Abstract
Increased Body Mass Index (BMI) is associated with reduced all cause and cardiovascular (CV) mortality in hemodialysis patients (HD), while CV risk increases with BMI in the general population. In the general population obesity is associated with inflammation, decreased HDL, increased LDL and triglycerides (TGs), all risk factors for CV disease. LDL does not predict CV risk in HD, whereas increased C-reactive protein (CRP), interleukin-6 (IL-6), low HDL or apo AI and increased fasting triglycerides (TG) do predict risk. Renal failure is associated with dyslipidemia and inflammation in normal weight patients. We hypothesized that effects of obesity may be obscured by virtue of renal failure in HD. We explored the relationship between adipose tissue pools and distribution, i.e., subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) (measured by MRI) and measures of inflammation (CRP, IL-6, ceruloplasmin and α 1 acid glycoprotein) HDL and LDL cholesterol, total TGs, apo A I, apo B, apo C II (an activator of lipoprotein lipase (LPL)) and CIII (an inhibitor of LPL) and the adipokines, leptin and adiponectin in 48 patients with prevalent HD. Total TG concentrations were positively correlated with VAT controlled for age, sex and weight. Both apo C II and apo C III correlated only with VAT. Adiponectin was inversely correlated with VAT and leptin was positively associated with SAT. CRP and α 1 acid glycoprotein were weakly associated with SAT while ceruloplasmin was strongly associated with VAT by multiple regression analysis. In contrast, apo B, LDL, apo A I and HDL, and IL-6 were not correlated with any measure of body composition potentially mitigating the effects of obesity in HD
Keywords: Inflammation, Apo A I, Apo C II, Apo C III, adipokines, leptin, adiponectin, HDL, LDL, triglycerides, ceruloplasmin
Introduction
While the relative risk of mortality is high among dialysis patients, one factor that predicts survival is having a higher BMI (1,2,3). BMI is imprecise as a measure of body composition and does not distinguish between muscle and fat mass. Nevertheless, the lack of an increase in mortality among a population with a very high BMI (>35 kg/m2) strongly supports a hypothesis that adiposity either in some way protects dialysis patients from mortality or that risk factors normally associated with adiposity may not be associated with adiposity among dialysis patients. Indices associated with lean body mass, such as total body water, approximated by the volume of distribution of urea (V) independently predict survival (4,5). Similarly, total body potassium, a measure of intracellular volume, predict survival in a variety of chronic diseases (6,7,8). While the positive association between lean body mass and survival can be explained by better preserved nutritional reserves or lower co-morbidities, the basis for improved survival among obese patients is not obvious, although a similar survival advantage is observed in patients with other chronic diseases (9).
Several cardiovascular risk factors are linked metabolically to body composition among subjects having normal kidney function (10). LDL cholesterol levels and triglyceride levels are positively associated with adiposity while HDL is inversely associated with adiposity among subjects having normal kidney function (11). Inflammation, as assessed by plasma CRP or IL-6 concentrations are also important cardiovascular risk factors both in dialysis patients as well as in subjects with normal kidney function (12,13,14). CRP and IL-6 levels are thought to be associated with adiposity in subjects with normal kidney function as a result of inflammation within or caused by increased visceral adipose mass (15,16). A third set of risk factors that are associated with adiposity both in the population having normal kidney function as well as in patients having kidney failure are the adipokines, leptin and adiponectin.
While some risk factors that predict cardiovascular disease among populations not having kidney failure are no longer predictive in dialysis patients, such as hypertension and Low Density Lipoprotein (LDL) cholesterol (17),other risk factors preserve their predictive values, such as C reactive protein (CRP), IL-6 (12,13,18,), and low levels of High Density Lipoprotein cholesterol (HDL) (17,19).
Elevated triglycerides (TG) are also associated with mortality among dialysis patients (19). While TG levels are associated with adiposity among subjects having normal kidney function, TG levels are also increased in dialysis patients by factors that may not be linked to body composition, such as increased levels of lipoprotein lipase inhibitors such as apolipoprotein C III (apo C III) (20,21) which is also linked both to insulin resistance and to increased body adiposity
A third cluster of risk factors associated with body composition modulating cardiovascular risk are adipokines (22,23). Leptin is secreted by adipocytes, and additionally is increased in dialysis patients beyond what would be anticipated by total fat mass, primarily because of reduced renal clearance of leptin (24,25), although the relationship to adiposity persists within populations of patients having renal failure (24,25). Leptin is associated with insulin resistance and increased circulating leptin has been directly associated with vascular disease (26,27,28,29). Circulating concentrations of a second adipokine, adiponectin, are inversely proportion to fat mass (30), and specifically inversely related to the important visceral adipose tissue mass (31,32). Adiponectin, like HDL has been reported to be inversely associated with vascular disease in subjects with normal renal function (33). The relationship between adiponectin levels and cardiovascular risk in patients having kidney disease is controversial. High levels have been associated with increased cardiovascular risk in some studies of dialysis patients (23) as well as in patients having stage 3 and 4 chronic kidney disease (34) while high levels have been reported to be protective in other studies (22).
