Abstract
Objective
To determine serum [PFOA] in residents near a fluoropolymer production facility: the contributions from air, water and occupational exposures, personal and dietary habits, and relationships to age and gender.
Methods
Questionnaire and serum PFOA measurements in a stratified random sample and volunteers residing in locations with the same residential water supply but with higher and lower potential air PFOA exposure.
Results
Serum [PFOA] greatly exceeded general population medians. Occupational exposure from production processes using PFOA and residential water had additive effects, no other occupations contributed. Serum [PFOA] depended on the source of residential drinking water, and not potential air exposure. For public water users the best-fit model included age, tap water drinks per day, servings of home-grown fruit and vegetables, and carbon filter use.
Conclusions
Residential water source was the primary determinant of serum [PFOA].
INTRODUCTION
Fluoropolymers are used in a variety of industrial and consumer products, including protective coatings for carpets and apparel, consumer housewares, paper coatings, electronics, insecticide formulations, surfactants, aerospace and other applications.
Perfluorooctanoate (PFOA, CF3, (CF2)6 C00−, CAS No 3825-26-1) has commercial use primarily as ammonium perfluorooctanoate, an essential surface-active agent in the production of various fluoropolymers, including tetrafluoroethylene. PFOA is a contaminant in other fluorochemicals and telomer products (1). According to manufacturers, it is typically not present in finished consumer articles. Ammonium perfluorooctanoate is fully dissociated into the anion form, perfluorooctanoate, in environmental media and biological fluids.
Organofluorine compounds behave very differently to the more widely studied organochlorines and organobromines and have unusual partitioning properties (2). Perfluorofatty and perfluorosulfonic acids, particularly PFOA and perfluorooctane sulfonate (PFOS), are now found ubiquitously in marine animals inhabiting widely spread geographical biospheres (3) and in human serum from widely disparate groups (4–7). PFOA and PFOS persist in the environment and resist biological, environmental or photochemical degradation (3M 2001). They have no known natural sources (8).
In the general US population, median serum PFOA values are around 4 to 5 ng/mL, occasional values are above 20 ng/mL (4,5,9) with no significant gender differences. Analyses of blood samples from residents near Washington County, Maryland found a 2-fold increase in serum PFOA levels between 1974 and 1989 (6). Kannan et al (7) have reported differences in blood serum PFOA levels among populations from different countries.
PFOA toxicology has recently been reviewed (1). PFOA is well absorbed by rats following both oral and inhalation exposure. Fecal excretion in male rats is increased by feeding cholestyramine resin, suggesting enterohepatic circulation (10). Dermal penetration is significant in rats but is low to negligible in humans (11). In rats, PFOA is a peroxisome proliferator activated receptor (PPAR) agonist causing liver toxicity (12, 13) with hepatomegaly and hepatic necrosis, and biochemical effects characteristic of PPAR agonists (14). PFOA promotes liver carcinogenesis in rats (15), and causes Leydig-cell testicular tumors and acinar cell pancreatic tumors (16, 17), through non-genotoxic mechanisms (18,19) with questionable human relevance. The human half-life of PFOA was between four and five years for retirees with previous heavy occupational exposure (20), much longer than in laboratory animals.
Control of human exposure to PFOA has been limited by the lack of information on sources and pathways. As the US Environmental Protection Agency states: "At present, there aren't any steps that EPA recommends that consumers take to reduce exposure to PFOA because the sources of PFOA in the environment and the pathways by which people are exposed are unknown. The limited geographic locations of fluorochemical plants making or using the chemical suggest that there may be additional sources of PFOA in the environment and exposures beyond those attributable to direct releases from industrial facilities. But whether human exposures are due to PFOA in the air, the water, on dusts or sediments in dietary sources or through some combination of routes is currently unknown" (21).
PFOA has been used in the manufacturing of fluoropolymers at a facility in Washington, WV since 1951. Potential airborne PFOA exposure was modeled using information on releases from the plant, meteorological conditions and topography. The wind rose-map, which shows the frequency and strength of winds from different directions, for the plant indicates the primary wind direction, toward the north/northeast, would carry airborne emissions into neighboring Ohio. PFOA was also released to the Ohio River, adjacent to the plant, as well as disposed in landfills and surface impoundments in the vicinity. According to the facility, total PFOA emissions from the facility have been reduced from 87,000 lbs (31,000 air, 56,000 water) and 80,000 lbs (31,000 air, 49,000 water) in 1999 and 2000 respectively, to 11,000 lbs (6,000 air, 5,000 water) and 1,700 lbs (200 air, 1,500 water) in 2003 and 2004 respectively.
PFOA has been detected in public and private drinking water supplies near the facility. The highest levels reported in public water supplies in the US to date have been in the Little Hocking water system, in operation since 1968, which draws water from wells across the Ohio river from the facility. The average [PFOA] in Little Hocking system distribution water for 2002–2005 has been 3.55 ng/mL (range 1.5 ng/mL to 7.2 ng/mL).
The objectives of the present study were to measure serum PFOA levels in a stratified random sample of the population served by the Little Hocking water service to determine: how the serum PFOA levels compared with levels measured in other populations; the relative contributions of air and water exposure to serum PFOA levels; and to determine the effects, if any, of demographic variables, occupational exposures, personal habits, use of water filters and dietary factors such as the ingestion of locally-harvested game and fish and of homegrown vegetables.
MATERIALS AND METHODS
Eligibility Criteria
Eligibility criteria for participation in the study were:
Residence in the area serviced by the Little Hocking Water Association for at least the past two years, as of July 2004
Ages two or older (changed to ages four or older after the study commenced to minimize participant discomfort) and
Not known to have a bleeding disorder (in order to diminish any risk from phlebotomy).
Selection of Households for Sampling Frame
Two populations of residents were identified for participation in the stratified random sampling. One population represented those whose residence was potentially exposed to PFOA in both air and water, the other whose residence was potentially exposed to PFOA in water but had very minimal potential for exposure in air. The sampling randomly selected households from each of these strata.
To identify areas where there was higher exposure to PFOA in the air, we used an air dispersion model that estimated the air concentration for PFOA emanating from the PFOA source plant. Inputs into the air dispersion model included the amounts of air emissions for the plant, wind velocities, and topographic contours. The air concentrations had been modeled for years 2002 & 2003 on an annual basis; the model produced very similar results for each of these years. To identify areas in the Little Hocking water service distribution area, a map of the water distribution system was obtained for the Little Hocking water service. The potential air and water exposure group comprised all those who had resided for at least two years in the water distribution system area of the Little Hocking water service and also within the contour line representing 0.2 µg/m3 PFOA in the air as a yearly average for 2002. These households were all located in portions of Zip Codes 45714 (Belpre) and 45742 (Little Hocking).
