residual limb skeletal structures; thus, this control system should be natural and easy-to-use. In this study, simulations have been conducted in an upper arm model to assess the feasibility and performance of sensing the voluntary rotation of residual humerus with an implanted magnet. A sensitivity analysis of the magnet size and arm size was presented. The influence of relative position of the magnet to the magnetic sensors, orientation of the magnet relative to the limb axis, and displacement of the magnetic sensors on the magnetic field was evaluated. The performance of shielding external magnetostatic interference was also investigated. The simulation results suggest that the direction and angle of rotation of residual humerus could be obtained by decoding the magnetic field signals with magnetic sensors built into a prosthetic socket. This pilot study provides important guidelines for developing a practical interface between the residual bone rotation and the prosthesis for control of prosthetic rotation.
Index Terms: Computer simulation, finite-element (FE) model, magnetic field, permanent magnet, prosthesis, upper limb amputation
I. INTRODUCTION
Arm rotation is very useful for unilateral upper limb amputees and essential for bilateral amputees to perform tasks of daily living [1]–[3]. Most commercially available upper limb prostheses only provide passive rotation using a friction turntable incorporated into the prosthesis. Passive humeral or wrist rotation is set with one’s intact arm or by rotating the joint with an object in the environment. The only commercially available body-powered humeral rotation unit is controlled by the amputee’s muscle structure (HR Unit, RIMJET Corporation). Body-powered and myoelectric wrist rotators are available. Body-powered devices use shoulder protraction to sequentially operate all functions, including a wrist rotator. Using the remaining flexion and extension muscles of the residual limb not specifically wrist rotation muscle, myoelectric wrist rotators also use sequential control to operate all functions. Thus, current control of these rotators is cumbersome, slow, and unintuitive. Prosthetic rotators, like all other prosthetic components, lack proprioceptive feedback. The amputee cannot feel the orientation of their prostheses; they must use visual feedback to know how their artificial limb is positioned in space. This lack of proprioceptive feedback impedes prosthetic control, increases the cognitive burden of using a prosthesis, and impairs function.
One method to improve voluntary control of artificial arm rotation is to physically couple the rotation of the bones remaining in the residual arm to the prosthetic rotation. To create a physical connection between the residual humerus and the prosthesis for control of the prosthesis rotation, two interfacing mechanisms, osseointegration and artificial epicondyles, have been developed. Osseointegration [4], [5] is a direct structural connection between bone and the prosthesis. This technique involves implanting a titanium bolt into the bone of the residual limb. An abutment attaches to the bolt and protrudes through the skin to provide the attachment for the prosthesis and the manipulation including prosthesis rotation. Another attachment method is the use of artificial epicondyles [6] that are created by surgically inserting an implant into the humerus and covering the implant with soft tissue and skin. The artificial epicondyles can suspend the prosthesis and provide the function of humeral rotation of the prosthesis. Patients using these systems demonstrate excellent rotation control with preservation of proprioception for rotation of their artificial limb in the long axis of the residual limb. These approaches are promising, but have drawbacks. Direct skeletal attachment is challenged by infections at the skin interface and loosening of implants [7]. Loading of the skin over artificial epicondyles can cause skin breakdown and there is potential for loosening of the implants. Both systems require extensive surgical procedures and significantly delay the use of prosthesis as the implants integrate with the residual limb bone matrix.
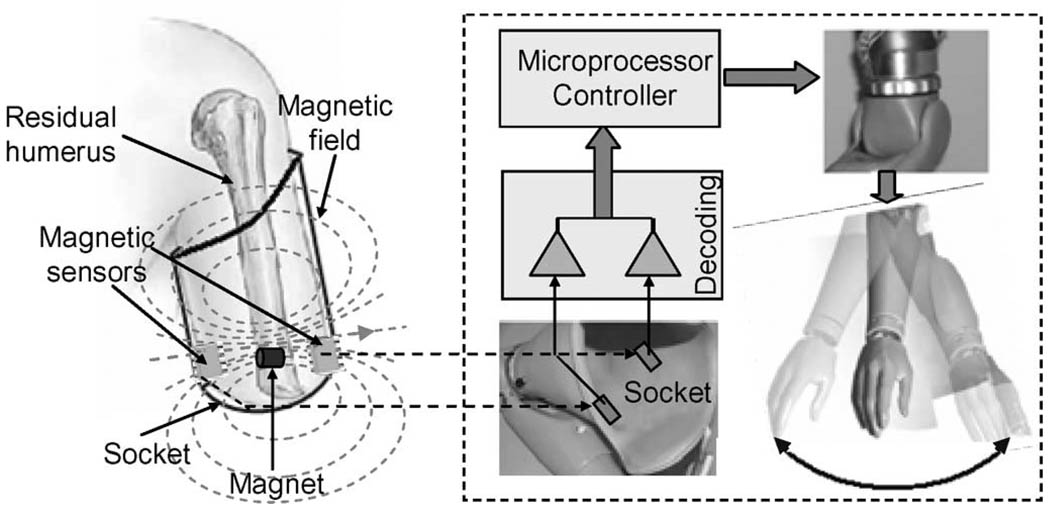
In this study, an alternative approach is proposed that has promise for improving the rotational control of artificial limbs. This new approach involves inserting a small permanent magnet into the distal end of residual bone of the subjects with an upper limb amputation (Fig. 1). The permanent magnet generates a magnetostatic field. When the amputee rotates their residual arm, the magnet will rotate with the residual bone. This rotation causes a change in magnetic field distribution of the magnet. This field change can be detected by magnetic sensors with fixed positions in a prosthetic socket, from which information on the residual arm rotation is derived and used as an input signal to control a powered rotator of the prosthesis. An important advantage of this approach may be that it potentially preserves some inherent proprioceptive awareness of arm rotation for amputees. Proprioception of rotation would come from the intact proximal joint including the muscle, tendons, and joint capsule where the primary proprioceptive afferent nerve fibers are located [8]. Thus, this control approach to arm rotation should be easier and more intuitive than traditional electromyogram (EMG) methods [9]–[11] or even recently proposed EMG pattern-recognition control methods [12]–[16] that require visual feedback for the amputees to know how their arm is positioned. This new approach does not compromise other applicable control sources, such as biceps and triceps EMG that can be used to operate other degrees of freedom such as wrist and hand movements. Finally, the surgery required to implant the magnet would be a relatively easy outpatient procedure and the delay in the prosthetic fitting would be minimal.
Fig. 1.
Schematic diagram of control system of prosthesis rotation by using a magnet to sense the rotation of residual arm.
A simulation study was conducted with finite-element (FE) models of the upper arm to evaluate the feasibility and performance of the new interfacing approach in sensing the rotation of residual humerus. While this technique also holds much promise for controlling pronation and supination of the forearm in a below-elbow amputee, the upper arm was chosen for this initial analysis due to its greater symmetry allowing a simpler model, and because the upper arm is larger in size than forearm, which would attenuate the magnetic fields so that a sensitivity analysis is more relevant. This analysis included the effects of arm size, magnet dimensions, relative position of the magnet to magnetic sensors, orientation of the magnet relative to the limb axis, displacement of the magnetic sensors on the magnetic field, and external magnetic interference on the distribution of magnetic field of the magnet. This simulation study provides important guidelines for developing a practical interface between the residual humerus and the prosthesis for the humeral rotation control of upper limb prostheses.