In the present study, we measured body adipose tissue compartments and lean body mass using MRI in a cohort of hemodialysis patients to investigate the relationship between the relationship between adiposity and a number of risk factors for cardiovascular disease.
Methods
Institutional Review Board approval was obtained and all subjects signed an informed consent prior to participation. Forty-eight prevalent hemodialysis patients consisting of 20 women and 28 men over the ages of 18 years were chosen so as to encompass a wide range of body mass indices and ages. Thirty-seven patients were African American, 3 were non black Hispanic, 3 were white, and 2 were Asian and 3 were other. Sixteen patients had diabetes mellitus. For analysis of the effect of race patients were coded as black or non black because of the small numbers in the other groups. All but one subject had been on maintenance hemodialysis for at least 3 months prior to study. One subject had been on dialysis for 2 months. They were studied on the day of a regularly scheduled hemodialysis session approximately 3 hours prior to initiation of a dialysis treatment.
Following an overnight fast, body weight was measured to the nearest 0.1 kg (Weight Tronix, New York), and height was measured to the nearest 0.5 cm by using a stadiometer (Holtain, Crosswell, United Kingdom). Whole-body MRI scans were prepared by using a 1.5 Tesla scanners (6X Horizon; General Electric, Milwaukee) to evaluate muscle and fat mass (35).
All assays were performed on serum obtained while the patient was in the fasting state. The inflammatory markers C-reactive protein (CRP) interleukin-6 (IL-6), the long lived acute phase proteins ceruloplasmin and, α 1 acid glycoprotein , the adipokines leptin and adiponectin, and the apolipoproteins (Apo) apo A I, apo C II, apo C III, apo B, triglycerides, total cholesterol and high density lipoprotein (HDL) low density lipoprotein (LDL) cholesterol were measured. CRP, ceruloplasmin, apo A I apo B and α 1 acid glycoprotein were measured with rate nephelometry using a Beckman Array automated nephelometer (36). Apo C II and apo C III were measured nephelometrically using a Hitachi chemical analyzer, leptin and adiponectin were measured by RIA (Millipore, St. Charles, MO). All nephelometric measurements were made in duplicate in each of two optical systems. The average of these values was used for calculations.
Statistical analysis methods
Data are presented as mean, standard deviation, median and range. Distribution of variables for normality was assessed by Kolmogorov-Smirnov test (37). Variables that were non-normally distributed were log transformed. Multivariate analysis employed multiple linear regression analysis, with backward elimination (P < 0.1 for parameter retention in the model) with biochemical markers as dependent variables. The independent variables were subcutaneous adipose tissue mass (SAT) or visceral adipose tissue mass (VAT) or total adipose tissue mass (TAT) measured by MRI adjusted for age, sex, presence of diabetes, weight and race. We also analyzed the effect of VAT on cardiovascular risk factors using VAT as a categorical variable. We divided the population into tertiles and performed an analysis of variance using Tukey's test for significance. We controlled for the effects of age, race, and sex. A two-sided P-value < 0.05 was considered significant. JMP 5.0.1 (SAS Campus Drive Cary, NC 27513 USA) was used for statistical analysis.
Results
Median BMI was 27.3 kg/m2 (19.4-46.6), median age was 54.5 years (33-80), median weight 78.1 kg (43.1-120). Median total adipose tissue mass was 24.3 kg (6.2-57.9), median subcutaneous adipose tissue mass was 20.3 kg (5.8-50.8), median visceral adipose tissue mass was 3.25 kg (0.13-8.88), median skeletal muscle mass (SMM) was 23.3 kg (12.2-36.9). Vintage ranged from two months to 15.3 years with a median vintage of 2.8 years. Residual renal function expressed as urea clearance ranged from zero to 7.7 ml/min. Only 9 patients had any urine output. Among those 9 the median residual clearance was 1.4 ml/min. Median and range of risk factor are shown in Table 1.
Table 1. Median and range of Risk Factors Among Dialysis Patients.
| CRP g/L | 5.0(0.1-260) |
| IL-6 (pg/ml) | 5.5 (1-22) |
| α 1 AG (mg/dl) | 106(46-187) |
| Ceruloplasmin (mg/dl) | 42(23-73) |
| HDL Cholesterol (mg/dl) | 43 (8-79) |
| Apo A I (mg/dl) | 131(89-239) |
| Triglycerides (mg/dl) | 133(36-436) |
| Apo C III (mg/dl) | 15.0(2.9-35.7) |
| Apo C II (mg/dl) | 3.2(0.45-7.66) |
| LDL Cholesterol (mg/dl) | 66(13-142) |
| Remnants Chol (mg/dl) | 3.7(2.1-9.1) |
| Total Cholesterol (mg/dl) | 157(83-236) |
| Apo B (mg/dl) | 60.9(14.9-120) |
| Adiponectin (μg/ml) | 18.4(5.9-59.4) |
| Leptin (ng/ml) | 9.85(0.2-127.2) |
Median and range of risk factors in hemodialysis patients.