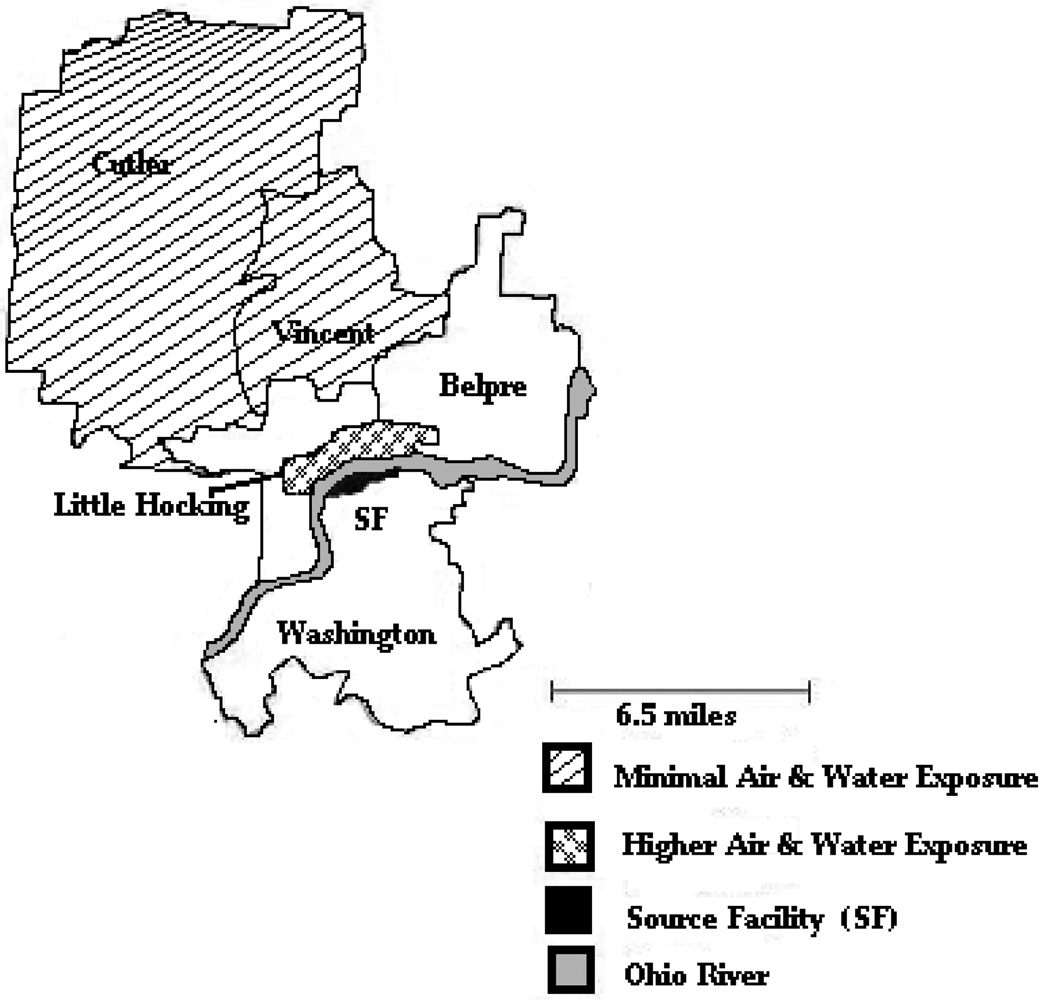
The potential water exposure group comprised residents who had resided for at least two years in the water distribution system area of the Little Hocking water service but in an area where air exposure to PFOA from the facility was negligible. The selected study area was zip codes 45724 (Cutler), and 45784 (Vincent). These areas were all at least several miles outside the lowest air concentration contours derived from the air dispersion model. Figure 1 shows the location of the residence areas for both the potential air and water exposure and the potential water only exposure zones.
Figure 1. Map showing the locations of the studied communities and the source facility.
Subjects for the minimal air exposure group were selected from the area shown in yellow, subjects for the higher air exposure group from the area shown in red. Residents in both of these areas obtained their water from the same public residential water supply. The location of the source facility is shown in black. The residents lived in Ohio, the source facility is located in West Virginia. The state boundary, the Ohio River, is shown in blue.
To identify households and residents in the zip codes of interest, demographic and other information was purchased from www.infousa, a proprietary database of detailed information on US consumer households compiled from thousands of public sources. The items used to select invitees were names of head of household, street address, city, state, ZIP Code, and length of residence.
Selection of Stratified Random Sample
For the area identified as having both air and water exposure 95 households in the www.infousa database met the requirements, all were invited to take part in the study. These included households with measured PFOA levels in potable well-water, measured by the Ohio Department of Environmental Protection and households using Little Hocking Water Association water. For the area identified as having only water exposure to PFOA, a stratified random sampling of households was performed, resulting in the selection of 342 households. All members of selected households who met the study eligibility criteria were invited to participate.
Invitations to participate
Invitation letters were sent from the University of Pennsylvania to each selected household. If no response was received, a second mailing was sent. If there was still no response after approximately 10 days, a telephone call was made to the household by staff of the Decatur Community Association. No participant chose an option for anonymous participation. On the weekend prior to the mailing of the invitation letter, a flyer was placed in the area weekend newspaper to announce that invitation letters were forthcoming. The principal local newspaper, the Marietta Times, independently wrote an editorial encouraging those selected to consider participation.
Community Volunteer Group
Because of great community interest, a lottery was conducted to select an additional sample of invitees from households that volunteered to participate in the study in response to a newsletter notice. Those households that met study criteria including residing in one of the areas used for stratified random sampling were included in the lottery.
Administration of Questionnaires
Administration of questionnaires and collection of blood samples were performed between July 2004 and February 2005, in nearby Parkersburg, WV. The questionnaires were developed and revised after review by the members of the Community Advisory Committee and an expert panel from the US EPA. The Community Advisory Committee, convened by the Decatur Community Association, comprised representatives of the townships in the Little Hocking Water Association Service District, representatives from the Ohio and US EPA, the Warren School District and the County Health Commissioner. Prior to finalization, the questionnaires were pilot tested on a representative group of 20 individuals from similar Southeastern Ohio or Western West Virginia communities, who did not live in the Little Hocking Water Association District.
Trained interviewers administered all questionnaires. Only one person from each household supplied household information. The household questionnaire elicited information to ensure that participants met the eligibility criteria, demographic information on eligible participants, household contact information, and sources of residential drinking water [private well, water district, cisterns, bottled water, hauled water, etc.], use of a home water filter, and water source and estimated usage for cooking, canning, and reconstituting canned soups and frozen juices.
All adults 18 years and older were administered the adult questionnaire that elicited demographic information, diet (including consumption of vegetables or fruit grown in your garden, meat or game grown locally, and fish caught locally), health conditions (liver, thyroid, bleeding disorders), current medications, current occupational or school if a full-time student, employment (including at a facility using PFOA, visiting or processing waste from that facility, work as a firefighter, in carpet cleaning or retreating carpets or rugs, or in professional carpet installation), and smoking and alcohol habits.
All children were administered a questionnaire that was similar to the adult questionnaire except that the questions about occupation and about smoking and alcohol habits were omitted.
Collection and Assay of PFOA Acid in Serum
Specimen collection
Twenty mls of blood were drawn into red-topped Vacutainer tube for PFOA analysis, immediately centrifuged, and the resulting serum was transferred to polypropylene aliquot tubes, labeled and shipped on dry ice to the analysis laboratory (Exygen Research) where it was stored at −80 °C pending analysis.
Standards and chemicals
The standard for perfluorooctanoic acid (99.2%) was obtained from Oakwood Products, Inc (West Columbia, SC) and characterized by DuPont (Newark, DE). Analysis by 19F NMR confirmed that the PFOA standard contained 98.7% straight chain PFOA and 0.53% branched PFOA isomers. The internal standard, [1,2-13C]-PFOA(C6F13CF213CO2H, 13C-PFOA) (96.4%) was provided by DuPont (Newark, DE).
Chemicals and reagents used in the sample preparation procedure or in the mobile phase were of reagent grade and were obtained from VWR Scientific (Bridgeport, NJ) and Sigma-Aldrich (St Louis, MO). Solvents used for the mobile phase (acetonitrile, water) were of HPLC grade and were obtained from EM Science (Gibbstown, NJ). The control human serum was purchased from Lampire Biological Laboratories, Inc (Pipersville, PA) and stored frozen at −20 °C. This fluid was used for the preparation of laboratory quality control samples with spiked-in PFOA.
