Abstract
Background/Aims
BALB/c mice with a homozygous deficiency in the Tgfb1 gene are a model of fulminant autoimmune hepatitis (AIH), spontaneously and rapidly developing Th1-mediated IFN-γ-dependent necroinflammatory liver disease. We sought to understand the molecular basis for fulminant Th1 liver disease and the specific role of the Ifng gene.
Methods
Global gene expression in livers from BALB/c Tgfb1−/− mice with and without an intact Ifng gene was assessed by microarray analysis. Expression patterns were confirmed by quantitative reverse transcriptase-polymerase chain reaction. Gene ontology clustering analysis was performed to identify altered pathways. The contributions of Ifng to altered expression pathways were quantified.
Results
Over 100 genes were strongly (> 10-fold) upregulated, most encoding proteins involved in immune function/response. Chemokines were the most prominently upregulated group, with eight chemokine genes upregulated > 10-fold. Ifng was necessary for the upregulation of CXC chemokines gene, but not of CC chemokine genes. By quantitative analysis, Ifng’s role in liver gene upregulation varied greatly among overexpressed genes.
Conclusions
Gene expression changes indicate a particularly important and heretofore unappreciated role for chemokines in fulminant AIH. Ifng has an important role in expression of some but not all genes. Ifng is dichotomous in the regulation of distinct chemokine subfamilies: specifically, Ifng is critical for overexpression of specific CXCL genes but dispensable for overexpression of specific CCL genes. These results provide a clearer understanding of the role of Ifng in the molecular basis of necroinflammatory liver disease.
Keywords: autoimmune hepatitis, chemokine, gene expression, interferon-γ– Th1
Introduction
The disease autoimmune hepatitis (AIH) is the clinical manifestation of break-through of normal hepatic tolerance mechanisms, with persistent immune-mediated destruction of hepatocytes (1). Disease progression is typically controlled through chronic use of immunosuppressive medications. However, as many as 10–20% of patients fail medical therapy (2, 3) and may require orthotopic liver transplantation. Prominent features of AIH include a strong genetic influence (4, 5), a failure of normal tolerogenic mechanisms in the liver (6–8) and a probable Th1 pathogenesis involving the accumulation of CD4+ T cells producing IFN-γ. Liver infiltrating CD4+ T cells from biopsies of AIH patients are found in portal tracts on biopsy and produce copious IFN-γ ex vivo (9, 10). Understanding the mechanisms responsible for the pathogenic activity of Th1 CD4+ T cells in the AIH liver is important to devise new rational therapies for this difficult disease.
The cytokine transforming growth factor-β 1 (TGF-β1) is a central player in the development and maintenance of immune tolerance (11). We have demonstrated that TGF-β1 plays a key role in the maintenance of immune tolerance in the liver. Mice homozygous for a targeted deletion of the Tgfb1 gene rapidly accumulate CD4+ T cells in the liver; furthermore, BALB/c-background Tgfb1null/null homozygotes (Tgfb1−/− mice) develop a severe fulminant necroinflammatory liver disease with widespread hepatocellular damage (12). The CD4+ T-cell subset is intimately involved in pathogenesis, as its monoclonal antibody-mediated depletion in presymptomatic mice prevents the development of necroinflammatory liver disease (13). CD4+ T cells isolated from Tgfb1−/− livers are highly skewed to the Th1 phenotype, producing copious IFN-γ (12). Ifng−/−Tgfb1−/− double knockout mice are largely protected from liver disease (12), establishing the Th1 cytokine IFN-γ as a key factor in the development of hepatocellular damage. However, the specific role of IFN-γ in the development of liver pathology in this mouse model has not been determined.
To further investigate the pathology involved in the development of fulminant necroinflammatory liver disease, we profiled liver gene expression in Tgfb1−/− mice using microarray. To advance our understanding of the pathological role of IFN-γ, we similarly examined livers from mice doubly deficient in both Ifng and Tgfb1.
Methods
Mice
Mice were bred at the Dartmouth Medical School in an American Association Accreditation of Laboratory Animal Care-accredited animal care facility and were treated humanely according to National Institutes of Health guidelines. The derivations of Tgfb1−/− mice and Ifng−/− Tgfb1−/− mice are published (12). Litters were screened by PCR of DNA from tail snips for Tgfb1 genotypes as described (14).
RNA purification and application of Affymetrix GeneChip® microarrays
Livers were dissected from 11-day-old BALB/c mice of the following genotypes (three mice per genotype): Ifng+/+Tgfb1+/+, Ifng+/+Tgfb1−/−, Ifng−/−Tgfb1+/+ and Ifng−/−Tgfb1−/−. Livers were homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and liver RNA prepared. Gene expression analysis used a total of 12 GeneChip® Mouse Genome 430 2.0 microarrays (Affymetrix, Santa Clara, CA, USA), which were processed with standard labelling, one-cycle amplification, hybridization, washes and scanning, completed at the Dartmouth Genomics and Microarray Laboratory.
Microarray data analysis
Resulting data were analysed using GeneTraffic™ version 3.2 (Stratagene, La Jolla, CA, USA). Expression values in CHP format were imported into the GeneTraffic suite and subjected to robust multi-array average (RMA) normalization using established algorithms (15). For multitesting, data in CEL format were uploaded into the Bioconductor Microarray Analysis server (Bioinformatics Shared Resource, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA) and similarly standardized using RMA. Significance was determined using a standard Wilcoxon’s rank sum test with Benjamini and Hochberg false discovery rate correction for multitesting (16). Resulting data were annotated and visualized using the exploratory visual analysis (EVA) program (17) by the Computational Genetics facility at Dartmouth-Hitchcock Medical Center (Lebanon, NH, USA). Reported statistical significance of cluster enrichment was determined using EVA. Cluster analysis was performed using GeneTraffic. Genes were clustered by hierarchical analysis using the Pearson correlation distance metric. Results were visualized using the imaging tools provided by GeneTraffic. Additionally, the lists containing genes that Bioconductor analysis identified as differentially expressed were uploaded to the Database for Annotation, Visualization and Integrated Discovery [DAVID; NIAID, NIH, http://david.abcc.ncifcrf.gov/, (18)]. Verification of clustering and pathway analysis was performed on the DAVID online suite.