II. METHODS
A. Element Modeling of Upper Arm and Permanent Magnet
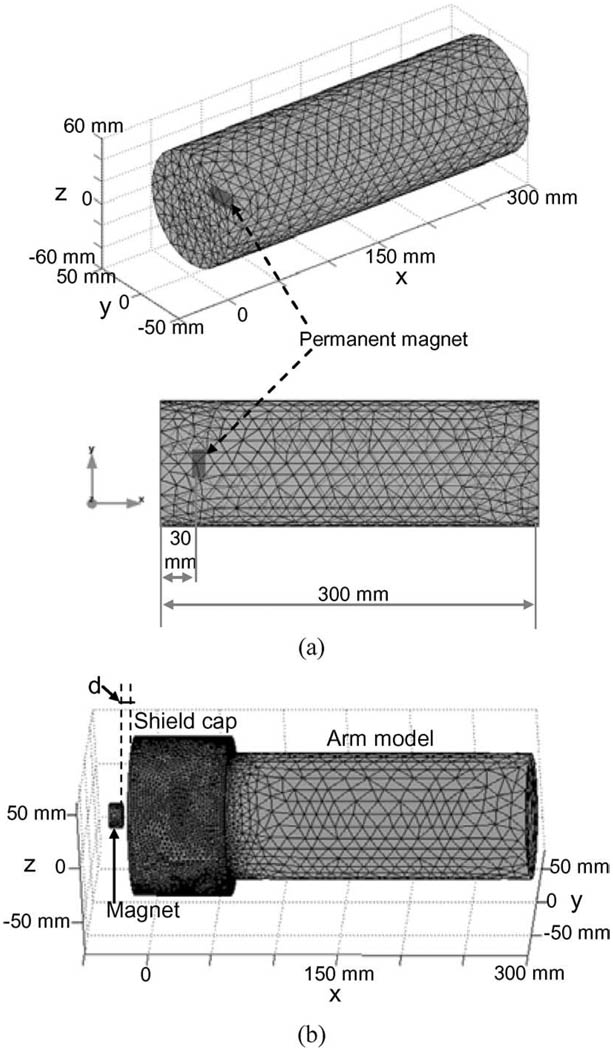
A residual upper arm was modeled as a cylinder, shown in Fig. 2(a). Considering the different tissues (bone, muscle, fat, and skin) as being magnetically homogeneous and having approximately the magnetic permeability of a free space [17], [18], this model had an identical magnetic permeability with a relative permeability of μr = 1 for all the tissues of upper arm. The radius of the cylindrical model was 50 mm (the average mid-upper arm circumference ≈ 300 mm for adults) [19] and the length was 300 mm [20]. In practical applications, a permanent magnet would be surgically attached to the distal end of residual humerus to produce the distribution of magnetic field. To model the permanent magnet, a small cylinder was inserted into the arm model [see Fig. 2(a)]. The cylindrical magnet model had an axis direction parallel to the cross section of the arm model and was located at the center of the limb model, 30 mm distant from the distal end of the arm. The average diameter of the humerus is 23 mm for adult men and 18 mm for women [21]. Thus, the magnet model had a radius of 5 mm and a length of 20 mm that would allow the magnet to be placed in the humerus much like a large orthopedic screw. This arm model with the embedded magnet model was considered as the basic model in the simulation study. To investigate the effects of the individual variation of residual arm sizes and magnet sizes on the distribution of magnetic field of the magnet, the basic model was accordingly modified to simulate these conditions.
Fig. 2.
(a) FE model of upper arm with an implanted permanent magnet. The 3-D FE model (top) and its 2-D view (bottom). The distance from the magnet to the distal end of the arm model is 30 mm. (b) FE model of upper arm with a magnetic shield cap. The shield cap covers the part of the arm model at distal side. The permanent magnet outside the shield cap was used to simulate the magnetostatic interference source. d is the distance between the magnet and the shield cap.
B. Sensitivity of Magnetic Field to Practical Variations
In practical implementation of the proposal approach, much variation is expected as the humerus moves within the residual soft tissue; thus, the magnet would move relative to the magnetic sensors. Orientation of the magnet relative to the limb axis and displacement of the magnetic sensors will affect the distribution of the magnetic field of the magnet. To investigate the sensitivity of the magnetic field to these variations, simulation studies were also performed to evaluate the effect of three kinds of variations on the magnetic field of the magnet.
Off-Center of the Magnet: The humerus would not remain exactly in the center of an arm when a prosthesis is used. For example, with flexion of the arm, the distal humerus moves forward in the residual limb and with abduction it moves laterally. Thus, the magnet implanted in the humerus will often be off-center. To evaluate the sensitivity of the magnetic field to the translational misalignment of the magnet, the magnet model in the basic model [see Fig. 2(a)] was shifted along the y-direction (parallel to the magnet central axis) and z-direction (perpendicular to the magnet central axis) [see Fig. 6(a)], respectively, and then, simulations were performed to calculate the magnetic field.
Orientation Declination of the Magnet: Another possible variation is that during surgical implantation the magnet may not be exactly perpendicular to the humeral axis. To estimate the sensitivity of the magnetic field to the orientation declination of the magnet, the magnet in the basic model [see Fig. 2(a)] was rotated on the x–y and y–z planes, respectively, and then, the simulations were performed to calculate the magnetic field.
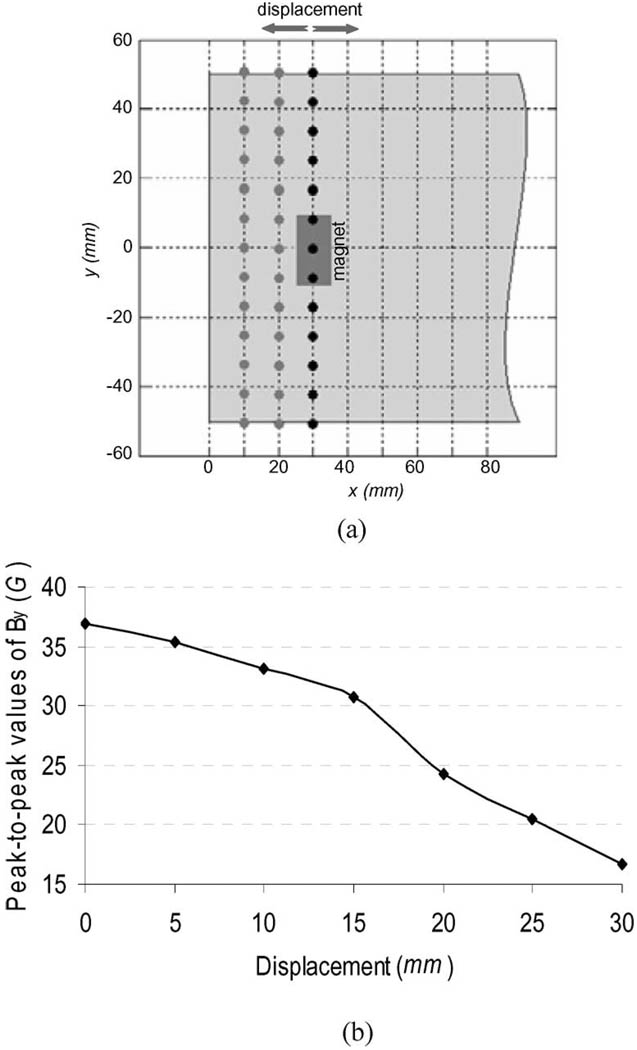
Displacement of Magnetic Sensors: It is impossible to ensure that the magnetic sensors are exactly at the same locations in everyday donning of a prosthesis. The residual limb often slips out of the prosthetic socket 10–20 mm. To investigate the sensitivity of the relative linear displacement between the magnets and the magnetic sensors, the strengths of the magnetic field were calculated over the surface observation points on different transverse cross sections in the y–z plane [see Fig. 7(a)].