Chromatographic and Mass spectrometric conditions
PFOA was analyzed through HPLC/tandem mass spectrometry by a slight modification of the method of Flaherty et al (22).
Standards, sample preparation and calibration
Controls and study subject samples were added 300 µL of acetonitrile. The samples were thoroughly mixed by vortexing, centrifuged and 5 µL of the cell- and protein-free supernatant used for analysis by the HPLC tandem mass spectrometer system. A 7-point calibration curve was analyzed throughout the analytical sequence for the fluorocompounds. The calibrators included normal human serum spiked with 0.5, 1, 5, 10, 20, 50, and 100 ng/mL of PFOA. The instrument response versus the calibrator concentration was plotted for each point. Linear regression with 1/x weighting was used to determine the slope, y-intercept and coefficient of determination (r2). Calibration curves were deemed acceptable if r2 ≥ 0.985. This is the external standardization method used for the determination of PFOA in the set of 408 samples described in this study. For samples with PFOA concentrations >100 ng/mL, the sample was diluted in 50:50 methanol/water and re-run. In addition the analysis of PFOA was done using 13C-perfluorooctanoic acid as an internal standard for a randomly selected set of 35 of the samples in order to certify that the external standardization method used provided equivalent PFOA concentration values. For these analyses the internal standard was mixed in acetonitrile at a concentration of 1 ng/mL. As described above for the externally standardized assay for sample preparation: to 100 µL of standards, controls and study subject samples was added 300 mL of acetonitrile containing the internal standard and the cell-and protein-free supernatants prepared as above. On comparison of the externally standardized with the internally standardized sets of results on the 35 selected samples, linear regression analysis showed excellent agreement between the two calibration procedures: Y(IS) = 1.073±0.0229*X(ext std) − 0.385±0.468; r2=0.985; Sy∙x=1.54.
Matrix spike samples and duplicate sample assays
One matrix spike for every 20 samples was prepared by adding a known concentration of the PFOA to the study subject serum sample for the purpose of assessment of the method’s accuracy throughout the set of study subject serum samples. The mean PFOA recovery for these spiked samples was 95% with an SD of 16.2%. In addition, one sample of every 10 was extracted and analyzed in duplicate in order to provide an assessment of the method’s precision throughout the set of samples. The average between assay %CV for PFOA duplicates was 5.7%. The lower limit of quantification of this method is 0.5 ng/mL. Validation of this LLOQ was conducted with replicate spiked samples of human serum with PFOA spiked into the samples at 0.5 ng/mL, the concentration of the lowest calibrator for this assay. The mean recovery ± SD was 101 + 2.7%.
Serum [PFOA] Philadelphia Volunteer Group
To help ensure that published general population serum PFOA levels were suitable for comparison purposes under the circumstances of the study, we identified a comparison group of 30 volunteers from the Philadelphia area. The Philadelphia volunteers, staff and students at the Hospital of the University of Pennsylvania, were paid $20 each to participate. Their mean age was 34.3, range 20–56; there were 9 men and 21 women. None identified previous or current occupational exposure to PFOA. Blood from these individuals was drawn, handled spun, stored, shipped and analyzed for PFOA in an identical manner to the blood obtained during the study. The mean serum PFOA levels for the Philadelphia comparison group was 6 ng/mL, IQR 5–10 ng/mL consistent with published values for the US population (4,5,6).
[PFOA] Water Sampling and comparison to serum levels
The concentration of PFOA in finished water in the Little Hocking water system has been measured approximately quarterly from 1/22/2002 to 5/18/2005 by the Ohio EPA. Fourteen measurements were available for this period, results before 11/29/04 had been reported as ammonium perfluorooctanate (APFO), and as PFOA from that date. PFOA concentration in private residential well water was publicly available for 9 individuals for whom private well water was their only reported source of residential drinking water. In one instance, 6 samples had been taken at regular intervals from 2002 through 2005. For this well, the values obtained were averaged to obtain a mean level over the period. For the remaining wells only one sample had been analyzed from a single point in time. The average PFOA concentration in Little Hocking system distribution water from January 2002 until May 2005 was 3.55 ng/mL (range 1.5 ng/mL to 7.2 ng/mL). For private wells used by study participants, PFOA concentrations ranged from not detectable (<0.010 ng/mL) to 14.0 ng/mL.
Statistical Analysis
To determine if serum PFOA levels differed by dietary or personal habits, water source, water usage, occupational exposure, etc., preliminary data analyses included the t-test for binary predictors or the analysis of variance (ANOVA) for greater than 2 exposure categories. Adjustment for multiple comparisons were made using Tukey-Kramer. To check the assumptions of the statistical approach used, various analyses were rerun with the exact test using Monte Carlo. Results were similar to that of the f test. Subsequent higher order analyses included analysis of covariance adjusting for age. Final multivariate analysis to assess the independent contribution of multiple variables was a generalized estimating equation (GEE) to adjust for household cluster. Only variables associated with serum PFOA levels on univariate analysis with a probability <.10 were included. To determine model of best fit, both forced entry and backward elimination were employed. All analyses were performed using SAS statistical software (Version 9.1, SAS Institute, Cary NC). A p<.05 was considered statistically significant. Serum PFOA levels Serum [PFOA] are presented as mean, median, and interquartile range (IQR).
To examine the effect of demographic variables (age, gender, duration lived at current residence) we excluded the 18 participants who reported substantial occupational exposure (defined below) to PFOA. To examine the effects of number of glasses of drinking water per day, use of a residential water filter and of dietary exposures we included only those residents whose sole source of residential drinking water was Little Hocking water system water. Only individuals who designated a single source of residential drinking water, and who did not have substantial occupational exposure to PFOA were included in these analyses.
Human Subjects Approvals
The study was approved by the Institutional Review Board (IRB) of the University of Pennsylvania. The study was voluntary and informed consent was obtained for all participants prior to any study. Minors under the ages of 17 were encouraged to give informed assent whenever feasible. A Certificate of Confidentiality was obtained from the NIH to ensure maximum protection of personal information and results.
A partnership between the University of Pennsylvania School of Medicine, The Decatur Community Association, a local community association in the Little Hocking water service area, and Grand Central Family Medicine in Parkersburg WV, a local health care provider, conducted the study through a grant from the Environmental Justice Program of NIEHS. The community was involved at all stages of the study. A local health-care provider informed each participant of his or her personal PFOA results together with any necessary explanation.
RESULTS
Response and Participation Rate
Stratified Random Sample
343 individuals from 169 households participated in the phlebotomy and questionnaire administration. One subject withdrew from the study, 6 subjects could not donate sufficient blood, one subject did not complete the questionnaire, and 11 subjects did not meet eligibility criteria because their household water service was received from a water system other than the Little Hocking Water Association. Accordingly, data was available for analysis from 324 subjects from 161 households selected through the stratified random selection process. The participation rate by location of household mailing address is given in Table 1.
Table 1.
Household Participation Rates for Randomly Selected Households by Community.
| Households Invited to Participate |
# Agreeing to Participate |
# Completing Data Acquisition |
Participation Rate |
|
|---|---|---|---|---|
| Little Hocking | 78 | 45 | 38 | 48.7 |
| Belpre | 17 | 8 | 7 | 41.2 |
| Cutler | 101 | 45 | 30 | 29.7 |
| Vincent | 241 | 115 | 86 | 35.7 |
| TOTAL | 437 | 213 | 161 | 36.8 |
Response and Participation - Community Volunteer Group
100% of the 37 households selected by lottery participated in the phlebotomy. However, 2 individuals from 2 households did not complete the questionnaire and were excluded from further analysis. Thus data from 54 individuals from 35 households was included in the final analysis. The racial and ethnic composition of both participants and volunteers was predominantly white non-Hispanic (97%, N=367), reflecting the composition of Washington County, Ohio.
