Abstract
pH-responsive polymers have been synthesised by grafting l-valine (PV-75), l-leucine (PL-75) and l-phenylalanine (PP-75) onto the pendant carboxylic acid moieties of a pseudo-peptide, poly(l-lysine iso-phthalamide), at a stoichiometric degree of substitution of 75 mol%. The effect of such modification on the pH-, concentration- and time-dependent cell membrane-disruptive activity of the grafted polymers has been investigated using a haemolysis model. At 0.025 mg mL−1, the grafted polymers were almost non-haemolytic at pH 7.4, but mediated considerable membrane lysis after 60 min in the pH range characteristic of early endosomes, which ranked in the order: PP-75 > PL-75 > PV-75 > poly(l-lysine iso-phthalamide). PP-75 was 35-fold more lytic on a molar basis than the membrane-lytic peptide melittin. With increasing concentration, the grafted polymers showed an increased ability to lyse cell membranes and caused noticeable membrane disruption at physiological pH. The mechanism of the polymer-mediated membrane destabilisation has been investigated. The in-vitro cytotoxicity of the grafted polymers has been assessed using a propidium iodide fluorescence assay. It has been demonstrated by confocal microscopy that the grafted polymers can induce a significant release of endocytosed materials into the cytoplasm of HeLa cells, which is a feature critical for drug delivery applications.
Keywords: pH-responsive polymer, Pseudo-peptide, Hydrophobic amino acid, Haemolysis, Cytotoxicity, Drug delivery
1. Introduction
Advances in genomics and proteomics have enabled the development of biomacromolecular drugs (e.g. nucleic acids and proteins) with potential for the treatment of a wide variety of diseases [1]. Amongst other problems, their clinical applications may be impaired by degradation by lysosomal enzymes during uptake into cells [2,3]. In order to achieve efficient intracellular delivery of such drugs, delivery systems are required that facilitate their release into the cytoplasm by destabilising endosomal membranes under mildly acidic conditions (pH 5.0–6.8) [4,5].
Recombinant viruses and fusogenic viral peptides have been used to mediate gene transfection [6–8], but their clinical use is potentially limited by safety issues and difficulties in large-scale production [9,10]. A variety of synthetic polymers have therefore been developed as non-viral vectors. Cationic polyethyleneimine [11], poly(2-(dimethylamino)ethyl methacrylate) [12] and polyamidoamine dendrimers [13] mediate gene delivery through the ‘proton sponge’ effect [14], but suffer from cytotoxicity and relatively low transduction efficiencies [15–17]. Anionic pH-responsive polymers are of interest as drug carriers because they can mimic the structure and pH-dependent membrane-lytic behaviour of endosomolytic viral peptides. The biomimetic polymers containing hydrophobic side chains and ionisable carboxyl groups undergo a change of conformation from extended charged chains to globular hydrophobic structures upon reduction of pH below their pKa ranges [18]. Hydrophobic interactions between the resulting hydrophobic domains and lipid bilayer membranes, and/or hydrogen bonding involving protonated carboxyl groups and lipid phosphodiester groups, lead to increased membrane binding of polymers and subsequent membrane disruption [19–23] through pore formation [24,25] or membrane solubilisation [22,26]. The hydrophobicity of polymers is one of the factors that influence their membrane-disruptive behaviour [23,27], and the balance between their hydrophobic and hydrophilic components is required to be manipulated.
Vinyl poly(α-alkylacrylic acid) polymers can be manipulated by controlling the hydrophobicity of pendant alkyl groups to disrupt lipid bilayer membranes at endosomal pH values, whilst being essentially non-lytic at physiological pH [3,17,28,29]. This has prompted their potential applications in cytoplasmic drug delivery [30–33]. However, vinyl polymers are not biodegradable, thus low molecular weights are required to allow renal excretion [34]. A biodegradable pseudo-peptidic polymer, poly(l-lysine iso-phthalamide), has been developed to overcome this limitation [35–37], but its use is limited by low cell membrane-lytic capacity over a relatively low pH range (4.6–5.0) [38]. This pseudo-peptide contains a hydrophobic backbone and pendant carboxyl groups, without hydrophobic side chains presented in fusogenic peptides and poly(α-alkylacrylic acid)s. Here, pH-responsive polymers have been prepared by grafting poly(l-lysine iso-phthalamide) through amide bonding with three different hydrophobic amino acids: l-valine, l-leucine and l-phenylalanine. The effects of the conjugated hydrophobic side chains on the cell membrane-disruptive activity and in-vitro cytotoxicity of the polymers are discussed in this paper. Their subcellular distributions and abilities to release endocytosed materials into the cytoplasm of mammalian cells are also evaluated.