Fig. 6.
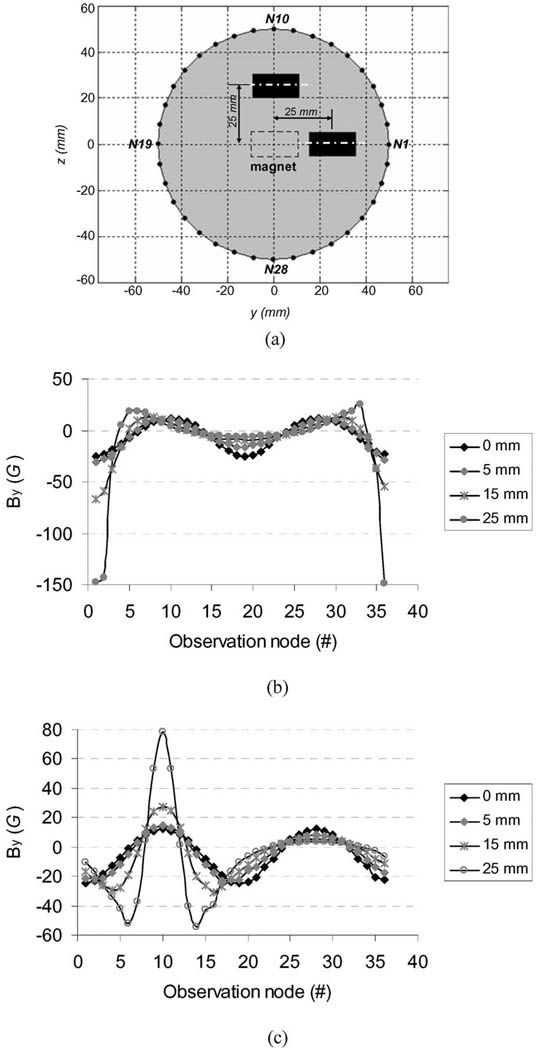
Sensitivity of the magnetic field to the off-center of the magnet. (a) Schematic diagram of the y- and z-direction shifts of the magnet in the simulation model. (b) Distributions of the y-direction component of the magnetic field over the surface observation nodes when the magnet model shifted 5, 10, and 15 mm along the y-direction, respectively. (c) Same as (b) along the z-direction.
Fig. 7.
Sensitivity of the magnetic field to the displacement of the magnetic sensors. (a) Schematic diagram of the shifted observation nodes along the x-direction in the simulation model. (b) Peak-to-peak values of the y-direction component of the magnetic field on arm surface. The horizontal axis is the displacement of the observation nodes relative to the observation nodes at x = 30 mm [black dots in (a)].
C. Deriving of Magnetic Field of Permanent Magnet
The magnetic field of a permanent magnet belongs to a magnetostatic field, where no currents are present. For a magnetostatic problem, the Maxwell’s equations [22] that govern the general electromagnetic field are simplified as
| (1) |
where H⃑ and B⃑ are vectors of magnetic field and magnetic flux density, respectively. M⃑0 is the premagnetization of the magnet. μ0 and μr are the permeability of free space and the relative permeability of medium, respectively. By defining a scalar magnetic potential (Vm) as H⃑ = −∇Vm, a partial differential equation (PDE) for the magnetic potential (Vm) can be derived from (1) as follows:
| (2) |
The finite-element method (FEM) was applied to numerically solve the PDE in the arm-magnet model. The 3-D basic arm-magnet model was meshed into about 25 000 tetrahedral elements [see Fig. 2(a)] using the FEMLAB electromagnetics module [23]. The basic model had about 4700 nodes. Given the premagnetization (M⃑0) of the permanent magnet, the scalar magnetic potentials (Vm) at all the FE model nodes were calculated by the PDE coefficient application mode of the FEMLAB [23]. The magnetic field vector (H⃑) at each node was obtained from Vm by H⃑ = −∇Vm, and then, the magnetic flux density vector (B⃑) at each node was computed by (1).
Magnetic flux density of permanent magnet ranges from 5 to 13 kG, depending on the material properties of the magnet. In the basic arm-magnet model shown in Fig. 2(a), the magnet had a residual magnetic flux density of 11 kG (NdFeB, a high-performance rare earth magnet with Br = 10–13 kG). The premagnetization (M⃑0) of the permanent magnet was derived from the residual magnetic flux density (B⃑r). The direction of residual magnetic field of the magnet was along the y-axis negative direction. The local magnetic field distribution produced by the magnet was obtained using the PDE solver in FEMLAB based on (2).
D. Shielding of Magnetostatic Interference
Other magnetic fields in the environment will inevitably interfere with the magnetic field of the permanent magnet implanted in the residual humerus. This may contaminate the magnetic field signals detected by sensors in the socket of the prosthesis. The magnetic interference sources may range from magnetostatic fields such as permanent magnet motors to time-varying magnetic fields such as high-frequency electromagnetic waves. Considering the magnetic field of the implanted permanent magnet as being a magnetostatic field, the time-varying magnetic field interference with the magnetic signals of the magnet would be easily removed or significantly attenuated using signal processing techniques such as low-pass filtering, whereas the magnetostatic field interference remains to be dealt with. Among all possible magnetostatic interference sources, permanent magnet motors embedded in the motorized prosthesis would likely cause the most severe magnetic interference due to the short distance between the motor and magnetic field detectors placed on the arm. To reduce the magnetic interference of such magnetostatic fields, a shield cap could be employed to cover the distal end of the residual arm. The performance of the shield cap in reducing the magnetostatic interference was evaluated by using an FE model shown in Fig. 2(b). A shield cap was formed by adding a cylinder to cover the part of the distal end of the arm-magnet model [see Fig. 2(a)] and a permanent magnet was placed outside the shield cap to mimic the magnetostatic interference source. The FE model was meshed using the FEMLAB electromagnetics module [23]. The magnetic field distribution on the arm surface produced by the outside magnet was calculated by giving the premagnetization of the permanent magnet and the distance (d) between the magnet and shield cap.
