Abstract
Objective
Determine whether certain biomarkers of toxicity and/or a past diagnosis of liver or thyroid disease were associated with serum perfluorooctanoate concentrations [PFOA] in a community with longstanding environmental exposure to PFOA.
Methods
Serum PFOA, hematologic and biochemical biomarkers and a questionnaire administered to 371 residents selected by stratified random sampling and a lottery amongst volunteers. Median PFOA was 354 ng/mL, interquartile range181 ng/mL to 571 ng/mL.
Results
No significant positive relationships between serum [PFOA] and liver or renal function tests, cholesterol, TSH, or with red cell indices, white cell or platelet counts. Mean serum [PFOA] was not increased in those with a history of liver or thyroid disease.
Conclusions
No toxicity from PFOA demonstrated using the measured endpoints, other endpoints need to be addressed.
INTRODUCTION
Perfluorooctanoate (PFOA, CF3, (CF2)6 C00−, CAS No 3825-26-1) is a persistent pollutant in the environment, and found at low concentrations in many diverse human populations globally. PFOA has defined toxicity to experimental species, but the toxicity to humans remains unclear. In this paper we report the first published study of possible health effects of PFOA in a non-occupational group.
PFOA has commercial use primarily as ammonium perfluorooctanoate, an essential surface-active agent in the production of various fluoropolymers, including tetrafluoroethylene. Fluoropolymers are used in a wide variety of industrial and consumer products, including protective coatings for carpets and apparel, consumer house wares, paper coatings, electronics, insecticide formulations, surfactants, aerospace and other applications. PFOA also occurs as a contaminant in other fluorochemicals and telomer products (1). PFOA is not detectable in fluoropolymer cookware samples studied under simulated cooking conditions (2). Ammonium perfluorooctanoate is fully dissociated into the anion form, perfluorooctanoate, in environmental media and biological fluids.
PFOA is a man-made chemical with no known natural source (3) that is persistent in the environment and is resistant to biological, environmental or photochemical degradation. PFOA, along with a related compound perfluorooctane sulphonate (PFOS), is now found both in marine animals inhabiting widely spread geographical biospheres (4) and in human serum from widely disparate groups. The median serum PFOA concentration ([PFOA]) in the US population is around 4 to 5 µg/L, with occasional values above 20 µg/L (5,6,7).
The toxicology of PFOA has recently been reviewed (1,8). In rats, PFOA is well absorbed following both oral and inhalation exposure (9,10) distributing primarily to the liver, plasma, and kidneys (11). PFOA binds covalently to proteins in the rat liver, plasma, and testes (11). The serum half-life in rodents is a few days with slower elimination in male rats than female (12,13). Urine and feces are the principal routes of excretion in male rats, urine only in females (14) and castrated male rats (14,15). In male rats fecal excretion of PFOA is increased by cholestyramine resin intake, suggesting enterohepatic circulation (16).
PFOA is not metabolized in mammals (9,12,17). A number of toxic effects have been observed in experimental species. PFOA is one of a group of compounds that activates the peroxisome proliferator activated receptor (PPAR) alpha in rats leading to a response characterized broadly as peroxisomal proliferation (1). In rats PFOA is strongly hepatotoxic (10, 18), male rats are more susceptible (19). Aged rats are also more susceptible to the liver damage and oxidative stress caused by PFOA (20). PFOA is immunotoxic to rats, resulting in decrease in spleen and thymus weights (21) as a result of both PPAR alpha dependent processes (22) and actions mediated through the brain (23). PFOA has been associated with increased serum estradiol and reduced testosterone in rats, possibly due to induction of hepatic aromatase activity (24).
Monkeys fed PFOA show decreased thyroid hormone levels, increases in liver weight and toxic hepatic changes (25), slight to moderate hypocellularity of the bone marrow, moderate atrophy of lymphoid follicles, and marked diffuse lipid depletion in the adrenals (19).
Carcinogenesis studies in rats fed PFOA show statistically significant increases in liver tumors, pancreatic acinar cell tumors, testicular Leydig cell adenomas (males) and mammary hyperplasia (females) compared with controls (26,27). In rodents, PFOA promotes liver carcinogenesis (28,29,30). While the significance of these tumors to humans is unclear, the International Agency for Research on Cancer (IARC) (31) has concluded that liver tumors induced in rodents by PPAR agents are unlikely to be operative in humans based on the current understanding of the mode of action in animals. Although tumor formation by PFOA was thought to occur only through non-genotoxic mechanisms (32), Yao and Zhou (33) have recently reported that PFOA exerts genotoxic effects on human hepatoma HepG2 cells mediated through intracellular reactive oxygen species and oxidative DNA damage.
Because of profound differences in PFOA half-lives between species, toxicokinetics of PFOA in humans cannot be predicted based on animal data (8). The half-life in the blood of PFOA in rats, following a single oral dose, was 4 hours in females, and 9 days in males (12). In rabbits, the serum half-life is in the order of 4 hours for both males and females (34). The serum half-life in cyanomolgus monkeys is approximately 20 days, with urine as the primary source of excretion (35, 36). The mean half-life in the serum of human retirees from the 3M Company who had previous heavy occupational exposure was 4.37 years (range 1.5 to 13.49 years SD=3.53) without substantial gender differences. Neither age, body mass index (BMI), nor number of years since retirement were significant predictors of the human serum half-lives in multivariate regression analysis (37). In humans the renal clearance of PFOA is 10−5 fold less than the glomerular filtration rate suggesting the absence of excretion by human kidneys (38). Thus the published half-life in human females is ~35,000 times longer than that for the female rat.