Determination of the relative contribution of Ifng to aberrant gene expression in Tgfb1−/− mice
For each gene, the following formula was used to calculate the contribution of Ifng to Tgfb1−/−-specific expression, and expressed as a ratio (R):
where E is the mean expression level (in arbitrary units) for three genotypically matched mice. For each gene, this formula incorporates the fold-change in expression when on an Ifng−/− background, calculating the ratio of expression changes on Ifng−/− vs. Ifng+/+ backgrounds. A ratio of ~1 indicates that upregulation of the gene in Tgfb1−/− liver is intact in the absence of functional IFN-γ and is therefore IFN-γ independent. A ratio of ~0 indicates that upregulation of the gene in Tgfb1−/− liver is eliminated in the absence of IFN-γ and is therefore IFN-γ dependent. A ratio between 0 and 1 indicates that upregulation of the gene is attenuated but not eliminated in the absence of IFN-γ and is therefore partially IFN-γ dependent.
Real-time quantitative reverse transcriptase-polymerase chain reaction
Reverse transcription was performed using the Taqman® RT Kit (Applied Biosystems, Foster City, CA) according to the manufacturer and used oligo-dT primers. PCR amplicons using specific primers [supporting information Table S1; (19–22)] were detected by SYBR Green. Each reaction was carried out in triplicate, and the mean was used in calculations. All values were measured in relation to the level of β-actin mRNA in the same sample. Gene expression was quantified relative to Tgfb1+/+ controls using the 2− (experimental CT − β-actin CT) method (23).
Results
Large changes in gene expression accompany fulminant hepatitis in Tgfb1−/− mice
We used microarray to identify genes whose expression level was altered in total liver RNA from Tgfb1−/− mice, all of whom exhibited fulminant liver necroinflammation. Of 28 287 genes queried, 111 (0.39%) were upregulated > 10-fold, and 43 and 11 were upregulated > 20- and 40-fold respectively (Table 1). Using equivalent cut-offs, 11, 4 and 0 unique genes were downregulated more than 10-, 20- and 40-fold respectively. Thus, fulminant necroinflammatory disease in Tgfb1−/− livers is more associated with gene upregulation than with gene downregulation.
Table 1.
Numbers of genes upregulated or downregulated in inflamed livers
| Genes |
||
|---|---|---|
| Fold change* | n† | %‡ |
| 40 | 11 | 0.039 |
| 20 | 43 | 0.15 |
| 10 | 111 | 0.39 |
| 5 | ND | ND |
| 1/5 | 57 | 0.20 |
| 1/10 | 11 | 0.039 |
| 1/20 | 4 | 0.014 |
| 1/40 | 0 | 0.0 |
Fold change indicates the threshold used to classify genes based on expression in Tgfb1−/− livers vs. Tgfb1+/+ livers.
The number of genes on the microarray significantly (P < 0.05 by Wilcoxon’s rank sum test) upregulated or downregulated at or above each fold change threshold was determined.
The percentage was calculated by dividing the number of genes indicated at each fold change by the total number of mouse genes (28 287; http://nar.oxfordjournals.org/cgi/reprint/33/suppl_1/D471.pdf).
ND, not determined.
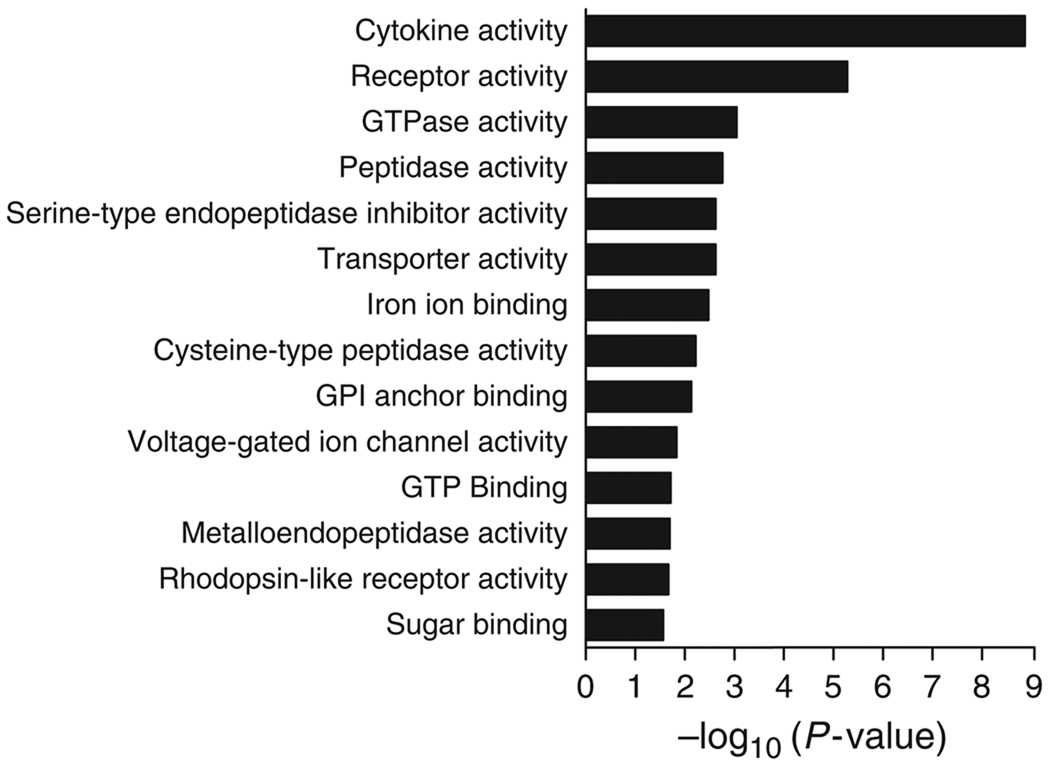
The 111 genes upregulated at least 10-fold were grouped using EVA according to their gene ontology (GO) function. Genes falling into the cytokine, receptor, GTPase and peptidase groups were the most highly enriched (P < 0.05; Fig. 1). From these four GO function groups, specific genes upregulated more than 10-fold are listed in Table 2. In the cytokine gene group, eight of nine genes thus identified are members of the chemokine class of signalling proteins, encoding four CXC chemokines, four CC chemokines, as well as IL-1β. The receptor activity group comprises a diverse list that includes Fc receptors, colony stimulating factor receptors, chemokine receptors and membrane-spanning 4A receptors. The GTPase activity group includes three guanylate nucleotide-binding proteins and a T-cell-specific GTPase. The peptidase activity group includes several granzymes,matrix metallopeptidases and cathepsins.