III. RESULTS
A. Distribution of Magnetic Field of Permanent Magnet on Arm Surface
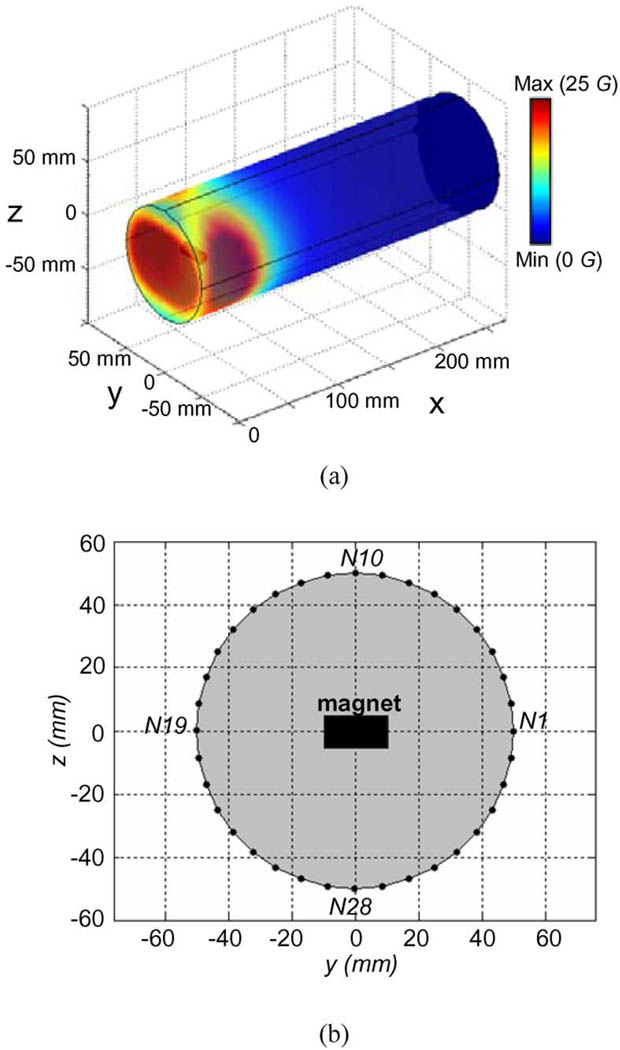
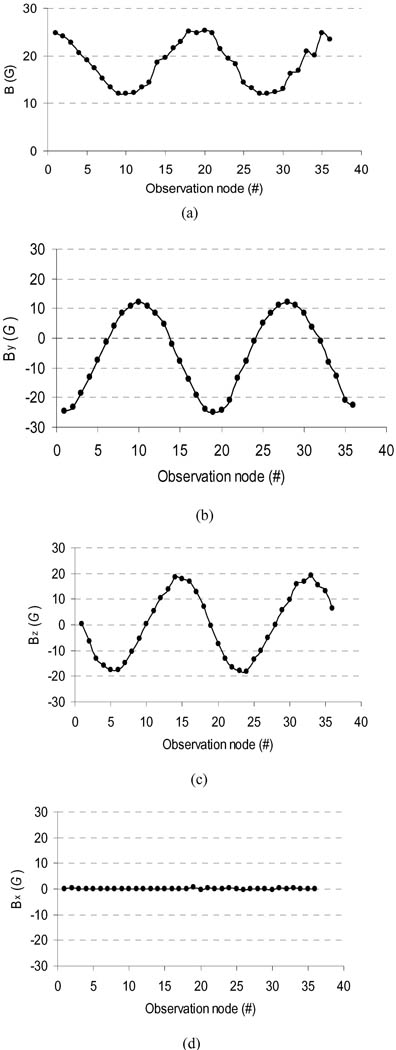
Fig. 3(a) shows a color map of the simulated magnetic flux density (B⃑) distribution on the arm model surface. To show the simulated magnetic field of the magnet around arm surface, 36 surface nodes on the circumference of a transverse cross section of the FE arm model were chosen as the observation points of the magnetic field [see Fig. 3(b)]. The transverse cross section was in the plane of the implanted magnet. These surface nodes labeled from N1 to N36 in Fig. 3(b) were uniformly distributed on the circumference surface with a 10° angle interval. The simulated B⃑ magnitude and its three components (Bx, By, and Bz) at these nodes are shown in Fig. 4. It can be observed from Fig. 4 that the magnitudes of B⃑ and its two components at y- and z-directions (By and Bz) significantly oscillated over these nodes, whereas its x-direction component (Bx)was almost unchanged over all the nodes. From the node N1 to the node N10, the magnitudes of B⃑ monotonously decreased from a maximum value of about 25 G to a minimum value of about 12 G and then strictly increased to about 25 G at the node N19. The two components, By and Bz, presented the similar monotonously varying feature to the B⃑ magnitude. Suitably placed magnetic sensors in the prosthetic socket could detect the magnetic field allowing the angle of residual humerus rotation to be measured and used to control the humeral rotation of the prosthesis.
Fig. 3.
Simulated magnetic flux density (B) of the magnet in the basic model shown in Fig. 2(a). (a) Color map of distribution of the B⃑ magnitude on the arm model surface. (b) Thirty-six observation nodes on the circumference of a transverse cross section of the FE model. The transverse cross section was located at x = 30 mm.
Fig. 4.
Magnitudes of simulated magnetic flux density and its three components (Bx, By, and Bz) over 36 observation nodes shown in Fig. 3(b). The jagged results over the nodes (31–36) in the magnitude values of magnetic field might be produced by the modeling errors. (a) B magnitude. (b) Component By. (c) Component Bz. (d) Component Bx.
B. Effect of Different Model Conditions on Magnetic Field
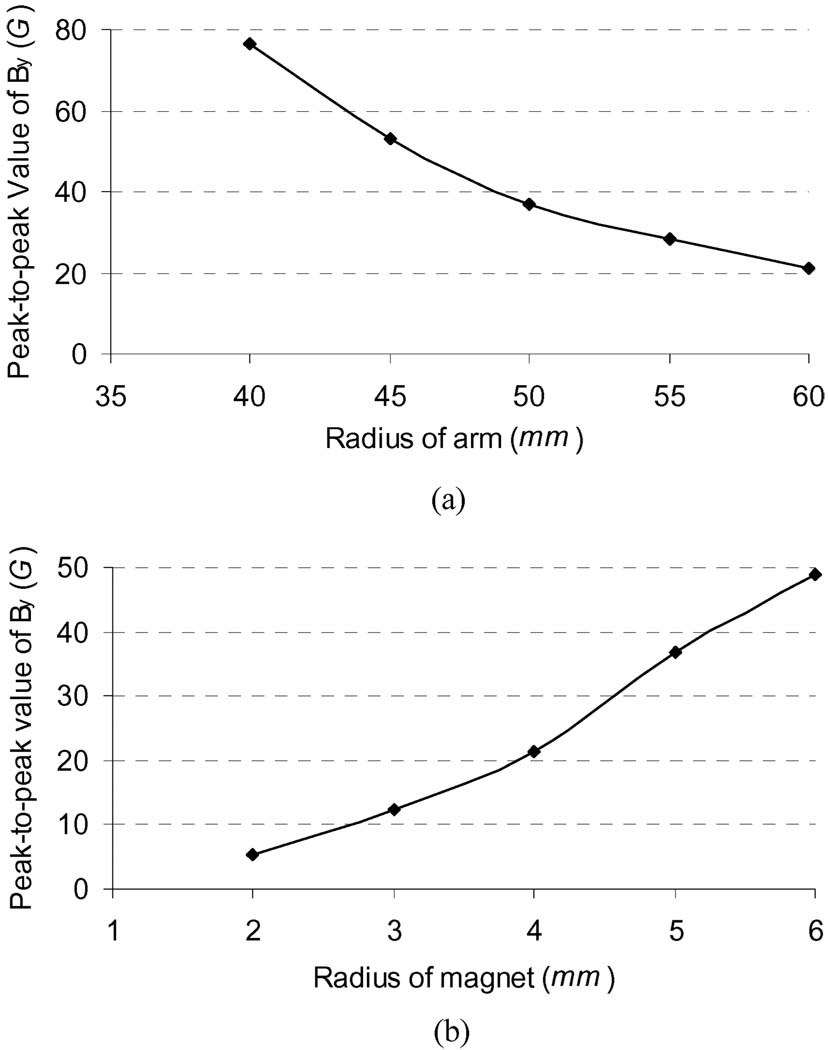
Effect of Arm Size: Upper arm size is variable across subjects with an average mid upper arm circumference of 320 ± 50 mm (radius ≈ 51 ± 8 mm for a cylindrical assumption of arm) for men and 280 ± 60 mm (radius ≈ 45 ± 9 mm) for women [19]. To assess the effect of arm size on the surface magnetic flux density (B⃑), four more cylindrical arm models with a radius of 40, 45, 55, and 60 mm, respectively, were used to implement the magnetic field simulation (60 mm radius would include up to approximately the 90th percentile in arm size [24]). In all these simulations, the permanent magnet had the same size (radius = 5 mm) and the same magnetic flux density (B⃑r) (11 kG). The simulation results showed that the distribution of the magnetic field from different arm sizes had the almost same sinusoidal patterns as those shown in Fig. 4. The effect of different arm sizes on the peak-to-peak strengths of the y-direction component (By) of the simulated B⃑ at the 36 surface nodes [see Fig. 3(b)] are plotted in Fig. 5(a). It can be seen from Fig. 5(a) that the By decreased along with the increase of the limb model radius. In the model with the smallest radius (40 mm), the peak-to-peak value of the component By was greater than 75 G, and in the model with the largest radius (60 mm), the peak-to-peak value of the component By was still greater than 20 G.