Human studies addressing potential PFOA toxicity are limited. Cross-sectional analysis of routine medical surveillance results from facilities producing both PFOA and PFOS have found significant positive association between serum PFOA and increased cholesterol, triglycerides and thyroid hormone (T3) levels (39). Cross-sectional studies of hormonal levels in workers at a PFOA production facility have found significant associations between serum hormones and PFOA in some years (40) but not other years (41). Elevated serum liver enzymes were associated with occupational exposure to PFOA but only in obese men (BMI> 35kg/m2) (42), and not in subsequent years (43). Preliminary results from two recent unpublished studies of workers occupationally exposed to PFOA, have also observed a positive association between serum [PFOA] and serum cholesterol (Dupont Company, personal communication).
A retrospective cohort mortality study at a plant producing PFOA found an elevated SMR for prostate cancer in chemical production workers, which was significantly associated with length of employment in chemical production. The relative risk of prostate cancer was 3.3 (95% CI 1.02–10.6) for workers employed in chemical manufacturing for 10 years or more (44). A follow-up study where workers were classified into multiple exposure groups did not confirm the association (45), but may have been limited by low statistical power to detect elevation in cancer rates in the smaller, reclassified groups. No epidemiologic studies of potential health effects in non-occupational groups have been reported.
We have performed an epidemiologic study of residents in the Little Hocking water district in Southeastern Ohio where there is significant environmental exposure to PFOA. Water supplied by the Little Hocking Water Association has been contaminated with PFOA for many years, over the last three years at a mean level of 3.5 ug/L. PFOA in the environment in the vicinity of Little Hocking is generally believed to be coming from a neighboring industrial facility where it is used as a solvent and dispersant for fluoropolymer production. We have shown that the residents of this water district have a median serum [PFOA] that is approximately 70 times that of the general US population, and that the major source of PFOA is water from either the public water supply or contaminated residential well-water (Emmett EA, Shofer FS, Zhang H, et al, submitted for publication). Serum [PFOA] was also influenced by age (higher in those ≤ 5 or ≥ 60), number of tap water drinks per day, number of weekly servings of home-grown fruits and vegetables and use of a carbon-based residential water filter. Residents of the area who also worked in the production area of the plant had the highest PFOA levels, with residential and occupational exposures appearing to be additive.
In this paper we explore whether certain biomarkers of toxicity and adverse health effects, potentially attributable to PFOA based on animal toxicologic studies, are associated with serum [PFOA] in Little Hocking water district residents. Specifically we examine serum liver function tests, cholesterol, renal function tests, Thyroid Stimulating Hormone (TSH) and various hematologic parameters. We also examine whether studied individuals reporting a previous clinical diagnosis of either liver or thyroid disease have elevated [PFOA] compared with study participants without such diagnoses.
METHODS
Selection and Study Group
The study group consisted of residents from a stratified random sample of persons from households who had resided in the Little Hocking Water Association district for at least two years, supplemented by a smaller group of volunteers meeting the same eligibility criteria. The selection of households for the sampling frame, selection of the stratified random sample of residents, process for distributing invitations to participate and participation rates are described elsewhere (Emmett EA, Shofer FS, Zhang H, et al. submitted for publication). For our previous studies of routes of exposure, 18 residents, who had substantial occupationally exposed to PFOA in the production area of the fluoropolymer production facility, and had been selected into the study by chance through the stratified random sampling process were excluded from some analyses. These 18 subjects were all included in the analyses reported in this paper.
Administration of Questionnaires
Administration of questionnaires and collection of blood samples were performed at the Grand Central Family Medicine Office in nearby Parkersburg, WV. Informed consent was first obtained from each subject, or parent or guardian in the case of minors. Minors under the ages of 17 were encouraged to give informed assent wherever feasible. Different questionnaires were administered to adults and children. Questionnaires were developed and revised after review by the members of the Community Advisory Committee and by a group of experts from the US EPA. Prior to finalization the questionnaires were pilot tested on a representative group of 20 individuals from similar Southeastern Ohio or Western West Virginia communities, who did not live in the Little Hocking Water Association District. Trained interviewers administered all questionnaires.
All adults 18 years and older were administered an adult questionnaire. The information elicited included demographic and occupational information, health conditions (i.e. have you ever been treated for or told by a doctor that you have any of the following health conditions: cirrhosis of the liver, hepatitis, any other liver condition; hyperthyroidism, hyperactive or overactive thyroid, goiter or enlarged thyroid, hypothyroidism or under active thyroid; history of bleeding disorder, smoking, and alcohol habits. All children were asked similar questions except that the questions about smoking and alcohol habits were omitted.
Blood and Samples
Phlebotomy was performed on all subjects. No subjects were given instructions to fast. Five mls of blood were taken into a purple-topped Vacutainer tube and sent for complete blood count (hemoglobin, hematocrit, red blood cell indices, white cell count and differential white cell count, platelet count). Thirty mls of blood were drawn into red-topped Vacutainer tubes: ten mls were immediately sent for serum chemistry determinations (Total Protein, Albumin, Blood Urea Nitrogen, Creatinine, Bilirubin, Alkaline Phosphatase, Aspartate Aminotransferase (SGOT), Alanine Aminotransferase (SGPT), Gamma Glutamyl Transpeptidase (GGT), Total Cholesterol, Thyroid Stimulating Hormone). Twenty mls of blood were immediately spun down and the serum frozen and stored pending shipping in batches to the Clinical Toxicology Laboratory at the University of Pennsylvania for PFOA analysis. The storage, shipping and handling of samples, and the assay procedure for [PFOA] are described elsewhere (Emmett EA, Zhang H, Shofer FS, Freeman D, Rodway MV, Desai C et al, submitted for publication). PFOA was analyzed using HPLC/tandem mass spectrometry by a modification of the method of Flaherty et al (46).