Fig. 1.
Enrichment of gene ontology (GO) function groups with genes upregulated in inflamed livers. Genes significantly upregulated (10-fold or greater, P < 0.05 as determined by Wilcoxon’s rank-sum test) in Tgfb1−/− liver RNA were grouped by GO function using the exploratory visual analysis program. Bars show the −log10 (significance) for the enrichment of each group.
Table 2.
Gene ontology grouping of genes induced > 10-fold
| Cluster | Symbol | Gene or protein name* | Fold induction |
|---|---|---|---|
| Cytokine activity, n = 9 | Cxcl11 | Chemokine (C–X–C motif) ligand 11 | 51.6 |
| Cxcl9 | Chemokine (C–X–C motif) ligand 9 | 29.5 | |
| Cxcl2 | Chemokine (C–X–C motif) ligand 2 | 25.9 | |
| Ccl6 | Chemokine (C–C motif) ligand 6 | 22.4 | |
| Ccl5 | Chemokine (C–C motif) ligand 5 | 17.9 | |
| Il1b | Interleukin 1 β | 16.8 | |
| Cxcl10 | Chemokine (C–X–C motif) ligand 10 | 11.1 | |
| Ccl3 | Chemokine (C–C motif) ligand 3 | 10.9 | |
| Ccl24 | Chemokine (C–C motif) ligand 24 | 10.5 | |
| Receptor activity, n = 22 | Ms4a6d | Membrane-spanning 4-domains, subfamily A, member 6D | 39.6 |
| Fcgr3a | Fc fragment of IgG, low affinity IIIa, receptor | 38.1 | |
| Marco | Macrophage receptor with collagenous structure | 26.8 | |
| Csf2rb1 | Colony stimulating factor 2 receptor, β1, low-affinity (g-m) | 26.3 | |
| Il1rn | Interleukin 1 receptor antagonist | 24.7 | |
| Cd274 | CD274 antigen | 24.0 | |
| Fpr-rs2 | Formyl peptide receptor, related sequence 2 | 22.1 | |
| Ccr1 | Chemokine (C–C motif) receptor 1 | 21.5 | |
| Csf2rb2 | Colony stimulating factor 2 receptor, β2, low-affinity (g-m) | 20.8 | |
| Cd300lf | CD300 antigen like family member F | 17.7 | |
| Pigr | Polymeric immunoglobulin receptor | 15.8 | |
| Ccr5 | Chemokine (C–C motif) receptor 5 | 13.4 | |
| Il2rg | Interleukin 2 receptor, γ-chain | 13.4 | |
| Fpr1 | Formyl peptide receptor 1 | 13.0 | |
| Fcgr3 | Fc receptor, IgG, low affinity III | 12.7 | |
| Ms4a6b | Membrane-spanning 4-domains, subfamily A, member 6B | 12.3 | |
| Gpr109a | G protein-coupled receptor 109A | 12.0 | |
| Clec4d | C-type lectin domain family 4, member d | 11.9 | |
| Procr | Protein C receptor, endothelial | 11.8 | |
| Fcgr1 | Fc receptor, IgG, high affinity I | 11.7 | |
| Ms4a4b | Membrane-spanning 4-domains, subfamily A, member 4B | 11.4 | |
| Ms4a8a | Membrane-spanning 4-domains, subfamily A, member 8A | 10.7 | |
| GTPase activity, n = 5 | 5830443L24Rik | RIKEN cDNA 5830443L24 gene | 27.7 |
| Gbp1 | Guanylate nucleotide-binding protein 1 | 23.6 | |
| Gbp2 | Guanylate nucleotide binding protein 2 | 19.2 | |
| Tgtp | T-cell-specific GTPase | 13.2 | |
| Gbp3 | Guanylate nucleotide-binding protein 3 | 12.6 | |
| Peptidase activity, n = 9 | Gzmb | Granzyme B | 44.3 |
| Mmp13 | Matrix metallopeptidase 13 | 39.3 | |
| Mmp12 | Matrix metallopeptidase 12 | 34.4 | |
| Ctsc | Cathepsin C | 18.0 | |
| Adam8 | A disintegrin and metallopeptidase domain 8 | 12.4 | |
| Gzma | Granzyme A | 10.7 | |
| Serpinb6b | Serine (or cysteine) peptidase inhibitor, clade B, member 6b | 10.5 | |
| Ctss | Cathepsin S | 10.4 | |
| Casp4 | Caspase 4, apoptosis-related cysteine peptidase | 10.2 |
Genes induced > 10-fold and populating the four most highly enriched clusters (Fig. 1) are listed.