Effect of Magnet Size: There is a paradox for the selection of magnet size: a large magnet would generate a stronger magnetic field that will benefit magnetic signal detection and processing, but may increase difficulty and complexity of the implanting surgery and packaging of the magnet for practical application. To provide some general guidelines for the selection of magnet size in the practical applications, four additional cylindrical magnet models with a radius (rm) of 2, 3, 4, and 6 mm were used to estimate the effect of magnet size on the distribution of magnetic field (B⃑). The magnetic flux density of these magnets was the same (11 kG). These simulations were performed in an identical arm model with a radius of 50 mm. Fig. 5(b) shows the peak-to-peak values of the component By of the simulated B⃑ from different size magnets. Similarly, the different size magnets affected the strength of the magnetic field at the arm surface. Cylindrical magnets with a radius of 2 mm or greater can produce a magnetic field component in the y-direction greater than 5 G.
Effect of Off-Center of the Magnet: The effects of the magnet shift on the y-direction component of the magnetic field over the surface observation points are illustrated in Fig. 6(b) and (c). When the magnet in the model was shifted by 5 mm, the distribution of the magnetic field in the y-direction still retained a sinusoidal pattern [Fig. 6(b) and (c)] and the peak-to-peak strength of the y-direction component only had a 4.5 G change (12% increase) for y-direction shift and a 1.3 G change (3.5% increase) for z-direction shift in comparison with the centered magnet. With a 10 mm off-center, the sinusoidal patterns of the y-direction component were distorted and the peak-to-peak strength had a change of about 20 G (54%)/13 G (35%) for y-, z-direction shift. When the off-center of the magnet was greater than 15 mm, the distribution of the y-direction component became a triphasic pattern [see Fig. 6(b) and (c)]. The peak-to-peak strength had an increase of up to 43 G for a y-direction shift and 21 G for a z-direction shift.
Effect of Orientation Declination of the Magnet: When the magnet was placed at 80° to the axis, i.e., 10° declination, the simulation result indicated a decrease of less than 4 G (11% change) in the peak-to-peak strength of the magnetic field on arm surface in the y-direction and no significant change in the sinusoidal distribution of the magnetic field.
Effect of Displacement of Magnetic Sensors: The peak-to-peak values of the y-direction component of the magnetic field for the different-distance shifts of the observation points are illustrated in Fig. 7(b). A 5 mm displacement caused a 1.5 G decrease of the peak-to-peak value of the magnetic field, about 4% change. With 10 and 20 mm displacements, the peak-to-peak values of the magnetic field in the y-direction had a decrease of about 3.8 G (10%) and 12 G (33%), respectively.
Fig. 5.
Effects of arm size and magnet size on the peak-to-peak strength of the y-direction component of magnetic flux density on arm surface. (a) Peak-to-peak values of the y-direction component from five different arm models. (b) Peak-to-peak values of the y-direction component from five different magnet models.
C. Performance of Shield Cap in Shielding Magnetostatic Interference
A shield cap model with an inner radius of 60 mm with respect to the basic arm model’s radius of 50 mm and a length of 70 mm was used in the simulation [see Fig. 2(b)]. The shield cap model had a wall thickness of 3 mm, which is the smallest value we could use in the FEMLAB simulation software [23], and a low relative permeability (5000). In practical application, very thin magnetic shielding materials with very high relative permeability (such as a magnetic shielding alloy with a thickness of 16 μm and with a permeability of 1 000 000, MetGlad, Less EMF, Inc., Albany, NY) would be employed and may provide the same or stronger shielding effects. The outside cylindrical permanent magnet [see Fig. 2(b)] had a radius of 10 mm and a length of 10 mm and represented a motor magnet that might be used for a powered humeral rotator. For example, an eight-pole high-torque brushless dc motor (HT02000 Emoteq, Inc.) has eight permanent magnets measuring 11 mm × 8.6 mm × 2.1 mm and the assembly of eight magnets are equivalent to a cylindrical magnet with a length of 10 mm and a radius of 7.1 mm. The residual magnetic flux density (Br) of the magnet was 11 kG (NdFeB magnet with Br = 10–13 kG, one of the commonly utilized high-performance rare earth motor magnets) [25]. This is likely an overestimate of the motor’s magnetic field since the eight permanent magnets would be separately mounted on the rotor surface and the motor would be generally covered by metal materials. The magnetic field distributions on the arm surface produced by the outside magnet were calculated using the FE model [see Fig. 2(b)] with and without the shield cap, respectively.