Role of Occupational Exposure
We established criteria for substantial occupational exposure to PFOA of: at least one years’ work in a production area within a facility in which PFOA was used in the production process; with the last such occupational exposure within the previous 10 years. Seventeen individuals from the stratified random sample, and one from the local volunteer sample met this definition for substantial occupational exposure. All had received their occupational exposure to PFOA in the same fluoropolymer manufacturing facility located in Washington, WV across the Ohio River from the study area. An additional 48 individuals reported past or current potential occupational exposure to PFOA as follows (individuals can be represented more than once): 18 individuals had worked in a fluoropolymer manufacturing facility in a non-production area, at the fluoropolymer production facility in a production area for less than one year total and/or more than ten years ago, or in a job for another employer that required visits to the fluoropolymer production facility, so did not meet the criteria for substantial occupational exposure; 8 individuals had worked in a job involving waste disposal or waste processing from the fluoropolymer manufacturing facility; 29 individuals had worked as firefighters (volunteer, military, as a company employee or paid) and 13 individuals had worked in carpet cleaning, retreating carpets or rugs, or in professional carpet installation. Compared to the no exposure group, none of these occupational exposure groups had statistically significant elevated serum PFOA levels (p>.05) (Table 2). Among those with potential occupational exposure, the highest median values were observed for firefighters. However, these values remained well below the concentrations of the substantial occupational exposure group. Since none of these groups had significantly elevated serum PFOA levels they were aggregated into one group (potential exposure) for statistical analysis purposes.
Table 2.
Serum [PFOA] ng/mL by Occupational Exposure Group
| Occupational Exposure | N | Median | Mean | IQR |
|---|---|---|---|---|
| NO OCCUPATIONAL EXPOSURE | 312 | 329 | 423 | 175–537 |
| POTENTIAL OCCUPATIONAL EXPOSURESa | 48 | 388 | 406 | 168–623 |
| Firefighter: voluntary, military, company employee or paid | 29 | 447 | 453 | 236–709 |
| Non-production area of fluoropolymer facility, in production area not meeting criteria for substantial occupational exposure, or requiring visits to facility. | 18 | 381 | 386 | 125–430 |
| Carpet cleaning, retreating carpets or rugs, or in professional carpet installation | 13 | 302 | 408 | 191–631 |
| Facility processing or disposing fluoropolymer production waste | 8 | 253 | 578 | 115–918 |
| SUBSTANTIAL OCCUPATIONAL EXPOSURE [Production area within a facility in which PFOA was used in the production process >1 year and last exposure having occurred within previous 10 years] | 18 | 775 | 824 | 422–999 |
Some individuals had more than one potential occupational exposure, therefore N for the potential occupational exposure subgroups does not total to 48.
When comparing substantial, potential, and no occupational exposure groups, the substantial occupational exposure group had a significantly higher median serum PFOA levels of 775 ng/mL than the potential exposure (388 ng/mL), and no occupational exposure groups (329 ng/mL) (p=.0002, p<.0001 respectively, Table 2).
As a result of this finding, the substantial occupational exposure group was removed from further analysis of PFOA exposure in the community. Since the serum PFOA levels for the potential exposure group were not different from the rest of the community, they were included in subsequent analyses of community exposures and treated for purposes of analysis as residents without substantial occupational exposure.
Role of Community Air Exposure: Serum [PFOA] by Community of Residence
The median serum PFOA level in the combined two areas with highest potential air exposure (Little Hocking and Belpre) was 326 ng/mL, compared to 368 ng/mL in the combined two areas with a potentially minimal contribution from PFOA through air pollution (Cutler and Vincent) (Table 3). This difference was not statistically significant (p=.32).
Table 3.
Serum [PFOA] in ng/ml by community area, for randomly selected participants and for all participantsa.
| Community Areas with Higher Expected Contribution from Air |
Randomly Selected Participants | All Participants (local volunteers and randomly selected) |
||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | Median | IQR | N | Mean | Median | IQR | |
| Belpre | 14 | 321 | 298 | 83–533 | 30 | 307 | 244 | 103–445 |
| Little Hocking | 74 | 478 | 327 | 187–572 | 92 | 458 | 311 | 175–567 |
| TOTAL | 88 | 453 | 326 | 176–568 | 122 | 421 | 298 | 155–556 |
|
Community Areas with Minimal Expected Contribution from Air |
||||||||
| N | Mean | Median | IQR | N | Mean | Median | IQR | |
| Cutler | 59 | 361 | 316 | 169–477 | 70 | 380 | 314 | 185–477 |
| Vincent | 160 | 439 | 370 | 190–570 | 168 | 438 | 370 | 188–577 |
| TOTAL | 219 | 418 | 368 | 182–555 | 238 | 421 | 361 | 186–555 |
18 subjects with substantial occupational exposure were excluded from analysis.
Additionally, the inclusion of local volunteers made no appreciable difference to the results (Table 3). Because of the similarity of serum PFOA levels in each community regardless of air pollution or the inclusion of volunteers, all communities and samples were combined in the subsequent analyses to examine the effects of water exposure on [PFOA].
Role of Exposure in Water: Serum [PFOA] and Primary Source of Residential Drinking Water
With regard to water exposure, the highest median serum PFOA level (374 ng/mL) was found for the group who used only Little Hocking system water as their residential drinking water source (Table 4). The lowest was found in those who currently used only bottled and/or cistern and/or spring water as the source of their residential drinking water. The serum PFOA levels in those who used bottled, spring or cistern water was significantly lower than those in both the Little Hocking water system only and the mixed Little Hocking plus another water source groups (p= .0004, and p= .007 respectively. The serum PFOA levels for those who used Little Hocking water system water only and the mixed Little Hocking and another water source were not statistically significantly different (p=.17).
Table 4.
Serum [PFOA] in ng/ml by primary residential source of drinking watera,b. All Participants (randomly selected and local volunteers)
| Drinking Water Source | N | Median | Mean | IQR | Range |
|---|---|---|---|---|---|
| Little Hocking system water only | 291 | 374 | 448 | 221–576 | 7–1950 |
| Little Hocking system plus bottled or spring | 26 | 320 | 358 | 206–370 | 72–1280 |
| Bottled and/or cistern and/or spring only* | 10 | 71 | 154 | 49–217 | 12–527 |
| Well water and well & other | 26 | 79 | 296 | 28–155 | 8–4520 |
Subjects with substantial occupational exposure to PFOA were excluded from these analyses
7 subjects did not indicate residential source of drinking water
Significantly different from Little Hocking water only (p=.003 ) and Little Hocking system plus bottled or spring water (p=.05)
The mean serum PFOA levels in those who used any well water as their sole residential drinking water source was variable; this group included some of the lowest and some of the highest PFOA serum concentrations.