Feedback of Results to Participants
Each participant was informed of his or her personal blood chemistry and hematological results as well as [PFOA], together with any necessary explanation by a local health-care provider (H.Z. or N.R.). Those few participants with markedly abnormal blood chemistry and/or hematological results were personally telephoned by H.Z. or N.R and the results discussed directly with the participant. Participants with abnormal laboratory results were advised to see their personal physician.
Statistical Analysis of Biomonitoring and Exposure Data
To determine if serum [PFOA] was correlated with any liver function or hematologic parameter, simple regression was used. To assess whether PFOA levels were increased in those with abnormal blood chemistries or hematologic values, binary groups were formed (normal vs. abnormal) and tested using Student’s t-test. Serum [PFOA] in participants with thyroid or liver disease was compared to those without disease using Student’s t-test. For the regressions, data are presented using correlation coefficients and p-values testing whether the slope of the line is 0. Serum [PFOA] is presented as mean, median, and interquartile range (IQR). All analyses were performed using SAS statistical software (Version 9.1, SAS Institute, Cary NC). A p<.05 was considered statistically significant.
Human Subjects Approvals
The study was approved by the Institutional Review Board (IRB) of the University of Pennsylvania. Participation was voluntary. Informed consent was obtained for all participants prior to any study. A Certificate of Confidentiality was obtained from the NIH to ensure maximum protection of personal information and results.
The study was conducted through a partnership between the University of Pennsylvania School of Medicine, the Decatur Community Association, a local community association in the Little Hocking water service area, and Grand Central Family Medicine in Parkersburg WV, a local health care provider, through a grant from the Environmental Justice Program of NIEHS. The community was involved at all stages of the study. The Community Advisory Committee included residents from the affected townships, representatives of the US and Ohio EPA and the Health Commissioner for Washington County, Ohio.
RESULTS
Demographics and Distribution of Serum [PFOA] in the Studied Population
Results were available from 371 individuals, 317 participants from the randomly selected sample and 54 from the volunteer group. Females represented 53.4% of the study sample (N=198). The age distribution of the study group is presented in Table 1. The median age of the study group was 50 (range 2.5 –89 years). There were 43 children under 18 years of age.
Table 1.
Age Distribution of 371 Residents of Little Hocking Water Service District Participating in Study
| Age | Frequency | Percent | Cumulative Percent |
|---|---|---|---|
| 2–10 | 20 | 5.39 | 5.39 |
| 11–20 | 29 | 7.82 | 13.21 |
| 21–30 | 20 | 5.39 | 18.60 |
| 31–40 | 42 | 11.32 | 29.92 |
| 41–50 | 80 | 21.56 | 51.48 |
| 51–60 | 93 | 25.07 | 76.55 |
| >60 | 87 | 23.45 | 100.00 |
| Total | 371 | 100.00 |
N = 371
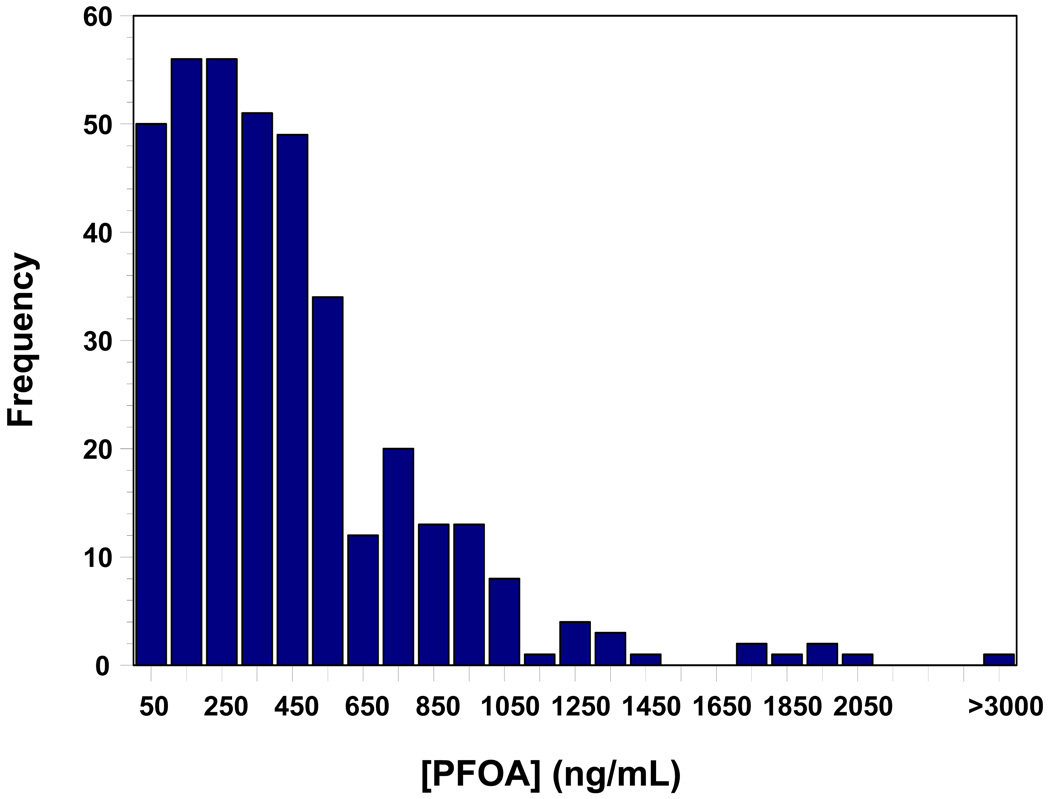
The distribution of serum [PFOA] in the studied individuals is presented in Figure 1. In the population, the median serum [PFOA] was 354ng/mL, and interquartile range was 184 to 571 ng/mL. There was a fairly uniform distribution of values through about 450ng/mL with a truncated distribution to around 2100ng/mL and one value above 4000ng/mL.