Inflammatory Markers
By simple linear regression analysis, CRP was positively associated with SAT (r2 = 0.11, P = 0.03, ceruloplasmin positively associated with VAT (r2 = 0.16 P = 0.01) and SAT (r2 = 0.2 P < 0.005) and α 1 acid glycoprotein was positively associated with SAT (r2 = 0.11, P = 0.03) (Table 2). After adjustment for demographic variables the association that remained significant were between both α 1 acid glycoprotein and log CRP and SAT and between ceruloplasmin and VAT (Table 3). IL-6 was not significantly related to any measure of adiposity.
Table 2. Relationship between body composition and cardiovascular risk factors in hemodialysis patients.
| Relationship between acute phase proteins and body composition by univariant analysis | ||
|---|---|---|
| VAT | SAT | |
| Log CRP | NS | P = 0.03 * |
| Log IL-6 | NS | NS |
| α 1 AG | NS | P = 0.03 * |
| Ceruloplasmin | P = 0.01 | P = 0.005 |
| Relationship between adipokines and body composition by univariant analysis | ||
| VAT | SAT | |
| Log Leptin | < 0.0001 | < 0.0001 |
| Log Adiponectin | < 0.0001* | 0.003 |
| Relationship between lipids and body composition by univariant analysis | ||
| VAT | SAT | |
| Total Cholesterol | NS | 0.012 * |
| Triglycerides | 0.0003 * | 0.03 |
| LDL | NS | NS |
| HDL | NS | NS |
| Apo C II | 0.001 * | 0.006 |
| Apo C III | 0.002 * | 0.007 |
| Apo B | NS | NS |
| Apo A I | NS | NS |
Univariant relationship between groups of risk factors, inflammatory markers, adipokines, lipoprotein levels and and measures of adipose mass, visceral adipose tissue (VAT), subcutaneous adipose tissue and (SAT) in hemodialysis patients. All tissue compartments are in kg and were measured by magnetic resonance imaging.
Table 3. Multivariate analysis of relationship between body composition and cardiovascular risk factors in hemodialysis patients.
| Dependent variable | Independent variable | β | Std. Error | t | P. | Adjusted R2 | Excluded variables |
|---|---|---|---|---|---|---|---|
| Log_CRP | Constant | -0.709 | 0.169 | -4.187 | .000 | 0.092 | Race, sex, age, VAT |
| SAT | 0.016 | 0.007 | 2.314 | .026 | |||
| α 1AG | Constant | 85.987 | 9.670 | 8.892 | .000 | 0.087 | Race, sex, age, VAT |
| SAT | .913 | 0.405 | 2.254 | .029 | |||
| Ceruloplasmin | Constant | 20.937 | 5.955 | 3.516 | .001 | 0.300 | Sex, Age, SAT |
| VAT | 3.181 | .809 | 3.933 | .0001 | |||
| Race (black) | 14.543 | 5.011 | 2.902 | .006 | |||
| Apo C II | Constant | 1.072 | .706 | 1.519 | .137 | .275 | Age, SAT, sex |
| VAT | .491 | .120 | 4.077 | .0001 | |||
| Race (black) | 1.123 | .576 | 1.949 | .059 | |||
| Apo C III | Constant | 9.114 | 1.714 | 5.317 | .0001 | .219 | Race, sex, SAT, age |
| VAT | 1.571 | .450 | 3.491 | .001 | |||
| Triglycerides | Constant | 85.400 | 21.689 | 3.938 | .000 | .262 | Race, SAT, sex, age |
| VAT | 21.965 | 5.769 | 3.808 | .001 | |||
| Log_Adiponectin | Constant | 1.618 | .079 | 20.480 | .000 | .429 | Sex, SAT, age |
| VAT | -.078 | .013 | -.5.850 | .0001 | |||
| Race (black) | -.133 | .065 | -2.056 | .046 | |||
| Log_Leptin | Constant | -.189 | .110 | -1.719 | .093 | .766 | Race, age, sex |
| SAT | .047 | .006 | 7.388 | <.0001 | |||
| VAT | .053 | .030 | 1.735 | .090 |
Lipid Markers
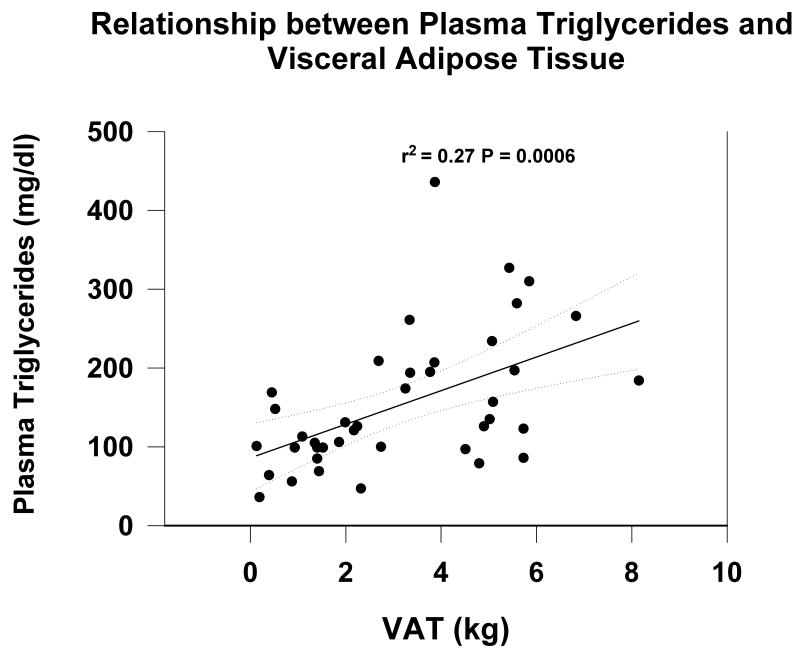
By univariate analysis triglycerides correlated with VAT (r2 = 0.27 P = 0.0006) (Figure 1) (Table 2). By multiple regression analysis, TGs were significantly correlated only with VAT after controlling for age, sex, and race (Table 3). When VAT was used as a categorical variable, TGs were significantly greater in tertiles 3 and 2 when compared with patients in the lowest tertile of VAT.