Relationship between [PFOA] in primary residential water supply and serum [PFOA] in residents
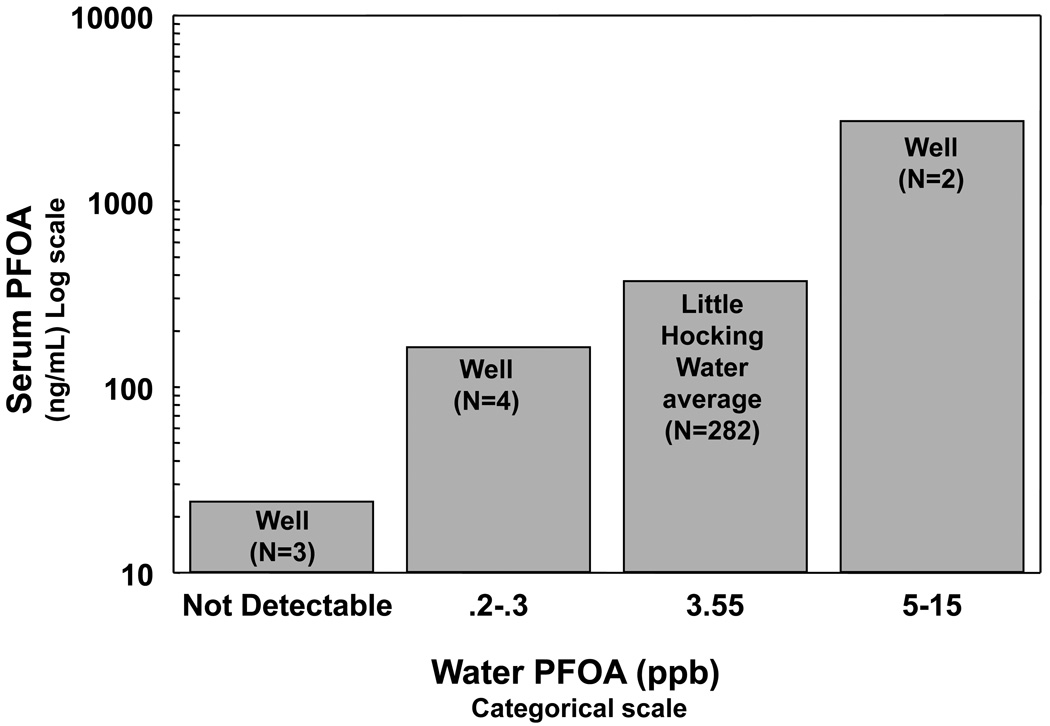
Figure 2 presents a graphical relationship between PFOA concentrations in drinking water and serum PFOA levels. Three individuals drank from wells where the PFOA was not detectable, their average serum PFOA level was 20.8 ng/mL, (range 13.6 to 31.4 ng/mL). Six individuals used a private well with measurable PFOA in water as their only source of residential drinking water. Although the numbers of individuals for whom the PFOA concentration in well water is known is small, there is an apparent strong relationship between the level of the serum PFOA levels and the PFOA concentration of the drinking water source.
Figure 2. Relationship of PFOA Concentration in Water Source (Little Hocking & Private Wells) to Serum PFOA Levels.
The numbers in parenthesis indicate the number of samples. Although the number of observations from persons using only residential well-water source is small, there is a marked and statistically significant relationship between the PFOA levels in serum and the PFOA concentration in the residential drinking water source. Only subjects 6 years of age or older using a single residential drinking water source were included in the analysis.
The median serum/drinking PFOA water ratio residents using only the Little Hocking water system was 105 (371/3.55), with an interquartile range between 62 (221/3.55) and 162 (576/3.55). For the six individuals who used a private well with measured [PFOA] as their only source of residential drinking water, the serum/drinking water PFOA ratios ranged from 142 to 855.
Serum PFOA levels and gender, age, years of residence, smoking and alcohol
Serum PFOA level was not significantly different by gender for participants without substantial occupational exposure (p=.32). The median [PFOA] for females was 320 ng/mL, IQR 161–509, and for males was 345, IQR 190 to 576.
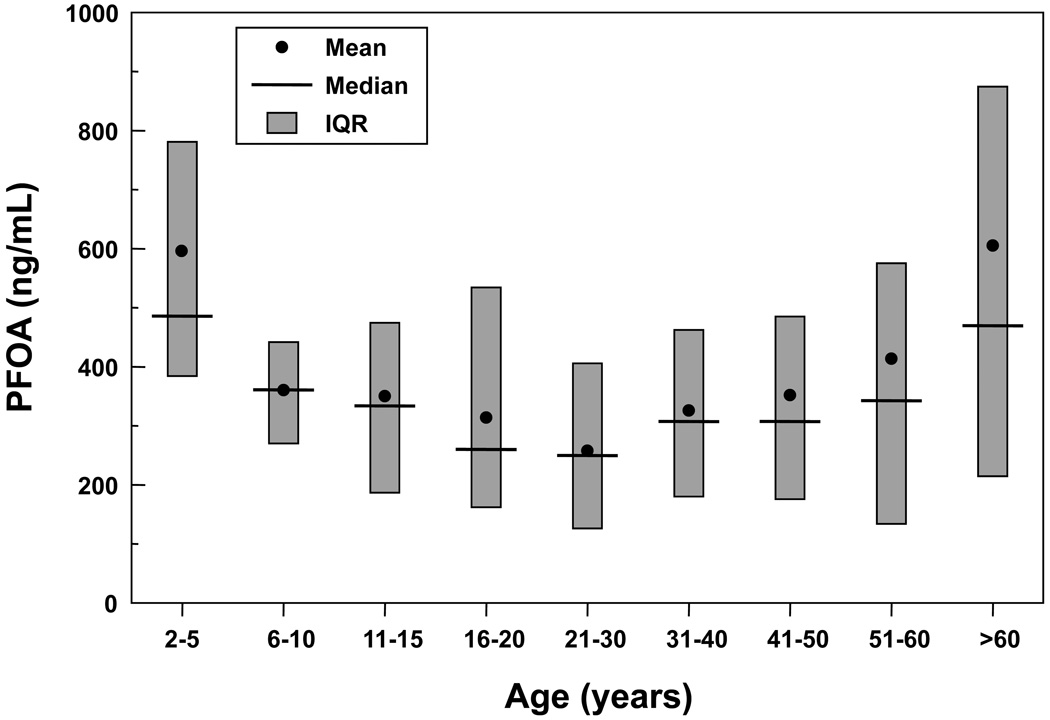
Serum PFOA concentrations were highest in those aged more than 60, followed by those aged from 2–5, and those aged 51–60 (Figure 3). Participants >60 years were significantly more likely to have higher serum PFOA levels compared to participants in all other age groups except children 2–5 years old (.0006< p <.02).
Figure 3. Distribution of Serum PFOA Levels in ng/mL by age.
Residents >60 years had significantly higher serum PFOA levels compared to all other age groups except children age 2–5 years old
With regard to residence, only participants over 18 years were examined. Years lived at current residence was grouped into 2–5 years, 6–10 years, 11–15 years, and >15 years. Age was also found to be correlated with years of residence (r= .6). Therefore, age was controlled for in the analysis for which no statistically significant association between years lived at current residence and serum PFOA levels was found (p=0.7).
The influence of alcohol consumption (consumption of beer wine or liquor in last thirty days) and smoking (current cigarette smoker) were evaluated in all adult participants ages 18 and over who did not have substantial occupational exposure. No significant association was found between serum PFOA levels and smoking (p=0.28) or serum PFOA levels and alcohol consumption (p=0.46)
Little Hocking Water System Users: Water Usage Variables Affecting Serum PFOA Concentrations
The effect of drinking tap water, eating local fruits and vegetables, meat or fish, or having a carbon water filter on serum PFOA concentrations in Little Hocking Water System Users is shown in Table 5. With increasing tap water drinks per day (at home or at work), PFOA levels increased (p=.004). Particularly, participants who drank 8 or more cups of tap water per day (at home or at work) had significantly higher serum PFOA levels compared to other drinking categories (.002 < p < .004).
Table 5.