The 57 genes downregulated at least fivefold are distributed widely among various GO groups, with more prominent representation among the oxidoreductase activity, lyase activity and transferase activity groups (data not shown). For the 11 genes downregulated > 10-fold, five are in the oxidoreductase activity group, including two cytochrome P450 familymembers (supporting information Table S2). Functional groups associated with upregulated genes were largely nonoverlapping with those associated with downregulated genes (P < 0.05; data not shown). Groups associated with upregulated gene expression are generally populated by immune genes and genes encoding proteases, whereas groups associated with downregulated gene expression are generally populated by genes encoding proteins involved in microsomal function and oxidative metabolism. This distinction was independently confirmed by annotation and analysis using the DAVID database (18) (data not shown).
Ifng is important for the upregulation of some but not all genes in inflamed livers from Tgfb1−/− mice
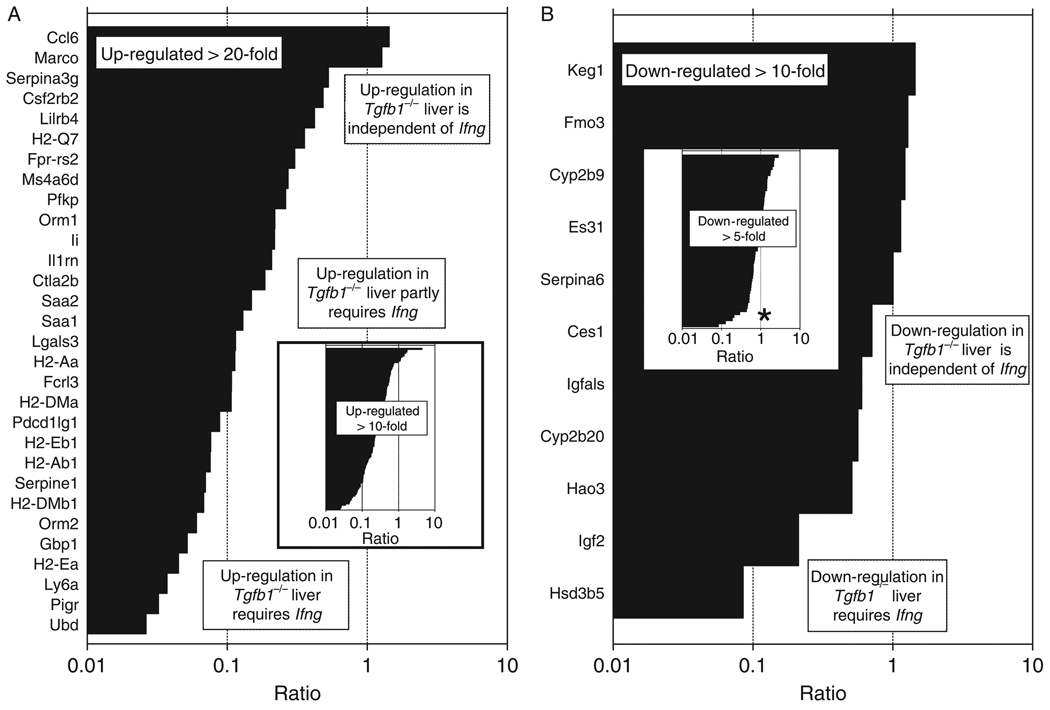
To more precisely define at the molecular level which changes in gene expression are dependent upon IFN-γ, we carried out microarray analyses of livers from Tgfb1−/− mice on an Ifng−/− background, along with livers from Tgfb1+/+(Ifng−/−) littermate control mice. Genes previously found to be upregulated > 10-fold in Tgfb1−/− (Ifng+/+) livers were then clustered using GeneTraffic according to expression in Ifng−/−Tgfb1−/− livers (data not shown). Eighteen percent of genes upregulated in livers from Tgfb1−/− mice were not upregulated in livers from Tgfb1−/− mice on an Ifng−/− background, whereas 13% of genes upregulated in livers from Tgfb1−/− mice were upregulated equivalently whether on an Ifng−/− background or a Ifng+/+ background. The remaining 69% were partially upregulated in livers from Tgfb1−/− mice on an Ifng−/− background. The magnitude of the specific contribution of Ifng is depicted visually in Figure 2A and can be seen to vary greatly for 30 individual genes upregulated > 20-fold in Tgfb1−/− liver (Fig. 2A); a similar pattern is seen for genes upregulated > 10-fold. A similar analysis of downregulated genes shows thatmost genes are suppressed equivalently in livers from Tgfb1−/− mice, whether they are on an Ifng+/+ background or on an Ifng−/− background. Thus, in general, Ifng has a much greater influence on gene upregulation than gene downregulation in this model of fulminant hepatitis.
Fig. 2.
Requirement of Ifng for gene overexpression in liver inflammation varies among upregulated genes. (A) For 30 genes upregulated > 20-fold in TGF-β1−/− livers, the contribution of Ifng to upregulation of each gene was calculated as in ‘Methods’ and expressed as a ratio. A high ratio (~1) indicates that the gene is upregulated equivalently in livers in Tgfb1−/− mice on either Ifng+/+ or Ifng−/− backgrounds; i.e. gene upregulation is IFN-γ independent. A low ratio (approaching 0) indicates that the gene is upregulated in livers in Tgfb1−/− mice on an Ifng+/+ background but not on an Ifng−/− background; i.e. gene upregulation is Ifng dependent. Intermediate ratios indicate partial Ifng dependence. A ratio > 1 indicates that Ifng actually suppresses gene upregulation, such that gene expression is greater when both Tgfb1 and Ifng are interrupted. Inset: The same analysis applied to over 100 genes upregulated > 10-fold. Gene identifiers are not shown, because of image resolution limitations. The data show that genes upregulated in Tgfb1−/− liver cannot be readily classified into Ifng-dependent and Ifng-independent groups. Rather, the sigmoidal shape of the ‘curve’ shows that Ifng’s involvement is different for each gene, and that a continuum best describes Ifng’s role in regulation. (B) A similar analysis was applied to 11 genes downregulated > 10-fold, and (inset) over 50 genes downregulated > five-fold (gene identifiers not shown). By comparison with upregulated genes, most downregulated genes are fairly refractory to the presence or absence of an intact Ifng gene. The asterisk indicates a natural ‘breakpoint’ in the curve. The few genes below this breakpoint more strongly depend upon Ifng for their downregulation.