Figure 1.
Relationship between serum triglycerides and visceral adipose tissue (VAT) measured by MRI in prevalent hemodialysis patients.
Apo C II correlated with VAT (r2 = 0.24 P = 0.001) and SAT (r2 = 0.18 P = 0.006) by univariate analysis (Table 2). By multiple regression analysis apo C II correlated either with VAT after adjustment for age, sex and race, (Table 3). Apo C II was significantly greater in patients in the upper two tertiles of VAT compared to tertile I.
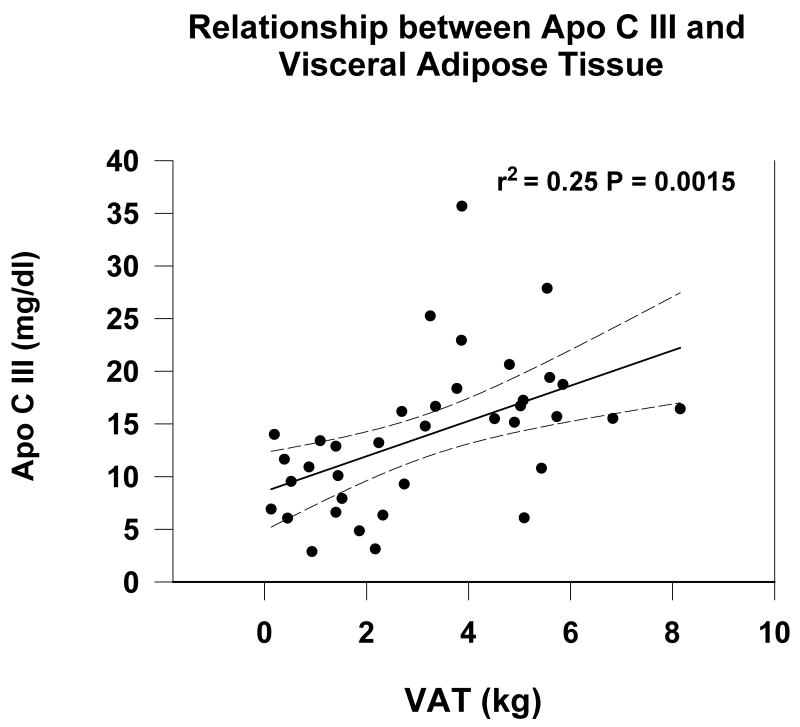
Similarly, apo C III correlated with VAT (r2 = 0.22 P = 0.0016) (Figure 2) and TAT (r2 = 0.2, P = 0.003) by univariate analysis Table 2). By multiple regression analysis apo C III correlated positively with VAT after adjustment for age, sex, race, (Table 3). Apo C III was significantly greater in patients in the upper two tertiles of VAT compared to tertile I.
Figure 2.
Relationship between serum apo C III and visceral adipose tissue (VAT) measured by MRI in prevalent hemodialysis patients.
Neither HDL cholesterol nor apo A I nor LDL cholesterol correlated with any measures of body composition. In contrast to other lipid markers the r2 values were approximately zero suggesting no effect of body composition on HDL or apo A I levels in these subjects.
Adipokines
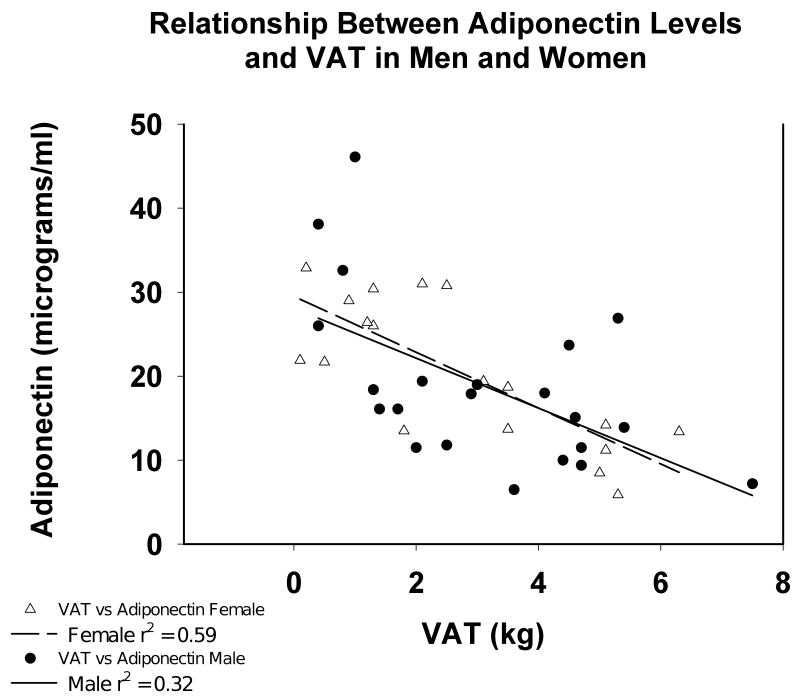
Adiponectin was significantly and inversely associated with SAT (r2 = 0.20, P = 0.0027) and VAT (r2 = 0.37 P < 0.0001). By multiple regression analysis, adiponectin was only negatively associated with VAT (P<0.00001) and was not affected by sex or any other anthropometric measurements (Table 3). The relationship between adiponectin and VAT (Figure 3) was essentially identical between men and women. Adiponectin was significantly greater in patients in the first tertile of VAT compared with the second (P <0.01) and was significantly greater in the first terile than in either the 2nd or the 3rd tertile.