Serum [PFOA] ng/mL, number of tap water drinks per day, consumption of local meat and game, fish, vegetables and fruits and use of carbon water filtera
| Factor | N | Meanb | Median | IQR | pr > t | |
|---|---|---|---|---|---|---|
| Tap water drinks/day | 0 | 20 | 374 | 301 | 233–423 | <.0001 |
| 1–2 | 40 | 324 | 265 | 176–438 | ||
| 3–4 | 66 | 413 | 370 | 206–550 | ||
| 5–8 | 90 | 450 | 373 | 242–373 | ||
| >8 | 55 | 565 | 486 | 294–486 | ||
| Local Meat | 0 | 157 | 389 | 329 | 179–498 | 0.018 |
| 1–20 | 49 | 488 | 451 | 246–690 | ||
| >20 | 77 | 516 | 424 | 295–595 | ||
| Local Fish | No | 273 | 448 | 374 | 221–571 | 0.8958 |
| Yes | 18 | 458 | 398 | 290–681 | ||
| Fruit and vegetables from your garden | 0 | 133 | 356 | 295 | 174–485 | <.0001 |
| 1–20 | 75 | 458 | 420 | 264–661 | ||
| >20 | 77 | 571 | 469 | 308–802 | ||
| Carbon Water Filterc | Yes | 64 | 360 | 318 | 170–482 | 0.0005 |
| No | 209 | 493 | 421 | 258–631 |
Little Hocking water source only
Means adjusted for age unless otherwise indicated
Not adjusted for age
A secondary analysis has been performed, examining air exposure and local vegetable/fruit intake. There was no effect of air exposure on PFOA (p=.16) or the interaction between air exposure and local vegetable/fruit intake (p=.73) As a result of the lack of association between these 2 variables, air exposure was not included in the GEE model. Similarly, there was a statistically significant increase (p=0.0002) in the mean serum [PFOA] associated with increasing numbers of weekly servings of fruits and vegetables from a local garden. Additionally, there was an increase in serum PFOA with servings of meat or game grown or harvested locally (p=.005). No association was found between local fish consumption and serum PFOA concentrations.
With regard to water filtration systems, residents using only Little Hocking water system water as their residential drinking water source were divided into 2 groups: those using a home water filter system based on carbon (N=64) and those who had no home water filtration system or used a system not known to remove PFOA, or used a system whose type and composition could not be verified (N=209). Residents using carbon water filters had significantly lower median serum PFOA levels (318 ng/mL), compared with residents using Little Hocking System water who did not use carbon water filtration (421 ng/mL) (p= .008)
Serum PFOA levels and Household Cooking Use of Tap Water
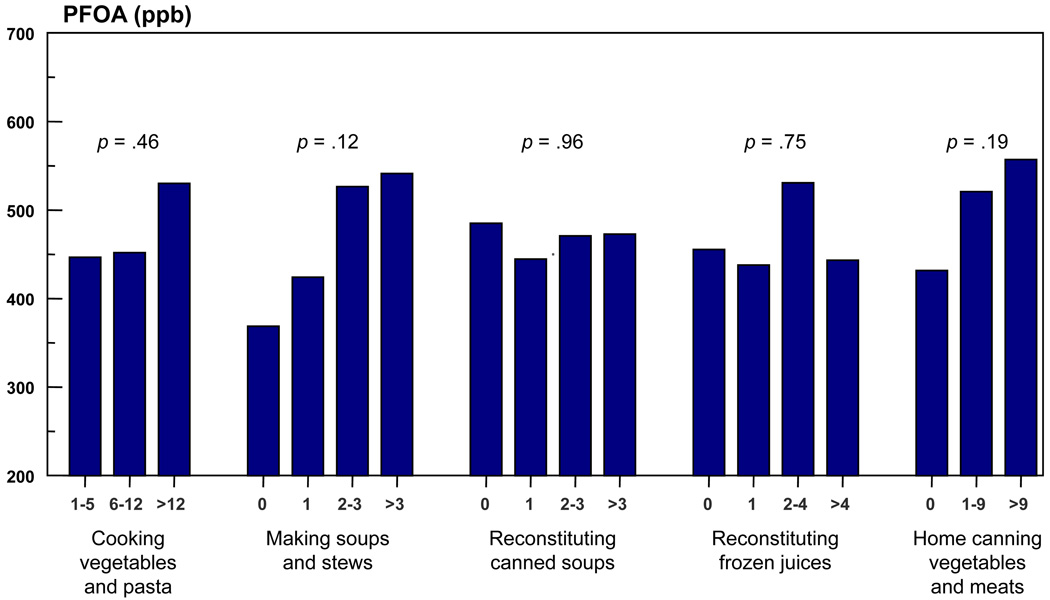
There was no relationship between serum [PFOA] and the use of tap water in cooking for those households using only Little Hocking water system water (Figure 4). When cooking vegetables and pasta, making soups and stews, reconstituting canned soups, reconstituting frozen fruit juices and home canning of vegetables and meats were examined, no statistically significant relationship with serum PFOA levels was found. However a linear trend of increasing serum PFOA levels was observed with increasing use of water for making soups and stews and for home canning of vegetables and meats.
Figure 4. Distribution of serum PFOA levels in ng/mL, within householda for cooking tap water usageb (Amounts are servings per week).
a PFOA levels represents average household value
b Households using Little Hocking water system only
Little Hocking Water System Users: Multivariate Analysis Adjusting for Household Clustering
The model of best-fit included age, tap water drinks per day, fruit and vegetable servings per week from your garden, and use of a carbon filter (Table 6). Eating meat and game grown or harvested locally was not found to be associated with serum PFOA levels in the multivariate analysis.
Table 6.
Results of Application of General Estimating Equations (GEE)
| Parameter | Estimate | Standard Error |
95% Confidence Limits | Z | Pr > |Z| | |
|---|---|---|---|---|---|---|
| Intercept | 110.54 | 58.10 | −3.34 | 224.42 | 1.9 | 0.0571 |
| Vegetable and fruit from your garden servings/week | 62.31 | 20.96 | 21.23 | 103.39 | 2.97 | 0.0029 |
| Tap water drinks/day | 5.93 | 2.02 | 1.97 | 9.88 | 2.94 | 0.0033 |
| Age (yrs) | 3.53 | 1.03 | 1.50 | 5.55 | 3.42 | 0.0006 |
| No carbon filter use | 104.92 | 35.86 | 34.65 | 175.20 | 2.93 | 0.0034 |
Note: This analysis includes only participants from households using Little Hocking water system only. Participants with substantial occupational exposure were excluded
DISCUSSION
We found that median serum PFOA levels in randomly selected residents of the Little Hocking water service district ranged from 298 to 370 ng/mL, in the order of 60 to 75 times the median levels of approximately 5 ng/mL previously described for general US populations (4,5,6). The majority of serum PFOA levels in these residents exceeded the maximums reported in previous community studies in other geographic locations. For example, the range of serum PFOA levels for 645 U.S. adult blood donors was from 1.9 ng/mL to 52.3 ng/mL (4), for 238 elderly volunteers in Seattle was 1.4 ng/mL to 16.7 ng/mL (5) and for 598 children from across the US was from 1.9 ng/mL to 56.1 ng/mL (9). The serum PFOA levels for the thirty comparison subjects for the Philadelphia area in our study all fell within previously reported normal population ranges.