Confirmation of expression patterns by quantitative reverse transcriptase-polymerase chain reaction
To confirm expression patterns revealed by microarray, we examined the expression of selected genes by quantitative reverse transcriptase-polymerase chain reaction (QRT-PCR) using liver RNA isolated from an additional four to six mice per genotype group. By microarray analyses, the genes H2Ea and CCL3 are both strongly upregulated in Tgfb1−/− liver. However, overexpression of H2Ea is largely dependent upon Ifng, whereas the over-expression of CCL3 is independent of Ifng. Similar patterns of expression were observed when these genes were specifically measured by QRT-PCR (supporting information Fig. 1A). Moreover, we examined three genes downregulated on microarray in Tgfb1−/− liver (Keg, SerpinA6 and Ces); all yielded similar patterns of expression when analysed using QRT-PCR (supporting information Fig. 1B). As another demonstration of quality, the measured expression of the housekeeping gene β-actin was similar among microarray samples (supporting information Fig. 1C).
Specific chemokine genes are strongly overexpressed in hepatitic Tgfb1−/− livers
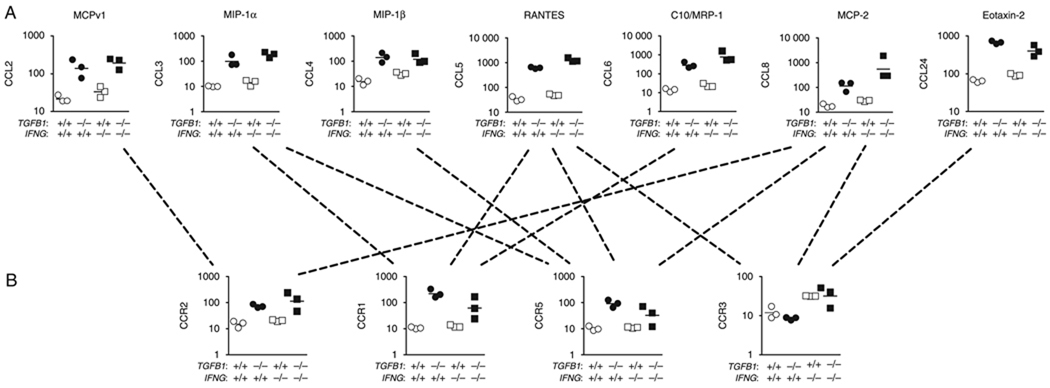
Because chemokines represented eight of nine genes in the cytokine GO function group, we more thoroughly examined the expression of chemokines and their receptors. Chemokines are a diverse family of small secreted proteins further categorized based on the relative positions of N-terminal cysteine residues. Among the CXC sub-family, four (CXCL2/Gro-β, CXCL9/Mig, CXCL10/IP-10 and CXCL11/ITAC) were upregulated in Tgfb1−/− livers > 10-fold, and expression was strongly dependent upon Ifng (Table 3, Fig. 3A). Other CXCL chemokines were either not detected or not elevated in inflamed livers (Table 3). Expression of genes encoding receptors for CXC chemokines (CXCR1–5) was similar between Tgfb1−/− livers and control livers (data not shown). Among the other chemokine families, seven CCL chemokines (CCL2, 3, 4, 5, 6, 8, 24) were upregulated (Fig. 4A). Unlike the four strongly upregulated CXCL chemokines, upregulation of each CCL chemokine was not dependent on Ifng (Fig. 3B). Among known or probable receptors for these chemokines, three (CCR1, 2, 5) were upregulated; expression was independent of Ifng (Fig. 4B). The CCR3 receptor, which binds CCL8 and CCL24, was not upregulated in Tgfb1−/− liver. No change in expression was observed for ligands in the C or CX3C subfamilies (data not shown).
Table 3.
CXCL chemokine expression in inflamed livers
| Change in expression in Tgfb1−/− liver |
||||
|---|---|---|---|---|
| Receptor | Cognate ligands | Alternate names | Ifng+/+ | Ifng−/− |
| CXCR1 | CXCL5* | ENA-78 | ||
| CXCR2 | CXCL1 | KC/Gro-α | ++ | + |
| CXCL2 | Gro-β | ++ | ||
| CXCL5* | ENA-78 | |||
| CXCL7 | NAP-2 | |||
| CXCR3 | CXCL9 | Mig | ++ | |
| CXCL10 | IP-10 | ++ | ||
| CXCL11 | I-TAC | ++ | ||
| CXCR3-B† | CXCL4 | PF4 | + | |
| CXCR4 | CXCL12 | SDF-1α | − | − |
| CXCR5 | CXCL13 | BCA-1 | ++ | |
| CXCR6 | CXCL16 | − | + | + |
| Unknown | CXCL14 | BRAK | ||
| Unknown | CXCL15 | Lungkine | ||
CXCL5 binds to both CXCR1 and CXCR2.
Alternative splice form of CXCR3.
+, increase of two- to 10-fold; ++, increase of > 10-fold; −, decrease of two- to 10-fold.
Fig. 3.
Ifng is differentially required for the overexpression of distinct chemokines. Selected genes were reanalysed by quantitative real-time reverse transcriptase-polymerase chain reaction (QRT-PCR) using an additional four to six 11-day-old mice per genotype. (A) Four upregulated CXCL genes (CXCL2, 9, 10 and 11) and (B) three upregulated CCL genes (CCL4, CCL5, CCL8) were measured by QRT-PCR. QRT-PCR data are normalized to a β-actin control QRT-PCR. Results from microarray and QRT-PCR are shown side-by-side. Units are arbitrary. The y-axes are in log10 format.