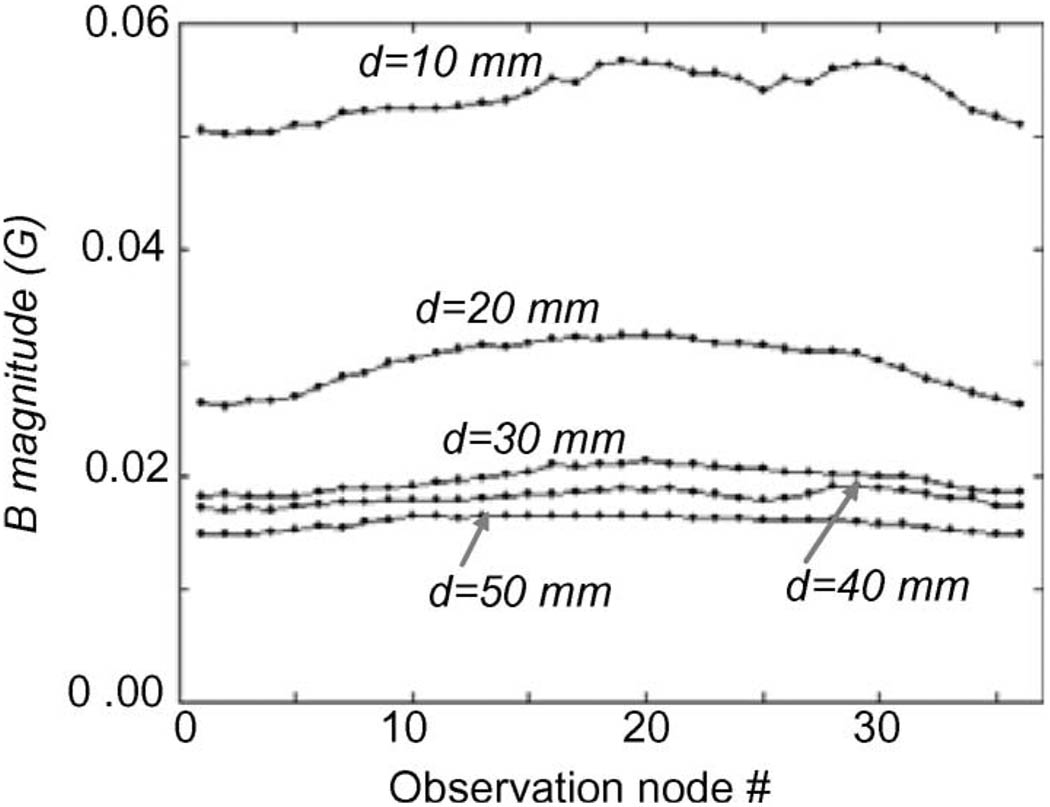
Without the shield cap, the maximum strength of the magnetic interference from the outside magnet was around 11 G. This would cause a significant influence on the magnetic field of the implanted magnet where the peak-to-peak value of the B⃑ magnitude was about 13 G [see Fig. 4(a)]. With the shield cap, when the distance (d) between the magnet and shield cap was set to 10, 20, 30, 40, and 50 mm, respectively, the simulated B⃑ magnitudes are graphically summarized in Fig. 8. The maximum strength of the magnetic interference was less than 0.06 G, a reduction of 99.5% compared to that without the shield cap.
Fig. 8.
Attenuation of magnetostatic interference with shielding. Magnetic flux density magnitudes induced by the permanent magnet outside the shield cap are plotted over the 36 surface observation nodes. The distance (d) between the magnet and shield cap was 10, 20, 30, 40, and 50 mm, respectively.
IV. DISCUSSION
In this present study, we attempted to address the challenge of humeral rotation control of prosthesis with a system that may provide some proprioceptive feedback to the amputees. To measure the rotation of the residual humerus, we proposed to surgically implant a permanent magnet into the distal end of the humerus to sense its rotation, and then to use sensors mounted in the prosthetic socket over the residual arm surface to sense the field of the magnet. From these detected magnetic field signals, the position of the residual humerus rotation could be derived as an input signal for control of powered humeral rotation in an upper limb prosthesis. The feasibility and performance of the newly proposed approach have been evaluated through a series of computer simulations.
The best case scenario may be considered when the humerus is exactly in the center of the residual arm. For an averaged limb size (radius = 50 mm) with an implanted magnet having a residual magnetic flux density of 11 kG, the peak-to-peak magnetic field strength [see Fig. 4(b) and (c)] was about 35 G over 90°. This provided a positioning resolution of about 0.39 G/° rotation of residual humerus. Current cable-activated locking humeral rotators can provide up to 180 locking positions (2° each). For an electrical powered humeral rotator, it is possible to position a prosthesis at any angle through a dc geared motor. In practical use, 1° angular resolution should be good enough for positioning of a prosthesis. If a higher resolution is necessary for humeral rotation, the magnetic field still could be detectable with high-sensitivity magnetic sensors for more accuracy control of humeral rotation of a prosthesis.
The change in field strength can be readily detected with available sensors and would allow a control resolution of less than 1°. Anisotropic magnetoresistive and giant magnetoresistive sensors, Hall-effect magnetic sensors, or fiber-optic magnetometers [26] are small and light magnetic sensors, which have a very high sensitivity in the detection of the magnetic field, a low and stable offset, a low sensitivity to mechanical stress, and only require very low power consumption. They therefore provide an excellent means of measuring the magnetic field of the magnet on arm surface. For instance, the Hall-effect sensor A3515 (Allegro MicroSystems, Inc.), is a sensitive and temperature-stable linear magnetic sensor with greatly improved offset characteristics. It can provide a linear sensitivity range of up to ±800 G, which completely covers the variation range of the magnetic field on the arm surface in all the simulated conditions. With a 5 mV/G output sensitivity (supply voltage = 5 V), when the residual humerus rotates 1°, the sensor could produce an analog output voltage of around 1.95 mV with respect to the positioning resolution of 0.39 G/° described earlier for an average sized arm with an 11 kG magnet. This analog voltage could be easily amplified with a high-performance instrumentation-amplifier and processed with conditioning circuitry. In addition, the sensor is packaged in a plastic three pin ultramini-SIP “UA” package with dimensions of (4.17 mm × 3.10 mm × 1.57 mm) and requires a total power dissipation of less than 50 mW (maximum supply current = 10 mA).
A sensitivity analysis of limb and magnet sizes was performed to assess the range of field strengths that would be produced on arm surface. In the worst case that a given-size magnet was implanted into the residual humerus in a big upper arm with a radius of 60 mm (arm circumference ≈ 377 mm), the magnet still generated a magnetic field change rate of about 0.22 G/° humerus rotation in the y-direction on arm surface. Using a Hall-effect magnetic sensor such as A3515 (Allegro MicroSystems, Inc.) to detect the magnetic field, this would produce an analog voltage change rate of about 1.1 mV/° humerus rotation. This may be adequate for signal amplification; however, a larger magnet may be needed for higher quality signals. With a given magnet size, a smaller limb will have larger magnetic fields on the limb surface. Similarly, smaller diameter magnets will produce smaller magnetic fields. The sensitivity analysis could serve as a guide for determining the needed magnet size with respect to the limb size and bone diameter. The data indicate that considerably smaller magnets could be used, if needed, with smaller residual limbs and smaller bones.
In practical application, the distal humerus and magnet will not be perfectly located in the middle of the residual limb. When wearing a prosthetic socket, the distal humerus will move anteriorly with arm flexion, laterally with abduction, posteriorly with extension and medially with adduction. How far the off-center depends on how well the humerus is secured with myodesis and myoplasty, the thickness of the overlying soft tissue layer (fat) and the loads on the socket. Our simulation results showed that when the off-center of the magnet was less than 5 mm that approximately corresponds to 2 lb load on the arm according to [27], the magnetic field distribution over the arm surface retained a similar sinusoidal pattern and the peak-to-peak strength of the y-direction component of the magnetic field had a 4.5 G increase over 90°. When the distal humerus was displaced greater than 15 mm that corresponds to about 5.2 lb load on the arm [27], the distribution of the y-direction component became a triphasic pattern, and its peak-to-peak strength increased greatly to 43 G for y-direction shift and 21 G for z-direction shift. Humeral displacement causes much greater magnetic fields to be produced at the limb surface and the loss of the simple sinusoidal pattern. The key issue is to accurately determine the vector of the magnetic field with an array of surface sensors, regardless of where the distal humerus is positioned within the residual limb. Further analyses need to be performed to design an optimal sensor array and controller. The number of sensors will affect magnetic vector angle resolution. Sensors may be desirable in more than one plane to accommodate the residual limb slippage in the socket. This research needs to be done, considering the large variation in the shape of residual limbs and issues of practical implementation with current prosthetic sockets.