Figure 3.
Relationship between serum adiponectin level and VAT measured by MRI in prevalent hemodialysis patients by sex. Men are represented by the open circles and women by the open triangles.
Leptin (following log transformation) was positively associated with SAT (r2 = 0.766 P < 0.0001), VAT (r2 = 0.47, P <0.001) (Table 2) by univariate analysis but only with SAT by multivariate analysis (r2 for the model 0.766, P < 0.0001) (Table 3).
Discussion
Mortality is increased with increasing BMI after a minimum mortality at BMIs between 23 and 26 in normal subjects (38,39). Adiposity, especially visceral adiposity, is associated with cardiovascular disease (39,40), hypertension (41) and other cardiovascular risk factors (increased triglycerides, LDL cholesterol, decreased HDL cholesterol and inflammation (10) in patients with normal kidney function. Obesity identified as waist to hip ratio is also a risk factor for incident chronic kidney disease (CKD) (42). Among those patients who develop CKD, waist to hip ratio also was a risk factor for cardiovascular events (43). However, the mean GFR in this cohort was 51.1 ml/min, so that extrapolation of risk to that of prevalent dialysis patient population may not be applicable.
The causal link between cardiovascular mortality and adiposity is proposed at least in part to be a consequence of alterations in blood lipid levels and in inflammation (15,16,44). In contrast to patients having normal renal function, BMI is associated with increasing survival among dialysis patients even at BMI values greater than 39 (1). Thus, obesity in dialysis patients must either contribute some beneficial effect, or specific risk factors linking mortality and adiposity must be mitigated.
We found that components of this relationship, especially the linkage between HDL cholesterol and Apo A I, and to a lesser extent, the relationship between IL-6 and CRP and elements of adiposity were obscured among dialysis patients. By contrast the relationship between VAT and triglyceride-rich lipoproteins was essentially preserved as was the relationship between adiposity and adipokine levels.
The dysplipidemia of CKD, specifically of the apo-B containing lipoproteins, is characterized by reduced clearance (45). Decreased clearance of these lipoproteins is due in part to intrinsic defects in the capacity of triglyceride-rich lipoproteins to act as appropriate substrates for lipolytic enzymes (21) consistent with the presence of an intrinsic structural change in lipoproteins making them less susceptible to lipolysis by LPL. Apo C III is an inhibitor of the action of LPL on TG rich lipoproteins (46,47) and is increased in dialysis patients (20). However while the levels of apo C III were significantly greater than reported for normal subjects (48,49), we found that apo C III levels were associated with VAT within these subjects, similar to the relationship described in patients without renal failure (50). By contrast, LDL cholesterol was low for this population as a whole and was not significantly associated with adiposity.
Inflammation is common among dialysis patients (51) and is well above levels observed in non-dialysis populations (3,12). Inflammation is associated with the malnutrition, inflammation, atherosclerosis (MIA) syndrome (52) providing a basis for inflammation in non obese subjects, potentially obscuring an effect linked to adiposity. Among subjects having normal kidney function, the association between adiposity and either CRP and IL-6 is quite strong, however the median values are significantly lower than we report for here for dialysis patients (53). The upper tertile of CRP found among patients not having kidney disease begins at 3 mg/L (54), a value below the median value among the dialysis patients whom we studied. Similarly median IL-6 values were also well above the upper quartile (> 2.28 pg/ml) among the non dialysis population (14). Axelsson reported an association between truncal fat mass and serum IL-6 levels, however the r2 value was 0.044 (55). By contrast we found a strong association between the more long lived acute phase protein ceruloplasmin and VAT. Ceruloplasmin has been associated with central obesity in patients not on dialysis (56). Serum IL-6 and CRP values are highly variable temporally in hemodialysis patients, far more so than are the levels of α 1 acid glycoprotein or ceruloplasmin (57) possibly contributing to the decrease in association between these more variable proteins and the adipose pools. Thus, while inflammation and low HDL were indeed found in this population, the risk was either not linked to adiposity at all or only weakly linked to adiposity (CRP), primarily because of low HDL and increased inflammation, at least as reported by short lived makers of inflammation, among lean dialysis patients. The risk factors were present regardless of adiposity and present at a level associated with the highest level of cardiovascular risk in populations not having kidney failure. It is possible that other factors that we have not controlled for have obscured any effects of body composition.