Figure 1.
Frequency distribution of serum [PFOA] in the studied population of residents of the Little Hocking water service district. N = 371
Relationship Between Serum [PFOA] and Chemistry and Hematologic Biomarkers
The mean, median, and interquartile range for the studied blood chemistry variables in this population are presented in Table 2. Additionally, we present a regression slope and correlation coefficient testing the relationship between serum [PFOA] and each blood or serum chemistry variable. No significant correlation between any test and [PFOA] was observed (p>.05 for all). This remained true whether the relationship was explored for all individuals a group, or separately for adults aged 19 or older, and children/adolescents aged 18 or less.
Table 2.
Serum Chemistry Biomarkers in Study Population and Association with Serum [PFOA]
| Liver Function Test | Mean | Std Dev | Median | IQR | slope estimate |
r | p value* | |
|---|---|---|---|---|---|---|---|---|
| BUN | 14.97 | 5.32 | 14.0 | 12.0 | 18.0 | 0.0003657 | 0.028 | 0.59 |
| Creatinine, Serum | 0.83 | 0.33 | 0.8 | 0.7 | 0.9 | 0.00000755 | 0.010 | 0.86 |
| Protein, Total, Serum | 7.18 | 0.38 | 7.2 | 6.9 | 7.4 | 0.00008149 | 0.087 | 0.10 |
| Albumin, Serum | 4.32 | 0.27 | 4.3 | 4.1 | 4.5 | 0.00001197 | 0.017 | 0.74 |
| Bilirubin, Total | 0.47 | 0.34 | 0.4 | 0.3 | 0.6 | −0.00000467 | 0.000 | 0.92 |
| Alkaline Phosphatase, Serum | 101.50 | 70.99 | 82.0 | 67.0 | 101.0 | −0.00416 | 0.024 | 0.65 |
| AST (SGOT) | 23.94 | 19.53 | 21.0 | 18.0 | 27.0 | −.0007586 | 0.014 | 0.76 |
| ALT (SGPT) | 24.85 | 31.24 | 21.0 | 15.0 | 27.0 | −0.00183 | 0.024 | 0.65 |
| GGT | 25.21 | 33.07 | 20.0 | 14.0 | 27.0 | 0.00057711 | 0.010 | 0.89 |
| Cholesterol, Total | 198.01 | 38.86 | 194.0 | 172.0 | 220.0 | 0.00551 | 0.057 | 0.27 |
| TSH | 2.06 | 1.88 | 1.7 | 1.1 | 2.4 | 0.00021305 | 0.046 | 0.38 |
Tests slope = 0
N = 371
The mean, median, interquartile ranges, and the regression slope and correlation coefficient testing the relationship between serum [PFOA] and the studied hematologic variables are presented in Table 32. Only absolute monocyte count demonstrated a significant correlation with serum [PFOA] (p=.01). However the slope estimate was small (slope= 0.00005) and the correlation coefficient suggested a very weak positive correlation (r=.13). There was no significant relationship between serum [PFOA] and the percentage of monocytes in differential white cell counts.
We also evaluated whether the serum [PFOA] was significantly different between those with abnormal values for each of the serum chemistry and hematologic variables, compared with those who had a normal value for that test. For this purpose, abnormal values were defined with respect to the normal ranges for the individual ages, providing an additional check for age-related effects. Results for each variable were examined separately, as shown in Table 4. In three instances, AST (SGOT), percent neutrophils, and percent lymphocytes, there was a statistically significant difference (p=.03, .02, and .01 respectively). In each case, study individuals with abnormal values had lower serum [PFOA]s compared to individuals with normal values (AST: abn [PFOA] =263 vs. norm [PFOA] = 449; Neutrophils: abn [PFOA] =354 vs. norm [PFOA] =454; Lymphocytes: abn [PFOA] =327 vs. norm[PFOA] =450). In no instance was an abnormal value positively associated with [PFOA].
Table 4.
Comparison of Serum [PFOA] Between Those with Abnormal Values for Serum Chemistries and Hematologic Parameters and Those with Normal Values for That Parameter
| Parameter | Abnormal | Abn % | t-test* |
|---|---|---|---|
| BUN | 6 | 2% | 0.86 |
| Creatinine, Serum | 17 | 5% | 0.62 |
| Protein, Total, Serum | 2 | 0.5 | ND |
| Albumin, Serum | 7 | 2% | 0.83 |
| Bilirubin, Total | 4 | 1% | 0.70 |
| Alkaline Phosphatase, Serum | 6 | 2% | 0.63 |
| AST (SGOT) | 9 | 2% | 0.03 |
| ALT (SGPT) | 28 | 8% | 0.30 |
| GGT | 11 | 3% | 0.50 |
| Cholesterol, Total | 182 | 49% | 0.79 |
| TSH | 24 | 6% | 0.59 |
| White Blood Cell Ct | 18 | 5% | 0.64 |
| Red Blood Cell Ct | 17 | 5% | 0.18 |
| Hemoglobin | 16 | 4% | 0.66 |
| Hematocrit | 2 | 0.5 | ND |
| MCV | 19 | 5% | 0.43 |
| MCH | 18 | 5% | 0.97 |
| MCHC | 0 | 0% | ND |
| RDW | 25 | 7% | 0.31 |
| Platelets | 8 | 2% | 0.75 |
| Neutrophils | 35 | 9% | 0.02 |
| Lymphocytes | 18 | 5% | 0.01 |
| Monocytes | 39 | 11% | 0.09 |
| Eosinophils | 19 | 5% | 0.10 |
| Basophils | 0 | 0% | ND |
| Neutrophils (Absolute) | 12 | 3% | 0.23 |
| Lymphocytes (Absolute) | 3 | 1% | 0.59 |
| Monocytes (Absolute) | 7 | 2% | 0.85 |
| Eos (Absolute) | 22 | 6% | 0.85 |
| Basos (Absolute) | 0 | 0% | ND |
Tests for differences in PFOA values between normal and abnormal values
N = 371
Relationship Between Serum [PFOA] and Reported Liver or Thyroid Disease
Study individuals with liver disease (N=13) had higher levels of PFOA (527ng/mL) compared to individuals without liver disease (441 ng/mL) but this difference was not statistically significant (p=.5). Study individuals with thyroid disease (N=40) had lower levels of PFOA (387 ng/mL) compared to individuals without thyroid disease (451 ng/mL) but this difference was also not statistically significant (p=.3).