Other magnetic fields that exist around a subject could interfere with the magnetic field sensors in the prosthesis. The largest expected source would be the magnetostatic interference from the permanent magnet motor of an elbow actuator. A shield cap analysis of performance in attenuating magnetostatic interference was carried out by simulation. The strength of the magnetostatic inference displayed a plateau distribution around the arm surface (Fig. 8). The results demonstrate that the interference of the magnetostatic fields around a subject could be effectively attenuated by a shield cap at the distal end of the residual arm by over 99%. This magnetostatic interference would bias the magnetic field sensors causing a positioning error of less than 0.2° for prosthetic humeral rotation control with respect to the positioning resolution of 0.39 G/° described before for an average sized arm with an 11 kG magnet. Even able-bodied people cannot accurately position their arm with a 0.2° resolution. Thus, this 0.2° positioning error would not significantly affect the accurately positioning of an artificial arm. However, as noted earlier, the use of smaller magnets may be more adversely affected by interference. Shield walls extending more proximally should further reduce outside magnetostatic interference. Also, dynamic magnetic interference from the motor and other ambient time-varying magnetic fields could be attenuated by the shield cap and/or with a low-pass filter, if necessary. Further study is needed, especially if implanting smaller magnets is necessary.
Another pragmatic issue is placing the magnet in a hermetically sealed casing. This issue has already been addressed with implanted magnets into the body in previous studies [28], [29] and does not appear to be a difficult challenge. The magnet would most likely be incased in titanium, a nonmagnetic material. The casing may include screw threading so that the magnet could easily be secured into a bone much like any other orthopedic screw.
Humeral rotation is caused by the shoulder girdle muscles acting on the proximal humerus. Humeral rotation produces arm rotation in able-bodied people as the distal humerus (the condyle) causes the lower arm to turn. Transhumeral amputees can fully rotate their humeri just like able-bodied people and can feel the rotation angle and where their lost arm would be pointing. This is due to the proprioceptive elements of the shoulder complex: a complex system of feedback from muscle spindles, golgi tendon organs, joint capsule afferents, and other inputs. However, humeral rotation does not translate into arm rotation in the arm amputees. The skin may be adhered to the prosthetic socket with a vacuum fit, but the humeral rotation generally causes little prosthetic arm rotation because the bone simply rotates in a compliant soft tissue envelope. Thus, it is critical to measure the humeral rotation if we want the amputees to be able to intuitively rotate their prosthetic arm. Magnets or sensors placed in the muscle or under the skin would be insufficient to capture rotation of the bone. Another important application of this research would be for transradial amputees. The challenge remains in that little wrist rotation can be captured with current prosthetic sockets. The potential for providing an intuitive wrist control system with inherent position feedback is exciting for the transradial amputees as well. The anatomy is different in that the radius rotates over the ulnar. The analysis presented here shows promise for this level of amputation. The data indicate that an adequate field could be detected with the smaller forearm and likely with smaller magnets.
The proposed approach of implanting a magnet in residual limb bones has some risks. It would require surgery and would implant a foreign object into a bone. However, implanting the magnet could be as simple as implanting a single orthopedic bone screw with minimal surgical risks. A device embedded in bone could compromise the structural integrity of the bone; however, bones remodel to accept stresses and the residual upper limb bones of an amputee bear little if any weight. Foreign objects can be a source of infection. However, the infection risk of the implanted magnet should be not greater than for a regular orthopedic screw, which is routinely left in a patient for the rest of their lives. Thus, we believe that the minimal risks would be well worth the potential benefits. It is noteworthy that permanent magnets implanted in bones have been used for other medical applications such as the attachment of facial prostheses [30] and the measurement of wrist joint movements [28], [29]. These previous works provide practical evidence that it is surgically feasible to implant a permanent magnet into bones.
In summary, the present simulation study suggests that the newly proposed magnetic approach has the ability to sense the rotation of the residual humerus for control of a powered humeral rotator in an arm prosthesis. The simulation results are encouraging, suggesting potential clinical applications to improve the control of powered prostheses with preservation of physiological proprioceptive feedback. This study provides important guidelines for developing a practical humeral rotation control system and also implies that this technique may be beneficial to transradial amputees.
ACKNOWLEDGMENT
The authors would like to thank T. W. Williams for ideas and input for this project, Dr. N. Stoykov for his helps in using the FEMLAB software, and Dr. J. Sensinger for his assistance in preparation of the paper.
Biographies

Guanglin Li (M’01–SM’06) received the B.S. and M.S. degrees in electrical engineering from Shandong University, Jinan, China, in 1983 and 1988, respectively, and the Ph.D. degree in biomedical engineering from Zhejiang University, Hangzhou, China, in 1997.
He joined the Department of Electrical Engineering, Shandong University, where in 1998, he became an Associate Professor. During 1999–2002, he was a Research Fellow, and later, a Postdoctoral Research Associate in the Department of Bioengineering, University of Illinois. From 2002 to 2005, he was a Senior Research Scientist at BioTechPlex Corporation. Since 2006, he has been with the Rehabilitation Institute of Chicago (RIC), Chicago, where he is currently a Senior Research Scientist in the Neural Engineering Center for Artificial Limbs. He has also been a faculty member of the Northwestern University (NU), Chicago, where he is currently a Research Assistant Professor of physical medicine and rehabilitation. His current research interests include neuroprosthesis, neural-machine interface, biomedical signal analysis, and computational biomedical engineering.

Todd A. Kuiken (M’99–SM’07) received the B.S. degree in biomedical engineering from Duke University, Durham, NC, in 1983, and the Ph.D. degree in biomedical engineering and the M.D. degree from the Northwestern University, Chicago, IL, in 1989 and 1990, respectively. He completed his residency in physical medicine and rehabilitation at the Rehabilitation Institute of Chicago and Northwestern University Medical School, Chicago, in 1995.
During 1995, he was with the Rehabilitation Institute of Chicago (RIC) and the Northwestern University Medical School, Chicago, IL as a Residency Trainee in physical medicine and rehabilitation. He is currently the Director of the Neural Engineering Center for Artificial Limbs and of Amputee Services, Rehabilitation Institute of Chicago, Chicago. He is also an Associate Professor in the Department of Physical Medicine and Rehabilitation (PM&R) and Biomedical Engineering, Northwestern University. He is also the Associate Dean for Academic Affairs at Feinberg School of Medicine, Rehabilitation Institute of Chicago.
Contributor Information
Guanglin Li, Shandong University, Jinan 250100, China. He is now with the Neural Engineering Center for Artificial Limbs, Rehabilitation Institute of Chicago, Chicago, IL 60611 USA, and also with the Department of Physical Medicine and Rehabilitation, Northwestern University, Chicago, IL 60611 USA (guanglin-li@northwestern.edu).
Todd A. Kuiken, Neural Engineering Center for Artificial Limbs, Rehabilitation Institute of Chicago, Chicago, IL 60611 USA, and also with the Department of Physical Medicine and Rehabilitation, Northwestern University, Chicago, IL 60611 USA (tkuiken@northwestern.edu).