2. Materials and methods
2.1. Materials
Iso-phthaloyl chloride, potassium carbonate, N,N’-dicyclohexylcarbodiimide (DCC), 4-dimethylaminopyridine (DMAP), N,N-dimethylformamide (DMF), triethylamine, fluorescein 5-isothiocyanate (FITC), calcein, melittin (≥ 85%, HPLC), mPEG-5000 and PEG-10000 were purchased from Sigma-Aldrich (Dorset, UK). l-lysine methyl ester dihydrochloride, disodium hydrogen phosphate, trisodium citrate, dimethyl sulfoxide (DMSO) and d-glucose were obtained from Fisher (Loughborough, UK). l-valine methyl ester hydrochloride, l-leucine methyl ester hydrochloride and l-phenylalanine methyl ester hydrochloride were purchased from Alfa Aesar (Heysham, UK). Sterile defibrinated sheep red blood cells (RBCs) were purchased from TCS Biosciences (Buckingham, UK). RPMI-1640 medium (without l-glutamine), Dulbecco’s modified Eagle medium (DMEM) and propidium iodide were purchased from Invitrogen (Paisley, UK). Foetal bovine serum (FBS), Dulbecco’s phosphate buffered saline (D-PBS), penicillin-streptomycin solution (10,000 units mL−1 penicillin and 10 mg mL−1 streptomycin), l-glutamine solution (200 mM) and trypsin-EDTA solution (0.5 g L−1 porcine trypsin and 0.2 g L−1 EDTA·4Na) were obtained from Sigma-Aldrich (Dorset, UK).
2.2. Polymer preparation and composition determination
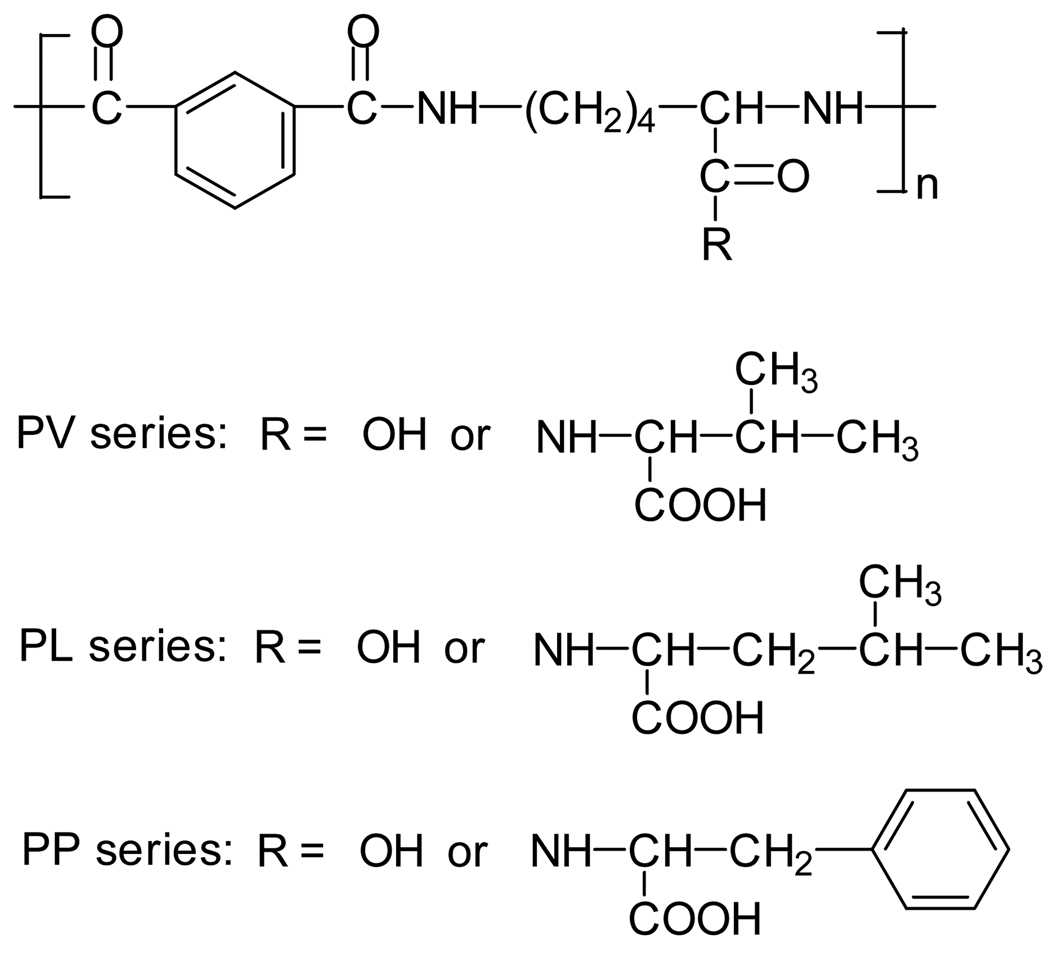
The synthesis of poly(l-lysine iso-phthalamide) has been reported previously [35–37]. l-valine, l-leucine and l-phenylalanine were grafted onto the pendant carboxylic acid moieties along the backbone of poly(l-lysine iso-phthalamide) (designated as PV, PL and PP series polymers respectively) (Fig. 1). Full details are to be published elsewhere, but in summary the grafted polymers were prepared from the coupling reaction between poly(l-lysine iso-phthalamide) and amino acid (l-valine, l-leucine and l-phenylalanine) methyl ester hydrochloride in anhydrous DMSO/DMF by DCC/DMAP mediated amide coupling chemistry [39,40] and subsequent hydrolysis with ethanolic sodium hydroxide. Three grafted polymers PV-75, PL-75 and PP-75 with the same stoichiometric molar percentage (75%) of amino acids relative to the pendant carboxylic acid groups