CONCLUSIONS
We have found no significant positive association between serum [PFOA] and markers of a number of potential health effects from PFOA, in a sample of residents from a community with markedly elevated serum [PFOA] compared with general population levels. The median serum [PFOA] in the studied residents was 354 ng/mL with an interquartile range of 184–571 ng/mL, compared with a median serum [PFOA] in the general US population of 4 to 5 ng/mL (5,6,7) and a median serum [PFOA] of 6 ng/mL, interquartile range of 5–10 ng/mL in 30 Philadelphia area residents (Emmett EA, Shofer FS, Zhang H, et al, submitted for publication). The median serum [PFOA] for 259 workers using PFOA at the fluoropolymer production facility neighboring the Little Hocking community was 490 ng/mL, and the median serum [PFOA] for 342 workers at that same facility who had never been assigned to PFOA areas was 110 ng/mL (47).
The biomarkers for effect and the diseases were chosen on the basis of the known toxicity in experimental animals and the results of a limited number of human occupational studies.
Although PFOA has been described as binding with human and rat serum albumin (48) we did not observe any association between serum [PFOA] and serum protein levels. We did not evaluate whether the concentration of PFOA was elevated in the protein compartment of serum, compared with other compartments.
In rats, PFOA administration results in PPAR alpha activation and is associated with reduction in serum cholesterol levels (1). The apparent association of increasing serum cholesterol with serum [PFOA] observed in three recent clinical studies of occupational groups is the opposite of what would be expected from PPAR alpha activation, suggesting a different mechanism in humans. In this study we did not observe any association between serum [PFOA] and serum cholesterol.
In rodents the liver appears to be the most sensitive target organ to the effects of PFOA. Subchronic and chronic toxicity studies show that PFOA administration produces increased liver size, diffuse hepatocellular hypertrophy and necrosis, and dose-dependent increases in serum alkaline phosphatase, ALT and AST levels (10,18). Male rats develop liver toxicity at lower levels than females perhaps reflecting slower elimination (19). Not only is the liver the most sensitive organ in rats, but in rodent studies of PFOA administration tumors have only been observed at PFOA dosages that result in overt hepatic damage including changes in ALT and AST. Therefore the potential for adverse changes in biomarkers of liver damage and any association between higher serum [PFOA] and past diagnosis or treatment for liver disease in our study participants, were of particular interest. We observed no significant positive associations between any of the studied biomarkers of potential liver toxicity (serum albumin, total bilirubin, alkaline phosphatase, ALT, AST or GGT) with serum [PFOA], and no significant elevation of serum [PFOA] in those with abnormal values for any of these biomarkers. Additionally, [PFOA] was not significantly elevated in those with a history of diagnosis or treatment of liver disease compared with those with no history of liver disease. We concluded that PFOA was not associated with demonstrable manifestations of increased liver disease in this population.
We did not evaluate cancer outcomes and therefore can make no firm conclusions with regard to potential carcinogenic outcomes in the study population. Nevertheless, since in rodents cancer from PFOA is always accompanied by evidence of frank liver damage, our failure to find evidence of liver damage could equate to a low-likelihood of cancer induction through non-genotoxic mechanisms.
We did not find evidence for an association of either BUN or serum creatinine and [PFOA] in the study population.
Reduced levels of thyroid hormones have been observed both in monkeys fed PFOA (25) and in occupationally exposed groups as part of their medical surveillance (39). We observed neither an association of serum [PFOA] with the levels of serum TSH nor an increased serum [PFOA] in those with a history of thyroid disease compared with those without such disease. A history of thyroid disease was quite prevalent in this population, being reported by 11% of participants, but we detected no contribution to this burden from PFOA exposure.
PFOA administration to monkeys is associated with bone marrow hypocellularity and there is evidence of immunotoxicity in both rats (21,22,23) and monkeys (19) fed PFOA. We did not observe changes in blood elements or in the differential white cell count associated with serum [PFOA]. We consider that the isolated weak but statistically significant positive association of absolute monocyte counts and serum [PFOA] may have been a chance finding. This association has not been previously noted in published studies of those working with PFOA, and we observed no corresponding association between the percentage monocyte count and serum [PFOA].
Developmental and reproductive studies in rats fed PFOA have not demonstrated developmental defects in offspring (49) despite observed toxicity in parents. However rat pup body weight was significantly reduced during lactation from PFOA treated mothers. A two generation reproductive study in rats found a slight but statistically significant: decrease in the lactation index for F1 male pups, increases in post lactation deaths in F1 females, delays in sexual maturation in F1 females, increase in estrous cycles in F1 females and delay in sexual maturation in F1 males, which could have resulted from compromised nutritional status (50). Thus, further investigation into potential reproductive and developmental effects of PFOA in humans is necessary.