Adiposity remained associated with triglycerides and with the cardiovascular risk factor Apo C III and visceral adiposity was inversely associated with adiponectin. In contrast to patients without kidney failure, adiponectin has been found to be directly associated with mortality in dialysis patients by some investigators, (23), indeterminate by some, (58), while a protective effect has been noted by others (22). It is indeed possible that adioponectin is not in the causal pathway linking body composition to outcome and is simply reflecting adiposity, thus explaining the apparent salutary effect of high adiponectin in the population of patients not having renal failure with a possible deleterious effect observed by some investigators among populations of patients having kidney failure (23).
We previously established in a much larger group of patients (approximately 26,000) that the relationship between HDL cholesterol and BMI was effaced as eGFR declined (59). The main limitation of the current study is that it is small, however we have directly measured both VAT, which is strongly associated with insulin resistance and dyslipidemia, as well as SAT and found that many, but not all risk factors associated with increased adiposity are increased in prevalent hemodialysis patients, regardless of total adiposity or visceral adipose mass. If indeed these risk factors are on the causal pathway to cardiovascular mortality, incremental risks imposed by these factors are not increased among obese dialysis patients. However, other risk factors, specifically associated with triglyceride-rich lipoproteins (TG and apo C III levels) retain the same qualitative relationship to body composition in dialysis patients as they do in subjects having normal renal function. Why obese dialysis patients avoid increased mortality risk despite the residual association between adiposity and these risk factors remains to be established.
Acknowledgments
Research was supported by the Renal Research Institute a grant from Dialysis Clinic Incorporated and by the WHNRC (Western Human Nutrition Research Center).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johansen KL, Young B, Kaysen GA, Chertow GM. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr. 2004;80:324–332. doi: 10.1093/ajcn/80.2.324. [DOI] [PubMed] [Google Scholar]
- 2.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, McAllister CJ, Shinaberger CS, Gjertson DW, Greenland S. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis. 2005;46:489–500. doi: 10.1053/j.ajkd.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Kaysen GA, Muller HG, Young BS, Leng X, Chertow GM. The influence of patient- and facility-specific factors on nutritional status and survival in hemodialysis. J Ren Nutr. 2004;14:72–81. doi: 10.1053/j.jrn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe RA, Ashby VB, Daugirdas JT, Agodoa LY, Jones CA, Port FK. Body size, dose of hemodialysis, and mortality. Am J Kidney Dis. 2000;35:80–88. doi: 10.1016/S0272-6386(00)70305-2. [DOI] [PubMed] [Google Scholar]
- 5.Gotch FA, Levin NW, Port FK, Wolfe RA, Uehlinger DE. Clinical outcome relative to the dose of dialysis is not what you think: the fallacy of the mean. Am J Kidney Dis. 1997;30:1–15. doi: 10.1016/s0272-6386(97)90558-8. [DOI] [PubMed] [Google Scholar]
- 6.Kotler DP, Tierney AR, Wang J, Pierson RN., Jr Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr. 1989;50:444–447. doi: 10.1093/ajcn/50.3.444. [DOI] [PubMed] [Google Scholar]
- 7.Kamat AM, Shock RP, Naya Y, Rosser CJ, Slaton JW, Pisters LL. Prognostic value of body mass index in patients undergoing nephrectomy for localized renal tumors. Urology. 2004;63:46–50. doi: 10.1016/j.urology.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Le Blanc K, Ringden O, Remberger M. A low body mass index is correlated with poor survival after allogeneic stem cell transplantation. Haematologica. 2003;88:1044–1052. [PubMed] [Google Scholar]
- 9.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, Kosiborod M, Portnay EL, Sokol SI, Bader F, Krumholz HM. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. American Heart Association; National Heart, Lung, and Blood Institute Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 11.Ooi EM, Watts GF, Farvid MS, Chan DC, Allen MC, Zilko SR, Barrett PH. High-density lipoprotein apolipoprotein A-I kinetics in obesity. Obes Res. 2005;13:1008–1016. doi: 10.1038/oby.2005.118. [DOI] [PubMed] [Google Scholar]
- 12.Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35:469–476. doi: 10.1016/s0272-6386(00)70200-9. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;18:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 16.Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87:2084–9. doi: 10.1210/jcem.87.5.8450. [DOI] [PubMed] [Google Scholar]