Fig. 4.
Strong upregulation of specific CC chemokines and their receptors in Tgfb1−/− livers. Microarray results for (A) seven CCL genes and (B) four CCR receptor genes are shown. Units are arbitrary. The y-axes are in log10 format. Dotted lines indicate known or putative ligand–receptor interactions, underscoring the substantial redundancy in this system.
Discussion
The strong consistent changes in gene expression combined with reproducibility at QRT-PCR using additional biological replicates indicate that the profiling data are high quality, and allow us to draw conclusions with confidence. The aggressive phenotype observed in BALB/c Tgfb1−/− mice, in which fulminant liver disease is 100% penetrant by 11 days of age (13), readily translates into robust gene expression differences. Most upregulated genes are involved in immune function or response, encoding chemokines, cytokines, their receptors, acute phase proteins, tetraspanins, major histocompatibility complex class II proteins, proteins important for antigen presentation and a variety of proteases and granzymes. These observations are consistent with the important role of the immune system in fulminant hepatitis and with previous histological and flow cytometric findings that T cells, macrophages and neutrophils are abundant in the liver in this model system (13, 24). By contrast, the downregulated genes are not involved in immune function per se but encode proteins involved in a variety of biological functions. Except for a few genes involved in oxidoreductase activity, functional relationships among downregulated genes are not readily apparent.
The Th1 cytokine IFN-γ is important for liver damage in Tgfb1−/− mice. Recent studies from our laboratory indicate that CD4+ T cells – but not CD8+ T cells, NK cells or other cell types – serve as the relevant source of IFN-γ for liver pathology in Tgfb1−/− mice (25). Notably, studies in AIH patient samples also implicate CD4+ T-cell-derived IFN-γ in disease pathogenesis (9, 10). However, whereas liver disease in Tgfb1−/− mice is significantly ameliorated when IFN-γ is absent, Ifng−/−Tgfb1−/− livers are not completely devoid of lesions and still exhibit T-cell accumulation in liver (25), some periportal inflammation (12) and a modest transaminitis developing after about 3 weeks of age (12). Thus, whereas IFN-γ is required for the full-blown necroinflammatory liver disease phenotype, there must be IFN-γ-independent pathways leading to dysregulation of the liver immune system and some liver pathology in Tgfb1−/− mice.
The microarray results provide molecular support for this contention; the requirement for Ifng varies greatly among overexpressed genes. For some genes [e.g. encoding the macrophage scavengerMarco or the GM-CSF-receptor subunit 2 (Csf2rb2)], upregulation is Ifng independent. For others [e.g. encoding the class II protein H2-Eα or the polymeric IgA receptor (Pigr)], upregulation is Ifng dependent. For others [e.g. encoding serum amyloid A1 (Saa1) or IL-1 receptor antagonist (IL1rn)], upregulation is partially Ifng dependent. Indeed, the role of Ifng in regulating gene expression in this system is remarkably complex. The curve in Figure 2A is continuous, with no obvious break to distinguish Ifng-dependent vs. Ifng-independent genes. Interestingly, downregulated genes behave differently, in that they are generally more refractory to the absence of Ifng and it is easier to define a ‘break point’ (asterisk in the inset to Fig. 2B) that distinguishes genes that do not require Ifng from those that do.
Strong chemokine upregulation is a prominent gene expression feature associated with fulminant liver disease in this mouse model. Chemokines are related members of a large family of small protein inflammatory mediators that potently elicit the chemotaxis of immune cells participating in inflammation. Chemokines are classified into four subfamilies based on the relative positions of N-terminal cysteine residues (CXC, CC, C and CX3C). Th1 cells preferentially express the chemokine receptors CXCR3 and CCR5 (26) and the ligands that interact with these receptors are chemoattractive for Th1 cells. CXCR3 is the exclusive receptor for three potent Th1 attracting ligands, CXCL-9, -10 and -11, all of which are strongly upregulated at the mRNA level in Tgfb1−/− liver in an Ifng-dependent fashion. CCR5 is an important receptor for the ligands CCL3, 4, 5, and 8, all of which are strongly upregulated, but in an Ifng-independent fashion. This dichotomy suggests that there are redundant pathways for the chemotaxis and accumulation of Th1 cells in Tgfb1−/− liver, one (via CXCR3) that is Ifng dependent, and one (via CCR5) that is Ifng independent.
Chemokine response pathways represent attractive therapeutic targets for autoimmune disease (27), so elucidating their roles may open up new treatment avenues. Chemokine signalling pathways have been implicated in the pathogenesis of a variety of autoimmune diseases including rheumatoid arthritis (28), systemic lupus erythematosis (29) and autoimmune thyroiditis (30), as well as in inflammation due to graft-versus-host disease (31, 32). In autoimmune demyelinating disease of the central nervous system, engagement of certain chemokine receptors is important for the homing of autoreactive T-lymphocytes to sites of demyelination and neuronal injury (33); however, other chemokines have been associated with neuroprotection (34), indicating complex roles for chemokines in the pathogenesis of autoimmune inflammatory disorders. Clinical trials using chemokine receptor antagonists in multiple sclerosis patients have met with limited success (35), although new approaches to the development of chemokine receptor antagonists may help to realize their therapeutic potential (36).