REFERENCES
- 1.Taylor C. The biomechanics of the normal and of the amputated upper extremity. In: Klopsteg PE, Wilson PD, editors. Human Limbs and Their Substitutes. New York: McGraw-Hill; 1954. pp. 169–221. [Google Scholar]
- 2.Engen TJ, Spencer WA. Development of externally powered upper extremity orthotics. Texas Inst. Rehabil. Res., Houston, TX, Final Rep. Proj. RD-1564. 1969 Jan. [Google Scholar]
- 3.Atkins DJ, Heard DCY, Donovan WH. Epidemiologic overview of individuals with upper-limb loss and their reported research priorities. J. Prosthet. Orthot. 1996;vol. 8:2–11. [Google Scholar]
- 4.Brånemark PI, Rydevik BL, Skalak R. Osseointegration in the Skeletal Reconstruction and Joint Replacement. Chicago, IL: Quintessence Publishing; 1997. [Google Scholar]
- 5.Bloebaum R. International symposium on osseointegration. presented at the 32nd Acad. Annu. Meet. Sci. Symp.; Chicago, IL.2006. [Google Scholar]
- 6.Witsø E, Kristensen T, Benum PL, Sivertsen S, Persen L, Funderud A, Magne T, Aursand HP, Aamodt A. Improved comfort and function of arm prosthesis after implantation of a humerus-T-prosthesis in transhumeral amputees. Prosthet. Orthot. Int. 2006;vol. 30:270–278. doi: 10.1080/03093640600605013. [DOI] [PubMed] [Google Scholar]
- 7.Fairley M. Osseointegation: In the wave of the future? O&P Edge. 2006 Sep. [Google Scholar]
- 8.Dietz V. Proprioception and locomotor disorders. Nature Rev. Neurosci. 2002;vol. 3:781–790. doi: 10.1038/nrn939. [DOI] [PubMed] [Google Scholar]
- 9.Parker PA, Scott RN. Myoelectric control of prostheses. Crit. Rev. Biomed. Eng. 1986;vol. 13:283–310. [PubMed] [Google Scholar]
- 10.Scott RN, Parker PA. Myoelectric prostheses: State of the art. J. Med. Eng. Technol. 1988;vol. 12:143–151. doi: 10.3109/03091908809030173. [DOI] [PubMed] [Google Scholar]
- 11.Graupe D, Salahi J, Kohn K. Multifunctional prosthesis and orthosis control via microcomputer identification of temporal pattern differences in single-site myoelectric signals. J. Biomed. Eng. 1982;vol. 4:17–22. doi: 10.1016/0141-5425(82)90021-8. [DOI] [PubMed] [Google Scholar]
- 12.Hudgins B, Parker PA, Scott RN. A new strategy for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 1993 Jan.vol. 40(no. 1):82–94. doi: 10.1109/10.204774. [DOI] [PubMed] [Google Scholar]
- 13.Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet. Orthot. Int. 2004;vol. 28:245–253. doi: 10.3109/03093640409167756. [DOI] [PubMed] [Google Scholar]
- 14.Englehart K, Hudgins B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 2003 Jul.vol. 50(no. 7):848–854. doi: 10.1109/TBME.2003.813539. [DOI] [PubMed] [Google Scholar]
- 15.Chu JU, Moon I, Mun MS. A real-time EMG pattern recognition system based on linear-nonlinear feature projection for a multifunction myoelectric hand. IEEE Trans. Biomed. Eng. 2006 Nov.vol. 53(no. 11):2232–2239. doi: 10.1109/TBME.2006.883695. [DOI] [PubMed] [Google Scholar]
- 16.Lake C, Dodson R. Progressive upper limb prosthetics. Phys. Med. Rehabil. Clin. North Amer. 2006;vol. 17(no. 1):49–72. doi: 10.1016/j.pmr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Stavroulakis P. Biological Effects of Electromagnetic Fields. New York: Springer-Verlag; 2003. [Google Scholar]
- 18.Uitert RV, Johnson C, Zhukov L. Influence of head tissue conductivity in forward and inverse magnetoencephalographic simulations using realistic head models. IEEE Trans. Biomed. Eng. 2004 Dec.vol. 51(no. 12):2129–2137. doi: 10.1109/TBME.2004.836490. [DOI] [PubMed] [Google Scholar]
- 19.Merck Manual. Introduction: Undernutrition. Merck Manual Online Medical Library. 2007 Jun. [Online]. Available: http://www.merck.com/mmpe/print/sec01/ch002/ch002 a.html.
- 20.Lowery MM, Stoykov NS, Taflove A, Kuiken TA. A multiple-layer finite-element model of the surface EMG signal. IEEE Trans. Biomed. Eng. 2002 May;vol. 49(no. 5):446–454. doi: 10.1109/10.995683. [DOI] [PubMed] [Google Scholar]
- 21.Heymsfield SB, Mc Manus C, Smith J, Stevens V, Nixon DW. Anthropometric measurements of muscle mass: Revised equations for calculating bone-free arm muscle area. Amer. J. Clin. Nutr. 1982;vol. 36:680–690. doi: 10.1093/ajcn/36.4.680. [DOI] [PubMed] [Google Scholar]
- 22.Malmivuo J, Plonsey R. Bioelectromagnetism: Principles and Applications of Bioelectric and Biomagnetic Fields. London, U.K.: Oxford Univ. Press; 1995. [Google Scholar]
- 23.FEMLAB Electromagnetics Module (Version 2.3) Munich, Germany: COMSOL; 2002. [Google Scholar]
- 24.Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Amer. J. Clin. Nutr. 1981;vol. 34:2540–2545. doi: 10.1093/ajcn/34.11.2540. [DOI] [PubMed] [Google Scholar]
- 25.Tsai W, Chang T. Analysis of flux leakage in a brushless permanent-magnet motor with embedded magnets. IEEE Trans. Magn. 1999 Jan.vol. 35(no. 1):543–547. [Google Scholar]
- 26.Lenz JE. A review of magnetic sensors. Proc. IEEE. 1990 Jun.vol. 78(no. 6):973–989. [Google Scholar]
- 27.Sensinger JW, Weir RFff. Modeling and preliminary testing socket-residual limb interface stiffness of above-elbow prosthese. IEEE Trans. Neural Syst. Rehabil. Eng. 2008 Apr.vol. 16(no. 2):184–190. doi: 10.1109/TNSRE.2008.918388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson MW, Peckham PH, Bhadra N, Kilgore KL, Gazdik MM, Keith MW, Strojnik P. Implantable transducer for two-degree of freedom joint angle sensing. IEEE Trans. Rehabil. Eng. 1999 Sep.vol. 7(no. 3):349–359. doi: 10.1109/86.788471. [DOI] [PubMed] [Google Scholar]
- 29.Bhadra N, Peckham PH, Keith MW, Kilgore KL, Montague F, Gazdik M, Stage T. Implementation of an implantable joint-angle transducer. J. Rehabil. Res. Dev. 2002;vol. 39:411–422. [PubMed] [Google Scholar]
- 30.Nestle B, Lukas D, Schwenzer N. Retention force of magnets in endosteal implants used for facial prosthesis. Int. J. Oral Maxillofac. Surg. 1999;vol. 28:41–44. [PubMed] [Google Scholar]