- 17.Hocher B, Ziebig R, Altermann C, et al. Different impact of biomarkers as mortality predictors among diabetic and nondiabetic patients undergoing hemodialysis. J Am Soc Nephrol. 2003;14:2329–37. doi: 10.1097/01.asn.0000081662.64171.9b. [DOI] [PubMed] [Google Scholar]
- 18.Bologa RM, Levine DM, Parker TS, Cheigh JS, Serur D, Stenzel KH, Rubin AL. Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis. 1998;32:107–114. doi: 10.1053/ajkd.1998.v32.pm9669431. [DOI] [PubMed] [Google Scholar]
- 19.Koch M, Kutkuhn B, Grabensee B, Ritz E. Apolipoprotein A, fibrinogen, age, and history of stroke are predictors of death in dialysed diabetic patients: a prospective study in 412 subjects. Nephrol Dial Transplant. 1997;12:2603–2611. doi: 10.1093/ndt/12.12.2603. [DOI] [PubMed] [Google Scholar]
- 20.Mekki K, Prost J, Bouchenak M, Remaoun M, Belleville J. Plasma lipoprotein lipase, hepatic lipase activities, VLDL, LDL compositions at different times of hemodialysis. Atherosclerosis. 2003;169:269–77. doi: 10.1016/s0021-9150(03)00011-x. [DOI] [PubMed] [Google Scholar]
- 21.Lee DM, Knight-Gibson C, Samuelsson O, Attman PO, Wang CS, Alaupovic P. Lipoprotein particle abnormalities and the impaired lipolysis in renal insufficiency. Kidney Int. 2002;61:209–218. doi: 10.1046/j.1523-1755.2002.00116.x. [DOI] [PubMed] [Google Scholar]
- 22.Zoccali C, Mallamaci F, Tripepi G, Benedetto FA, Cutrupi S, Parlongo S, Malatino LS, Bonanno G, Seminara G, Rapisarda F, Fatuzzo P, Buemi M, Nicocia G, Tanaka S, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:134–41. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 23.Ohashi N, Kato A, Misaki T, Sakakima M, Fujigaki Y, Yamamoto T, Hishida A. Association of serum adiponectin levels with all-cause mortality in hemodialysis patients. Intern Med. 2008;47:485–91. doi: 10.2169/internalmedicine.47.0614. [DOI] [PubMed] [Google Scholar]
- 24.Nordfors L, Lonnqvist F, Heimburger O, Danielsson A, Schalling M, Stenvinkel P. Low leptin gene expression and hyperleptinemia in chronic renal failure. Kidney Int. 1998;54:1267–1275. doi: 10.1046/j.1523-1755.1998.00088.x. [DOI] [PubMed] [Google Scholar]
- 25.Daschner M, Tonshoff B, Blum WF, Englaro P, Wingen AM, Schaefer F, Wuhl E, Rascher W, Mehls O. Inappropriate elevation of serum leptin levels in children with chronic renal failure. European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood. J Am Soc Nephrol. 1998;9:1074–1079. doi: 10.1681/ASN.V961074. [DOI] [PubMed] [Google Scholar]
- 26.Bodary PF, Gu S, Shen Y, Hasty AH, Buckler JM, Eitzman DT. Recombinant leptin promotes atherosclerosis and thrombosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005 doi: 10.1161/01.ATV.0000173306.47722.ec. [DOI] [PubMed] [Google Scholar]
- 27.Kougias P, Chai H, Lin PH, Yao Q, Lumsden AB, Chen C. Effects of adipocyte-derived cytokines on endothelial functions: implication of vascular disease. J Surg Res. 2005;126:121–129. doi: 10.1016/j.jss.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 28.Fruhbeck G. A heliocentric view of leptin. Proc Nutr Soc. 2001;60:301–318. doi: 10.1079/pns200196. [DOI] [PubMed] [Google Scholar]
- 29.Singhal A, Farooqi IS, Cole TJ, O'Rahilly S, Fewtrell M, Kattenhorn M, Lucas A, Deanfield J. Influence of leptin on arterial distensibility a novel link between obesity and cardiovascular disease? Circulation. 2002;106:1919–1924. doi: 10.1161/01.cir.0000033219.24717.52. [DOI] [PubMed] [Google Scholar]
- 30.Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Havel PJ. Diabetes. 2004;53 1:S143–51. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 31.Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 32.Physiological pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Swarbrick MM, Havel PJ. Metab Syndr Relat Disord. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–8. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 34.Menon V, Li L, Wang X, Greene T, Balakrishnan V, Madero M, Pereira AA, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ. Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2599–606. doi: 10.1681/ASN.2006040331. [DOI] [PubMed] [Google Scholar]
- 35.Lee RC, Wang Z, Heo M, Ross R, Janssen I, Heymsfield SB. Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. Am J Clin Nutr. 2000;72:796–803. doi: 10.1093/ajcn/72.3.796. [DOI] [PubMed] [Google Scholar]
- 36.Beckman Instructions 015-248545-F. Beckman Instruments, Inc.; Brea CA: Nov, 1994. [Google Scholar]
- 37.W H, Flannery BP, Teukolsky SA, Vetterling WT. Numerical Recipes in FORTRAN: The Art of Scientific Computing. 2nd. Cambridge, England: Cambridge University Press; 1992. Kolmogorov-Smirnov Test; pp. 617–620. [Google Scholar]
- 38.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 39.Pischon T, Boeing H, Hoffmann K, et al. General and Abdominal Adiposity and Risk of Death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 40.Wassink AM, Olijhoek JK, Visseren FL. The metabolic syndrome: metabolic changes with vascular consequences. Eur J Clin Invest. 2007;37:8–17. doi: 10.1111/j.1365-2362.2007.01755.x. [DOI] [PubMed] [Google Scholar]