Our random sampling of residents in the water district included a number of individuals who worked in the production area of a fluoropolymer manufacturing facility located across the Ohio River in Washington, WV. This facility is believed to be the primary source of PFOA pollution in the area. A recent study of workers at this plant found the median serum PFOA level of 490 ng/mL for 259 workers currently working in production areas where PFOA was used (23). We found a median serum PFOA level of 774 ng/mL for the 18 workers who had worked in the production area at the facility, lived in the Little Hocking water service area, and participated in our study. The median serum PFOA level for these 18 individuals was 284 ng/mL higher than the median reported for all production workers at the facility, suggesting a combination of residential water and occupational contributions to the PFOA body burden. Since all but one of the production workers we studied was selected through stratified random sampling, we consider it unlikely that selection bias could explain this elevation. Workers from non-production areas of the facility included in our sampling did not have significantly increased serum PFOA levels compared with other residents. The serum PFOA levels in non-occupationally exposed community residents in the Little Hocking water service district approached and frequently surpassed those measured in production workers exposed to PFOA at the source fluoropolymer manufacturing plant. These results illustrate that body burdens of pollutants sustained through community environmental exposures are not necessarily less than those sustained through occupational exposure.
We were able to explore other potential occupational exposure contributions to the serum PFOA levels. In addition to use in the manufacture of fluoropolymers, it has been suspected that PFOA may also be a breakdown product of fluorinated telomers. PFOA is used as a surfactant or surface treatment chemical in many products, including fire-fighting foams; personal care and cleaning products; oil, stain, grease and water repellent coatings on carpet; textile leather and paper (21). PFOA has had limited use as a fire suppressant. A study of PFOA in consumer products identified extractable PFOA in carpet-care solution treated carpeting (24). Because PFOA and related fluorinated compounds are currently unregulated, there is relatively little available information on the extent of their use. Based on a qualitative assessment of potential occupational exposure to PFOA in the Southeastern Ohio area, we explored occupational exposure in firefighting, carpet cleaning and carpet installation in addition to potential exposure in the disposal or incineration of PFOA and/or waste from the fluoropolymer manufacturing facility. We did not observe a significant increase in median serum PFOA concentration in any of these occupational groups. It remains possible that in a population with less exposure to PFOA from ambient contamination, and identifiable contributions to the body burden might be found from one or more of these occupational exposures.
Several observations support the conclusion that the major source of the PFOA in Little Hocking water district residents was drinking water. Serum PFOA levels were similar whether residents lived in the area proximate to the plant where the air plume would have been concentrated, or in an area which had the same water service but was located up to 20 miles from the plant and where air pollution with PFOA was estimated to be minimal. Serum PFOA levels were considerably lower in those residents who were currently using only bottled, spring, or cistern water as their drinking water source. Where the primary drinking water source was well water, serum PFOA levels varied in proportion with well water PFOA levels.
The median serum/drinking water PFOA ratio of 105 we observed in Little Hocking water users likely reflects both high PFOA absorption after oral ingestion and a long half-life of PFOA in human blood. In rats, the oral bioavailability of PFOA is approximately 100% (25). The serum half-life varies widely by species and sex: several hours for female rats, about 7 to 10 days for male rats (25): 20.9 days for male and 32.6 days for female cynomolgus monkeys (26). The half-life in humans appears to be much longer. In the one set of data that is available, a study of 9 retirees from a fluoropolymer production facility, the mean serum PFOA half-life was found to be 4.4 years (20). However, we did not find a relationship between serum PFOA levels and length of residence in the Little Hocking water district among study participants, all of whom had lived in the area for at least two years. If the half-life in the general community is in the order of 4 to 5 years we would have expected to find a significant relationship with duration of residence. Our results thus lead us to question whether the serum PFOA half-life in the general community is as long as that published for the small retired worker group (20). We expect to have more data on this subject from a follow-up study.
In residents who drank only Little Hocking system water the model of best-fit for serum PFOA levels included age, tap water drinks per day, fruit and vegetable servings per week from a local garden, and use of a carbon water filter. The finding that PFOA concentrations were higher in children aged 5 and below and in the elderly aged over 60 is disturbing, since these may represent groups particularly vulnerable to adverse health consequences (27, 28). The reason for the higher serum PFOA levels in those aged 60 and above is not entirely clear, multivariate analysis shows the increased consumption of drinking water in this group does not fully explain the observed increase. Both the elderly and those aged 5 and below may spend more time at home with exclusive use of residential water than working or school-age residents. Infants and young children may have proportionately greater exposure to water-borne pollutants since they drink more water per kg of body weight than do adults (28). The levels in the very young may also represent additional exposures as PFOA has been shown to cross the placenta and to be present in breast milk (at approximately 1/10 of the serum concentration) in Sprague Dawley rats (29), although comparable studies in humans are lacking. We are performing further studies to elucidate PFOA exposures in maternal milk and infant formula. A higher serum PFOA level for young children was previously observed by Olsen et al (9) who measured PFOA in the serum of 598 children aged 2–12 who participated in a nationwide US study of Group A Streptococcal infections, 645 adult blood donors from 6 US blood bank donation sites, and 238 elderly subjects in Seattle participating in a study of cognitive function. The geometric mean serum PFOA levels (4.6 ng/mL, 4.2 ng/mL, 4.9 ng/mL respectively) were similar in all groups. However in the children there was a statistically significant negative association with age, with the highest mean serum PFOA levels noted at age 4 and the lowest at age 12. Our failure to find gender differences is consistent with previous observations in the US general population.
The association with the number of servings of fruits and vegetables from the home garden was unexpected. Possible explanations include the use of PFOA containing water for cooking, canning and washing fruits and vegetables, PFOA in the raw fruits and vegetables, and different dietary and drinking habits in those who consume more homegrown fruits and vegetables. We consider it unlikely that PFOA is elevated in raw fruits and vegetables from the garden because as a result of the natural rainfall characteristics it is unusual to water gardens and fruit trees extensively with residential water in this district. Also the association between serum PFOA and servings of fruits and vegetables was not reduced by adjusting for residence in the areas with known higher airborne and soil levels of PFOA. We are undertaking further studies to better understand the observed association.
Individuals using carbon-type water filters for residential drinking water had a reduction of approximately 25% in median serum PFOA levels compared with those not using a filter. This reduction was much less than we have seen for those who drank only bottled, spring or cistern water. Because of limited effectiveness, potential reliability problems associated with the need to maintain the filter system, and potential health problems associated with the use of home filtration systems we do not recommend reliance on home filters to remove PFOA. New water filtration products to remove PFOA are currently being pilot tested, with prospects of wider use in the near future.
The high serum PFOA levels in our study as a result of the relatively high exposure in drinking water, may have limited our ability to detect relatively small increases associated with contributions from ambient air pollution. Thus we cannot exclude the possibility that exposure to PFOA in air could lead to a detectable contribution to the PFOA body burden in other populations with minimal water exposure.
Our finding that the major source of serum PFOA was residential drinking water has helped empower those in the community who may choose to lower their PFOA exposure, with a view to lowering their body burden. As a result of our preliminary findings that the levels of PFOA were abnormally high in residents of the Little Hocking water district, and that the major non-occupational PFOA source was residential drinking water, the option of free bottled drinking water has been made available through the Little Hocking Water Association to those with this water service. More than half of the residents are already taking advantage of this offer. In addition, a new water filtration system designed to remove PFOA is now planned. We would anticipate that these actions should result in reduced serum PFOA levels. We plan to monitor changes in serum PFOA levels in the study group over the next eighteen months, to determine the extent of any serum PFOA reductions.
Identification of water as the major route of community exposure to PFOA in this population should encourage efforts to define exposure sources in other populations, and should provide a basis for personal and regulatory efforts to reduce human exposure to a pollutant which is of concern because of remarkable persistence in both the environment and in humans.