We consider that our results would reflect the effects, if any, of long-term exposure to PFOA in this community setting. The plant considered the source of PFOA has been in operation since 1948, and has been involved in fluoropolymer production using PFOA since 1951. Contamination of the community water supply by PFOA was detected around 1984, although results were not publicly available until much later. PFOA levels in Little Hocking system water have been measured regularly for the past three years; there have been variations, but the levels have remained within a general range. Our study inclusion criteria included residence in the water system distribution area for at least two years prior to data collection. Thus all participants would be expected to have had exposure to PFOA over a minimum period of two years.
The population we studied had serum [PFOA] very far above the mean [PFOA] observed to date in samples from the general US population. Our failure to find an association between PFOA and the variables we studied makes it highly unlikely that these variables would be affected by the [PFOA] levels currently found in the general US population. Our study did not address the possibility that PFOA might be contributing to other effects. Based on the findings in experimental animals, other end-points, particularly cancer, reproductive and childhood development, require further study.
Table 3.
Hematologic Variables in Study Population and Association with Serum [PFOA]
| Hematologic Parameter |
Mean | Std Dev | Median | IQR | slope estimate |
r | p value* | |
|---|---|---|---|---|---|---|---|---|
| White Blood Cell Ct | 6.89 | 1.79 | 6.8 | 5.5 | 7.9 | 0.00039608 | 0.09 | 0.08 |
| Red Blood Cell Ct | 4.55 | 0.41 | 4.5 | 4.3 | 4.8 | 0.00004031 | 0.04 | 0.44 |
| Hemoglobin | 13.95 | 1.27 | 13.9 | 13.0 | 15.0 | 0.00017275 | 0.06 | 0.29 |
| Hematocrit | 40.80 | 3.58 | 40.7 | 38.2 | 43.6 | 0.00039606 | 0.04 | 0.39 |
| MCV | 89.77 | 4.74 | 90.0 | 87.0 | 92.0 | 0.00020047 | 0.02 | 0.74 |
| MCH | 30.72 | 1.74 | 30.8 | 29.7 | 31.9 | 0.00014134 | 0.03 | 0.53 |
| MCHC | 34.21 | 0.67 | 34.3 | 33.8 | 34.7 | 0.00010286 | 0.06 | 0.23 |
| RDW | 13.38 | 1.09 | 13.2 | 12.6 | 13.9 | −0.0001558 | 0.06 | 0.26 |
| Platelets | 256.80 | 60.61 | 248.0 | 218.0 | 285.0 | 0.00827 | 0.05 | 0.30 |
| Neutrophils (%) | 59.24 | 9.13 | 59.0 | 54.0 | 65.0 | 0.0004305 | 0.02 | 0.71 |
| Lymphocytes (%) | 30.98 | 8.20 | 31.0 | 25.0 | 36.0 | --0.0006401 | 0.03 | 0.54 |
| Monocytes (%) | 6.39 | 2.36 | 6.0 | 5.0 | 8.0 | 0.00023119 | 0.04 | 0.44 |
| Eosinophils (%) | 3.01 | 2.20 | 2.0 | 2.0 | 4.0 | −0.0000652 | 0.01 | 0.82 |
| Basophils (%) | 0.33 | 0.48 | 0.0 | 0.0 | 1.0 | 0.00003319 | 0.03 | 0.59 |
| Neutrophils (Absolute) | 4.14 | 1.45 | 3.8 | 3.1 | 5.0 | 0.00025301 | 0.07 | 0.17 |
| Lymphocytes (Absolute) | 2.10 | 0.70 | 2.0 | 1.6 | 2.5 | 0.00009406 | 0.05 | 0.29 |
| Monocytes (Absolute) | 0.42 | 0.16 | 0.4 | 0.3 | 0.5 | 0.00005008 | 0.13 | 0.01 |
| Eos (Absolute) | 0.21 | 0.16 | 0.2 | 0.1 | 0.2 | 0.00000252 | 0.00 | 0.90 |
| Basos (Absolute) | 0.03 | 0.04 | 0.0 | 0.0 | 0.1 | 0.00000586 | 0.05 | 0.30 |
Tests slope = 0
N = 371
Acknowledgments
Sources of Support:
This study was supported by grant ES12591 from the Environmental Justice Program and by grant number 1 P30 ES013508-01A1 from the National Institute of Environmental Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
REFERENCES
- 1.Kennedy GL, Butenhoff JL, Olsen GW, et al. The toxicology of perfluorooctanoate. Crit Rev Tox. 2004;34:351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- 2.Powley CR, Michalcyzyk MJ, Kaiser MA, et al. Determination of perfluorooctanoic acid (PFOA) extractable from the surface of commercial cookware under simulated cooking conditions by LC/MS/MS. Analyst. 2005;130:1299–1302. doi: 10.1039/b505377c. [DOI] [PubMed] [Google Scholar]
- 3.Gribble GW. Naturally occurring organofluorines. In: Neilson AH, editor. The Handbook of Environmental Chemistry. Volume 3N. Berlin: Springer-Verlag; 2002. pp. 121–136. [Google Scholar]
- 4.Kannan K, Koistinen J, Beckman K, et al. Accumulation of perfluorooctane sulfonate in marine mammals. Environ Sci Technol. 2001;35:1593–1598. doi: 10.1021/es001873w. [DOI] [PubMed] [Google Scholar]
- 5.Olsen GW, Church TR, Miller JP, et al. Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors. Environ Health Perspect. 2003;111:1892–1901. doi: 10.1289/ehp.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsen GW, Church TR, Hausen KJ, et al. Quantitative evaluation of perfluorooctanesulfonate (PFOS) and other fluorochemicals in the serum of children. J Children’s Health. 2004;2:53–76. [Google Scholar]
- 7.Olsen GW, Church TR, Larson EB, et al. Serum concentration of perfluorooctanesulfonate and other fluorochemicals in an elderly population from Seattle, Washington. Chemosphere. 2004;54:1159–1611. doi: 10.1016/j.chemosphere.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Kuto N, Kawashima Y. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J Toxicol Sci. 2003;28:49–57. doi: 10.2131/jts.28.49. [DOI] [PubMed] [Google Scholar]