- 41.Davy KP, Hall JE. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol. 2004;286:R803–13. doi: 10.1152/ajpregu.00707.2003. [DOI] [PubMed] [Google Scholar]
- 42.Elsayed EF, Sarnak MJ, Tighiouart H, Griffith JL, Kurth T, Salem DN, Levey AS, Weiner DE. Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis. 2008;52:29–38. doi: 10.1053/j.ajkd.2008.02.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elsayed EF, Tighiouart H, Weiner DE, Griffith J, Salem D, Levey AS, Sarnak MJ. Waist-to-hip ratio and body mass index as risk factors for cardiovascular events in CKD. Am J Kidney Dis. 2008;52:49–57. doi: 10.1053/j.ajkd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lang CH, Dobrescu C, Bagby GJ. Tumor necrosis factor impairs insulin action on peripheral glucose disposal and hepatic glucose output. Endocrinology. 1992;130:43–52. doi: 10.1210/endo.130.1.1727716. [DOI] [PubMed] [Google Scholar]
- 45.Ikewaki K, Schaefer JR, Frischmann ME, Okubo K, Hosoya T, Mochizuki S, Dieplinger B, Trenkwalder E, Schweer H, Kronenberg F, Koenig P, Dieplinger H. Delayed in vivo catabolism of intermediate-density lipoprotein and low-density lipoprotein in hemodialysis patients as potential cause of premature atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:2615–22. doi: 10.1161/01.ATV.0000188555.60475.c2. [DOI] [PubMed] [Google Scholar]
- 46.Wang C, McConathy WJ, Kloer HJ, Alaupovic P. Modulation of lipoprotein lipase activity by apolipoproteins: effect of apolipoproein C-III. J Clin Invest. 1985;75:384–390. doi: 10.1172/JCI111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown WV, Baginsky ML. Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem Biophys Res Commun. 1972;46:375–382. doi: 10.1016/s0006-291x(72)80149-9. [DOI] [PubMed] [Google Scholar]
- 48.Saland JM, Ginsberg HN. Lipoprotein metabolism in chronic renal insufficiency. Pediatr Nephrol. 2007;22:1095–112. doi: 10.1007/s00467-007-0467-5. [DOI] [PubMed] [Google Scholar]
- 49.Lee DM, Knight-Gibson C, Samuelsson O, Attman PO, Wang CS, Alaupovic P. Lipoprotein particle abnormalities and the impaired lipolysis in renal insufficiency. Kidney Int. 2002;61:209–218. doi: 10.1046/j.1523-1755.2002.00116.x. [DOI] [PubMed] [Google Scholar]
- 50.Lofgren I, Herron K, Zern T, West K, Patalay M, Shachter NS, Koo SI, Fernandez ML. Waist circumference is a better predictor than body mass index of coronary heart disease risk in overweight premenopausal women. doi: 10.1093/jn/134.5.1071. [DOI] [PubMed] [Google Scholar]
- 51.Kimmel PL, Phillips TM, Simmens SJ, Peterson RA, Weihs KL, Alleyne S, Cruz I, Yanovski JA, Veis JH. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998;54:236–44. doi: 10.1046/j.1523-1755.1998.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stenvinkel P, Heimburger O, Lindholm B, Kaysen GA, Bergstrom J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome) Nephrol Dial Transplant. 2000;15:953–60. doi: 10.1093/ndt/15.7.953. [DOI] [PubMed] [Google Scholar]
- 53.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Jr, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O'Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–41. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 54.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 55.Axelsson J, Rashid Qureshi A, Suliman ME, Honda H, Pecoits-Filho R, Heimbürger O, Lindholm B, Cederholm T, Stenvinkel P. Truncal fat mass as a contributor to inflammation in end-stage renal disease. Am J Clin Nutr. 2004;80:1222–9. doi: 10.1093/ajcn/80.5.1222. [DOI] [PubMed] [Google Scholar]
- 56.Cignarelli M, DePergola G, Picca G, Sciaraffia M, Pannacciulli N, Tarallo M, Laudadio E, Turrisi E, Giorgino R. Relationship of obesity and body fat distribution with ceruloplasmin serum levels. Int J Obes Relat Metab Disord. 1996;20:809–813. [PubMed] [Google Scholar]
- 57.Kaysen GA, Dubin JA, Müller HG, Mitch WE, Rosales L, Levin NW, HEMO Group Impact of albumin synthesis rate and the acute phase response in the dual regulation of fibrinogen levels in hemodialysis patients. Kidney Int. 2003;63:315–22. doi: 10.1046/j.1523-1755.2003.00721.x. [DOI] [PubMed] [Google Scholar]
- 58.Rao M, Li L, Tighiouart H, Jaber BL, Pereira BJ, Balakrishnan VS. Plasma adiponectin levels and clinical outcomes among haemodialysis patients. HEMO Study Group. Nephrol Dial Transplant. 2008;23:2619–28. doi: 10.1093/ndt/gfn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lo JC, Go AS, Chandra M, Fan D, Kaysen GA. GFR, body mass index, and low high-density lipoprotein concentration in adults with and without CKD. Am J Kidney Dis. 2007;50:552–558. doi: 10.1053/j.ajkd.2007.07.011. [DOI] [PubMed] [Google Scholar]