Acknowledgments
Sources of Support
This study was supported by grant ES12591 from the Environmental Justice Program of the US National Institute for Environmental Health Sciences (NIEHS), National Institutes of Health, and by P30 Core Center grant ES 013508 from the NIEHS.
REFERENCES
- 1.Kennedy GL, Butenhoff JL, Olsen GW, et al. The toxicology of perfluorooctanoate. Crit Rev Tox. 2004;34:351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- 2.Ellis DA, Cahill TM, Mabury SA, et al. Partitioning of organofluorine compounds in the environment. In: Neilson AH, editor. Organofluorines. The Handbook of Environmental Chemistry, Volume 3N. Berlin: Springer-Verlag; 2002. pp. 63–83. [Google Scholar]
- 3.Kannan K, Koistinen J, Beckman K, et al. Accumulation of perfluorooctane sulfonate in marine mammals. Environ Sci Technol. 2001;35:1593–1598. doi: 10.1021/es001873w. [DOI] [PubMed] [Google Scholar]
- 4.Olsen GW, Church TR, Miller JP, et al. Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors. Environ Health Perspect. 2003;111:1892–1901. doi: 10.1289/ehp.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen GW, Church TR, Larson EB, et al. Serum concentration of perfluorooctanesulfonate and other fluorochemicals in an elderly population from Seattle, Washington. Chemosphere. 2004;54:1159–1611. doi: 10.1016/j.chemosphere.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Olsen GW, Huang HY, Helzlsouer KJ, et al. Historical comparison of perfluorooctane, perfluorooctanoate, and other fluorochemicals in human blood. Environ Health Perspect. 2005;113:539–545. doi: 10.1289/ehp.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kannan K, Corsolini S, Falandysz J, et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Env Sci Technol. 2004;38:4489–4495. doi: 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- 8.Gribble GW. Naturally occurring organofluorines. In: Neilson AH, editor. Organofluorines. The Handbook of Environmental Chemistry, Volume 3N. Berlin: Springer-Verlag; 2002. pp. 121–136. [Google Scholar]
- 9.Olsen GW, Church TR, Hausen KJ, et al. Quantitative evaluation of perfluorooctanesulfonate (PFOS) and other fluorochemicals in the serum of children. J. Children’s Health. 2004;2:53–76. [Google Scholar]
- 10.Johnson JD, Gibson SJ, Ober RF. Cholestyramine enhanced fecal elimination of carbon-14 in rats after administration of ammonium [14C] perfluorooctanate or potassium [14C] perfluorooctanesulphonate. Fund Appl Toxicol. 1984;4:972–976. doi: 10.1016/0272-0590(84)90235-5. [DOI] [PubMed] [Google Scholar]
- 11.Fasano WJ, Kennedy GL, Szostek B, et al. Penetration of ammonium perfluorooctanoate through rat and human skin in vitro. Drug Chem Toxicol. 2005;1:79–90. doi: 10.1081/dct-39707. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy GL. Dermal toxicity of ammonium perfluorooctanoate. Toxicol Appl. Pharmacol. 1985;81(2):348–355. doi: 10.1016/0041-008x(85)90172-3. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy GL, Hall GT, Britelli MR, et al. Inhalation toxicity of ammonium perfluorooctanoate. Food Chem. Toxicol. 1986;24(12):1325–1329. doi: 10.1016/0278-6915(86)90066-9. [DOI] [PubMed] [Google Scholar]
- 14.Uy-Yu N, Kawashima Y, Kozuka H. Comparative studies on sex-related difference in biochemical responses of livers to perfluorooctanoic acid between rats and mice. Biochem Pharmacol. 1990;39:1492–1495. doi: 10.1016/0006-2952(90)90434-m. [DOI] [PubMed] [Google Scholar]
- 15.Abdellatif AG, Preat V, Taper HS, et al. The modulation of rat liver carcinogenesis by perfluorooctanoic acid, a peroxisome proliferator. Toxicol Appl Pharmacol. 1991;111:530–537. doi: 10.1016/0041-008x(91)90257-f. [DOI] [PubMed] [Google Scholar]
- 16.Cook JC, Murray SM, Frame SR, et al. Induction of Leydig cell adenomas by ammonium perfluorooctanate: a possible endocrine related mechanism. Toxicol Appl Pharmacol. 1992;192(113):209–217. doi: 10.1016/0041-008x(92)90116-a. [DOI] [PubMed] [Google Scholar]
- 17.Biegel LB, Hurtt ME, Frame SR, et al. Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicological Sciences. 2001;60:44–45. doi: 10.1093/toxsci/60.1.44. [DOI] [PubMed] [Google Scholar]
- 18.Clegg ED, Cook JC, Chapin RE, et al. Leydig cell hyperplasia and adenoma formation: mechanisms and relevance to humans. Reproduct. Toxicol. 1997;11:107–121. doi: 10.1016/s0890-6238(96)00203-1. [DOI] [PubMed] [Google Scholar]
- 19.Liu SC, Sanfilippo B, Perroteau I, et al. Expression of transforming growth factor (TGFa) in differentiated rat mammary tumors: estrogen induction of TGFa production. Mol. Endocrinol. 1987;1:683–692. doi: 10.1210/mend-1-10-683. [DOI] [PubMed] [Google Scholar]
- 20.Burris JM, Lundberg JK, Olsen GW, Simpson C, Mandel J. Interim Report #2. 3M Medical Department. Washington DC: U.S. EPA Public Docket AR-226-1086; 2002. Determination of serum half-lives of several fluorochemicals. [Google Scholar]
- 21.U.S.EPA. OPPT Fact Sheet- PFOA Q's and A's. [Accessed August 1, 2005]; www.epa.gov/oppt/pfoa/
- 22.Flaherty JM, Connolly PD, Decker ER, et al. Quantitative determination of perfluorooctanoic acid in serum and plasma by liquid chromatography tandem mass spectroscopy. J Chromatog B. 2005;819:329–338. doi: 10.1016/j.jchromb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 23.U.S.EPA. U.S. EPA Public Docket AR-226-1922. Washington DC: 2005. Jan 11, Results to date from the PFOA Worker Health Study. [Google Scholar]
- 24.Washburn ST, Bingman TS, Braithewaite SK, et al. Exposure assessment and risk characterization for perfluorooctanoate in selected consumer articles. Env Sci & Tech. 2005;39:3904–3910. doi: 10.1021/es048353b. [DOI] [PubMed] [Google Scholar]
- 25.Kemper RA. U.S. EPA Public Docket AR-226. Washington DC: 2003. Perfluorooctanoic acid: toxicokinetics in the rat. [Google Scholar]
- 26.Thomford PJ. U.S. EPA Public Docket AR-226-1052a. Washington DC: 2001. 26 week capsule toxicity study with ammonium perfluorooctanoate (APFO) in cynomolgus monkeys. [Google Scholar]
- 27.Landrigan PJ, Etzel RA. Chemical Pollutants. In: Behrman RE, Kleigman RM, Jenson HB, editors. Nelson Textbook of Pediatrics 16th Edition. Philadelphia: WB Saunders; 2000. pp. 2152–2154. [Google Scholar]
- 28.Cooper RL, Goldman JM, Harbin TJ. Aging and Environmental Toxicology. Baltimore: Johns Hopkins University Press; 1991. [Google Scholar]
- 29.Hindlighter PM, Mylchreest E, Gannon SA, et al. Perfluoroctanoate: placental and lactational transport pharmacokinetics in rats. Toxicology. 2005;211:139–148. doi: 10.1016/j.tox.2005.03.010. [DOI] [PubMed] [Google Scholar]