- 9.Ophaug RH, Singer L. Metabolic handling of perfluorooctanoic acid in rats. Proc Soc Exp Biol Med. 1980;163:19–23. doi: 10.3181/00379727-163-40715. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy GL, Hall GT, Britelli MR, et al. Inhalation toxicity of ammonium perfluorooctanoate. Food Chem Toxicol. 1986;24:1325–1329. doi: 10.1016/0278-6915(86)90066-9. [DOI] [PubMed] [Google Scholar]
- 11.Vanden Heuvel JP, Kuslikis BI, Peterson RE. Covalent binding of perfluorinated fatty acids to proteins in the plasma, liver and tests of rats. Chem Biol Interact. 1991;82:317–328. doi: 10.1016/0009-2797(92)90003-4. [DOI] [PubMed] [Google Scholar]
- 12.Vanden Heuvel JP, Kuslikis BI, Van Rafelghem ML, et al. Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J Biochem Toxicol. 1991;6:83–92. doi: 10.1002/jbt.2570060202. [DOI] [PubMed] [Google Scholar]
- 13.Ylinen M, Kojo A, Hanhijdrvi H, et al. Disposition of perfluorooctanoic acid in the rat after single and subchronic administration. Bull Environ Contam Toxicol. 1990;44:46–53. doi: 10.1007/BF01702360. [DOI] [PubMed] [Google Scholar]
- 14.Vanden Heuvel JP, Davis JW, Sommers R, et al. Renal excretion of perfluorooctanoic acid in male rats: Inhibitory effect of testosterone. J Biochem Toxicol. 1992;7:31–36. doi: 10.1002/jbt.2570070107. [DOI] [PubMed] [Google Scholar]
- 15.Kudo N, Katakura M, Sato Y. Sex hormone regulated renal transport of perfluorooctanoic acid. Chem Bio Interactions. 2002;139:301–316. doi: 10.1016/s0009-2797(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 16.Johnson JD, Gibson SJ, Ober RE. Cholestyramine-enhanced fecal elimination of carbon-14 in rats after administration of ammonium [14C] perfluorooctanoate or potassium [14C] perfluorooctanesulfonate. Fund Appl Toxicol. 1984;4:972–976. doi: 10.1016/0272-0590(84)90235-5. [DOI] [PubMed] [Google Scholar]
- 17.Ylinen M, Hanhijarvi H, Jaakonaho I, et al. Stimulation by estradiol of the urinary excretion of perfluorooctanoic acid in the male rat. Pharmacol Toxicol. 1989;65:274–277. doi: 10.1111/j.1600-0773.1989.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy GL. Dermal toxicity of ammonium perfluorooctanoate. Toxicol Appl Pharmacol. 1985;81:348–355. doi: 10.1016/0041-008x(85)90172-3. [DOI] [PubMed] [Google Scholar]
- 19.Griffith FD, Long JE. Animal toxicity studies with ammonium perfluorooctanoate. Am Ind Hyg Assoc J. 1980;41(8):576–583. doi: 10.1080/15298668091425301. [DOI] [PubMed] [Google Scholar]
- 20.Badr MZ, Birnbaum LS. Enhanced potential for oxidative stress in livers of senescent rats by the peroxisome proliferalpha agonist perfluorooctanoic acid. Mech Aging and Devel. 2004;125:69–75. doi: 10.1016/j.mad.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Yang Q, Xie Y, DePierre JW. Effects of peroxisome proliferators on the thymus and spleen of mice. Clin Exp Immunol. 2000;122:219–226. doi: 10.1046/j.1365-2249.2000.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Q, Xie Y, Alexson, et al. Involvement of peroxisomal-poliferator-activate receptor alpha in the immunomodulation caused by peroxisome proliferators in mice. Biochem Pharmacol. 2002;63:1893–1900. doi: 10.1016/s0006-2952(02)00923-1. [DOI] [PubMed] [Google Scholar]
- 23.Taylor BK, Kreidt C, Nagalingam S. Central administration of perfluorooctanoic acid inhibits cutaneous inflammation. Inflammation Research. 2005;54:235–242. doi: 10.1007/s00011-005-1350-0. [DOI] [PubMed] [Google Scholar]
- 24.Cook JC, Murray SM, Frame SR, et al. Induction of Leydig cell adenomas by ammonium perfluorooctanate: a possible endocrine related mechanism. Toxicol Appl Pharmacol. 1992;113:209–217. doi: 10.1016/0041-008x(92)90116-a. [DOI] [PubMed] [Google Scholar]
- 25.Butentoff J, Costa G, Elcombe C, et al. Toxicity of ammonium perfluorooctanoate (APFO) in male cynomologus monkeys after oral dosing for six months. Toxicol Sci. 2002;69:244–257. doi: 10.1093/toxsci/69.1.244. [DOI] [PubMed] [Google Scholar]
- 26.Riker. Riker laboratories Inc. Experiment No 0281CR0012. Washington DC: 1987. Aug 29, Two year oral (diet) toxicity/carcinogenicity study of fluorochemical FC-143 in rats. U.S. EPA Public Docket AR-226-0437. [Google Scholar]
- 27.Biegel LB, Hurtt ME, Frame SR, et al. Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicolog Sci. 2001;60:44–45. doi: 10.1093/toxsci/60.1.44. [DOI] [PubMed] [Google Scholar]
- 28.Abdellatif AG, Preat V, Taper HS, et al. The modulation of rat liver carcinogenesis by perfluorooctanoic acid, a peroxisome proliferator. Toxicol Appl Pharmacol. 1991;111:530–537. doi: 10.1016/0041-008x(91)90257-f. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson R, Beije B, Preat V, et al. On the mechanism of the hepatocarcinogenicity of peroxisomal proliferators. Chem Biol Interact. 1991;78:235–250. doi: 10.1016/0009-2797(91)90017-2. [DOI] [PubMed] [Google Scholar]