Only a small number of studies have specifically examined chemokines in autoimmune liver diseases. Plasma levels of CXCL9 and CXCL10 are significantly increased in primary biliary cirrhosis (PBC) patients and rise with disease progression. Portal areas of PBC livers stain for both chemokines. In addition, CXCR3-positive cells are more numerous in peripheral blood and found in portal areas of diseased livers (37). CXCL10 is highly upregulated in sera of AIH patients, and hepatocytes around portal tracts in AIH liver strongly express CXCL10 mRNA. Successful treatment of AIH with prednisolone correlates with a sharp decrease in serum CXCL10 levels (38). Fulminant hepatic failure is associated with early and strong upregulation of CCL2, 3, 4 and 5 (39). Together, these data indicate that several chemokines are overexpressed in patients with autoimmune liver disease in serum and (where investigated) in liver. The results presented here provide additional impetus to understand the role of chemokines in the pathogenesis of autoimmune inflammatory liver diseases.
Supplementary Material
Figure S1. Quantitative RT-PCR verification of microarray expression data. Selected genes were reanalyzed by quantitative real-time RT-PCR using an additional 4–6 eleven-day old mice per genotype. (A, B) Transcripts for (A) two up-regulated genes, encoding H2-Eα and CCL3, and (B) three down-regulated genes, encoding Keg, SerpinA6, and Ces, were measured by QRT-PCR. QRT-PCR data are normalized to a β-actin control QRT-PCR. Results from microarray and QRT-PCR are shown side-by-side. (C) β-actin expression levels from the microarrays are shown. (A, B, C) Units are arbitrary. The y-axes are in log10 format.
Table S1. Primer sequences used in Real-time RT-PCR.
Table S2. Gene ontology grouping of genes suppressed > 10-fold.
Acknowledgements
This work was supported by National Institutes of Health Grants AI053056 (J. D. G.), AI078195 (J. D. G.), DK073904 (J. D. G.) and P20RR16437 from the COBRE Program of the National Center for Research Resources, as well as by a grant from the Hitchcock Foundation (J. D. G.). J. W. was supported by a Samuel A. Hamacher Autoimmune Hepatitis Postdoctoral Research Fellowship from the American Liver Foundation. R. T. R. was supported by National Institutes of Health Training Grants T32AI07363 and T32AR07576. We are indebted to Christine Kretowicz and Beverly Gorham for expert breeding and screening of mice.
Footnotes
Accession number for microarrays: GSE9892; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=jjmtrseosyqownu&acc=GSE9892.
Supporting Information
Additional supporting information may be found in the online version of this article:
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54–66. doi: 10.1056/NEJMra050408. [DOI] [PubMed] [Google Scholar]
- 2.Czaja AJ, Davis GL, Ludwig J, Baggenstoss AH, Taswell HF. Autoimmune features as determinants of prognosis in steroid-treated chronic active hepatitis of uncertain etiology. Gastroenterology. 1983;85:713–717. [PubMed] [Google Scholar]
- 3.Czaja AJ, Davis GL, Ludwig J, Taswell HF. Complete resolution of inflammatory activity following corticosteroid treatment of HBsAg-negative chronic active hepatitis. Hepatology. 1984;4:622–627. doi: 10.1002/hep.1840040409. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson PT. Genetics in autoimmune hepatitis. Semin Liver Dis. 2002;22:353–364. doi: 10.1055/s-2002-35705. [DOI] [PubMed] [Google Scholar]
- 5.Czaja AJ, Donaldson PT. Genetic susceptibilities for immune expression and liver cell injury in autoimmune hepatitis. Immunol Rev. 2000;174:250–259. doi: 10.1034/j.1600-0528.2002.017401.x. [DOI] [PubMed] [Google Scholar]
- 6.Longhi MS, Ma Y, Bogdanos DP, et al. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol. 2004;41:31–37. doi: 10.1016/j.jhep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Vergani D, Mieli-Vergani G. Autoimmune hepatitis. Autoimmun Rev. 2003;2:241–247. doi: 10.1016/s1568-9972(03)00017-x. [DOI] [PubMed] [Google Scholar]
- 8.Lau AH, de Creus A, Lu L, Thomson AW. Liver tolerance mediated by antigen presenting cells: fact or fiction? Gut. 2003;52:1075–1078. doi: 10.1136/gut.52.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lohr HF, Schlaak JF, Lohse AW, et al. Autoreactive CD4+LKM-specific and anticlonotypic T-cell responses in LKM-1 antibody-positive autoimmune hepatitis. Hepatology. 1996;24:1416–1421. doi: 10.1002/hep.510240619. [DOI] [PubMed] [Google Scholar]
- 10.Hussain MJ, Mustafa A, Gallati H, et al. Cellular expression of tumour necrosis factor-alpha and interferon- gamma in the liver biopsies of children with chronic liver disease. J Hepatol. 1994;21:816–821. doi: 10.1016/s0168-8278(94)80244-0. [DOI] [PubMed] [Google Scholar]
- 11.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 12.Gorham JD, Lin JT, Sung JL, Rudner LA, French MA. Genetic regulation of autoimmune disease: BALB/c background TGF-beta 1-deficient mice develop necroinflammatory IFN-gamma-dependent hepatitis. J Immunol. 2001;166:6413–6422. doi: 10.4049/jimmunol.166.10.6413. [DOI] [PubMed] [Google Scholar]
- 13.Rudner LA, Lin JT, Park IK, et al. Necroinflammatory liver disease in BALB/c background, TGF-beta 1-deficient mice requires CD4+ T cells. J Immunol. 2003;170:4785–4792. doi: 10.4049/jimmunol.170.9.4785. [DOI] [PubMed] [Google Scholar]
- 14.Lin JT, Kitzmiller TJ, Cates JMM, Gorham JD. MHC-independent genetic regulation of liver damage in a mouse model of autoimmune hepatocellular injury. Lab Invest. 2005;85:550–561. doi: 10.1038/labinvest.3700246. [DOI] [PubMed] [Google Scholar]
- 15.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 16.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc. 1995;57:289–300. [Google Scholar]