- 30.Abdellatif A, Al-Tonsy AH, Awad ME, et al. Peroxisomal enzymes and 8-hydroxyguanosine in rat liver treated with perfluorooctanoic acid. Disease Markers. 2003;19:19–25. doi: 10.1155/2003/135859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.IARC. International Agency for Research on Cancer. Lyon, France: 1995. Peroxisome Proliferation and its Role in Carcinogenesis. IARC Technical Report No. 24. [Google Scholar]
- 32.Clegg ED, Cook JC, Chapin RE, et al. Leydig cell hyperplasia and adenoma formation: mechanisms and relevance to humans. Reproduct Toxicol. 1997;11:107–121. doi: 10.1016/s0890-6238(96)00203-1. [DOI] [PubMed] [Google Scholar]
- 33.Yao X, Zhong L. Genotoxic risk and additive DNA damage in hepG2 cells exposed to perfluorooctanoic acid. Mutation Res. 2005;587:38–44. doi: 10.1016/j.mrgentox.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Johnson JD. Final Report. Analytical intravenous pharmacokinetic study of T-6067 in rabbits. Study No. AMDT-120694. 3M Environmental Toxicology Services. St Paul Minnesota: 1995
- 35.Butenhoff JL, Kennedy GL, Jr, Hindliter PM, et al. Pharmakokinetics of perfluorooctanoate in cyanomolgus monkeys. Toxicol Sci. 2004;82:394–406. doi: 10.1093/toxsci/kfh302. [DOI] [PubMed] [Google Scholar]
- 36.Noker P. Southern Research Institute Study ID: 99214. Washington DC: Southern Research Institute; 2003. A pharmacokinetic study of potassium perfluorooctanoate in the cynomologus monkey. U.S. EPA Public Docket AR-226-1228. [Google Scholar]
- 37.Burris JM, Lundberg JK, Olsen GW, et al. 3M Medical Department. Washington DC: 2002. Determination of serum half-lives of several fluorochemicals. Interim Report #2. U.S. EPA Public Docket AR-226-1086. [Google Scholar]
- 38.Harada K, Inoue K, Akiko M, et al. Renal clearance of perfluorooctane sulphonate and perfluorooctanoate in humans and their species specific excretion. Environ Research. 2005;99:253–261. doi: 10.1016/j.envres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Olsen GW, Burris JM, Burlew MM, et al. Epidemiologic assessment of worker serum perfluorosulphoanate (PFOS) and perfluorooctanoate concentrations and medical surveillance examinations. J Occup Env Med. 2003;45:260–270. doi: 10.1097/01.jom.0000052958.59271.10. [DOI] [PubMed] [Google Scholar]
- 40.Gilliland FD. PhD dissertation. University of Minnesota; 1992. Fluorocarbons and human health: studies in an occupational cohort. [Google Scholar]
- 41.Olsen GW, Gilliland FD, Burlew MM, et al. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perflourooctanoic acid. J Occup Environ Med. 1998;40:614–620. doi: 10.1097/00043764-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Gilliland FD, Mandel JS. Serum perfluorooctanoic acid and hepatic enzymes, lipoproteins, cholesterol: a study of occupationally exposed men. Am J Ind Med. 1996;29:560–568. doi: 10.1002/(SICI)1097-0274(199605)29:5<560::AID-AJIM17>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 43.Olsen GW, Burris JM, Burlew MM, et al. Plasma cholecystokinin and hepatic enzymes, cholesterol and lipoproteins in perfluorooctanoate production workers. Drug Chem Toxicol. 2000;23:603–620. doi: 10.1081/dct-100101973. [DOI] [PubMed] [Google Scholar]
- 44.Gilliland FD, Mandel JS. Mortality among employees of a perfluorooctanoic acid production plant. J Occup Med. 1993;35:950–954. doi: 10.1097/00043764-199309000-00020. [DOI] [PubMed] [Google Scholar]
- 45.Alexander BH, Olsen GW, Burris JM, et al. Mortality of employees of a perfluorooctanesulphonyl fluoride manufacturing facility. Occup Environ Med. 2003;60:722–729. doi: 10.1136/oem.60.10.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flaherty JM, Connolly PD, Decker ER, et al. Quantitative determination of perfluorooctanoic acid in serum and plasma by liquid chromatography tandem mass spectroscopy. J Chromatog B. 2005;819:329–338. doi: 10.1016/j.jchromb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 47.USEPA. Washington DC: 2005. Results to date from the PFOA Worker Health Study, January 11. U.S. EPA Public Docket AR-226-1922. [Google Scholar]
- 48.Han X, Snow TA, Kemper RA, et al. Binding of perfluorooctanoic acid to rat and human plasma proteins. Chem Res Toxicol. 2003;16:775–781. doi: 10.1021/tx034005w. [DOI] [PubMed] [Google Scholar]
- 49.Staples RE, Burgess BA, Kerns WD. The embryo-fetal toxicity and teratogenic potential of ammonium perfluorooctanoate (APFO) in the rat. Fund Appl Tox. 1984;4:429–440. doi: 10.1016/0272-0590(84)90200-8. [DOI] [PubMed] [Google Scholar]
- 50.Butentoff JL, Kennedy GL, Frame SR, et al. The reproductive toxicology of ammonium perfluorooctanoate in the rat. Toxicol. 2004;196:95–116. doi: 10.1016/j.tox.2003.11.005. [DOI] [PubMed] [Google Scholar]