- 17.Reif DM, Dudek SM, Shaffer CM, Wang J, Moore JH. Exploratory visual analysis of pharmacogenomic results. Pac Symp Biocomput. 2005;10:296–307. [PubMed] [Google Scholar]
- 18.Dennis G, Jr, Sherman BT, Hosack DA, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:R60.1–R60.11. [PubMed] [Google Scholar]
- 19.Kimura H, Kimura M, Rose NR, Caturegli P. Early chemokine expression induced by interferon-gamma in a murine model of Hashimoto’s thyroiditis. Exp Mol Pathol. 2004;77:161–167. doi: 10.1016/j.yexmp.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Jiankuo M, Xingbing W, Baojun H, et al. Peptide nucleic acid antisense prolongs skin allograft survival by means of blockade of CXCR3 expression directing T cells into graft. J Immunol. 2003;170:1556–1565. doi: 10.4049/jimmunol.170.3.1556. [DOI] [PubMed] [Google Scholar]
- 21.Overbergh L, Valckx D, Waer M, Mathieu C. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 1999;11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- 22.Du M, Irani RA, Stivers DN, Lee SJ, Travis EL. H2-Ea deficiency is a risk factor for bleomycin-induced lung fibrosis in mice. Cancer Res. 2004;64:6835–6839. doi: 10.1158/0008-5472.CAN-04-1678. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(− Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Robinson RT, French MA, Kitzmiller TJ, Gorham JD. Restriction of the CD4(+) T-cell receptor repertoire prevents immune pathology in TGF-beta1 knockout mice. Lab Invest. 2006;86:815–828. doi: 10.1038/labinvest.3700439. [DOI] [PubMed] [Google Scholar]
- 25.Robinson RT, Wang J, Cripps JG, et al. End-organ damage in a mouse model of fulminant liver inflammation requires CD4(+) T cell production of IFN-gamma but is independent of Fas. J Immunol. 2009;182:3278–3284. doi: 10.4049/jimmunol.0803417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Proudfoot AE, Power CA, Wells TN. The strategy of blocking the chemokine system to combat disease. Immunol Rev. 2000;177:246–256. doi: 10.1034/j.1600-065x.2000.17721.x. [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto T, Okamoto H, Toyama Y, Momohara S. Molecular aspects of rheumatoid arthritis: chemokines in the joints of patients. FEBS J. 2008;275:4448–4455. doi: 10.1111/j.1742-4658.2008.06580.x. [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni O, Anders HJ. Chemokines in lupus nephritis. Front Biosci. 2008;13:3312–3320. doi: 10.2741/2927. [DOI] [PubMed] [Google Scholar]
- 30.Kimura H, Caturegli P. Chemokine orchestration of autoimmune thyroiditis. Thyroid. 2007;17:1005–1011. doi: 10.1089/thy.2007.0267. [DOI] [PubMed] [Google Scholar]
- 31.Piper KP, Horlock C, Curnow SJ, et al. CXCL10-CXCR3 interactions play an important role in the pathogenesis of acute graft-versus-host disease in the skin following allogeneic stem-cell transplantation. Blood. 2007;110:3827–3832. doi: 10.1182/blood-2006-12-061408. [DOI] [PubMed] [Google Scholar]
- 32.Choi SW, Hildebrandt GC, Olkiewicz KM, et al. CCR1/CCL5 (RANTES) receptor-ligand interactions modulate allogeneic T-cell responses and graft-versus-host disease following stem-cell transplantation. Blood. 2007;110:3447–3455. doi: 10.1182/blood-2007-05-087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohler RE, Comerford I, Townley S, et al. Antagonism of the chemokine receptors CXCR3 and CXCR4 reduces the pathology of experimental autoimmune encephalomyelitis. Brain Pathol. 2008;18:504–516. doi: 10.1111/j.1750-3639.2008.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omari KM, Lutz SE, Santambrogio L, Lira SA, Raine CS. Neuroprotection and remyelination after autoimmune demyelination in mice that inducibly overexpress CXCL1. Am J Pathol. 2009;174:164–176. doi: 10.2353/ajpath.2009.080350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zipp F, Hartung HP, Hillert J, et al. Blockade of chemokine signaling in patients with multiple sclerosis. Neurology. 2006;67:1880–1883. doi: 10.1212/01.wnl.0000244420.68037.86. [DOI] [PubMed] [Google Scholar]
- 36.Horuk R. Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov. 2009;8:23–33. doi: 10.1038/nrd2734. [DOI] [PubMed] [Google Scholar]
- 37.Chuang YH, Lian ZX, Cheng CM, et al. Increased levels of chemokine receptor CXCR3 and chemokines IP-10 and MIG in patients with primary biliary cirrhosis and their first degree relatives. J Autoimmun. 2005;25:126–132. doi: 10.1016/j.jaut.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Nishioji K, Okanoue T, Itoh Y, et al. Increase of chemokine interferon-inducible protein-10 (IP-10) in the serum of patients with autoimmune liver diseases and increase of its mRNA expression in hepatocytes. Clin Exp Immunol. 2001;123:271–279. doi: 10.1046/j.1365-2249.2001.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leifeld L, Dumoulin FL, Purr I, et al. Early up-regulation of chemokine expression in fulminant hepatic failure. J Pathol. 2003;199:335–344. doi: 10.1002/path.1298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Quantitative RT-PCR verification of microarray expression data. Selected genes were reanalyzed by quantitative real-time RT-PCR using an additional 4–6 eleven-day old mice per genotype. (A, B) Transcripts for (A) two up-regulated genes, encoding H2-Eα and CCL3, and (B) three down-regulated genes, encoding Keg, SerpinA6, and Ces, were measured by QRT-PCR. QRT-PCR data are normalized to a β-actin control QRT-PCR. Results from microarray and QRT-PCR are shown side-by-side. (C) β-actin expression levels from the microarrays are shown. (A, B, C) Units are arbitrary. The y-axes are in log10 format.
Table S1. Primer sequences used in Real-time RT-PCR.
Table S2. Gene ontology grouping of genes suppressed > 10-fold.