of poly(l-lysine iso-phthalamide) are considered here. The structures of poly(l-lysine iso-phthalamide) and these grafted polymers were confirmed using a Nicolet Nexus FTIR spectrometer (Thermo Fisher Scientific, USA) and a Bruker Advance 500 MHz NMR spectrometer (Bruker Biospin GmbH, Germany). The molecular weight of poly(l-lysine iso-phthalamide) (Mw = 35,700, Mn = 17,900, polydispersity = 1.99) was determined using an aqueous gel permeation chromatography (GPC) system (Viscotek, UK), which was calibrated with polyethylene glycol standards. The degrees of grafting of PV-75, PL-75 and PP-75 were measured from their 1H-NMR spectra, and used to calculate their molecular weights. The compositions of these polymers are shown in Table 1. Fluorophore-labelled polymers (1.0 mol%) were prepared by coupling FITC-NH-(CH2)2-NH2, an amino derivative of FITC from the reaction with ethylene diamine using dibutyltin dilaurate as a catalyst, to the carboxylic acid groups of the polymers using standard DCC/DMAP mediated coupling techniques. The FITC-labelled polymers were purified by dialysis against deionised water and then lyophilised. A low level of labelling was selected here to avoid significant modulation of the polymer properties and fluorescence quenching.
Figure 1.
Repeat unit structures of poly(l-lysine iso-phthalamide)s grafted with l-valine (PV series), l-leucine (PL series) and l-phenylalanine (PP series).
Table 1.
Composition measurements for poly(l-lysine iso-phthalamide)s grafted with l-valine, l-leucine and l-phenylalanine.
| Poly(l-lysine iso-phthalamide) |
PV-75 | PL-75 | PP-75 | |
|---|---|---|---|---|
| [NH2]/[COOH] (%)a | 0 | 75 | 75 | 75 |
| DS (mol%)b | 0 | 62.1 | 61.2 | 63.2 |
| Weight percentage of amino acid grafts (wt%) | 0 | 21.4 | 23.1 | 28.1 |
| Mn (103) c | 17.9 | 22.8 | 23.3 | 24.9 |
Stoichiometric molar percentage of amino acids (l-valine, l-leucine and l-phenylalanine) relative to the carboxylic acid groups along the backbone of poly(l-lysine iso-phthalamide).
Degree of substitution, defined as the number of amino acid grafts per 100 carboxylic acid groups and determined by 1H-NMR in d6-DMSO at room temperature.
Calculated based on the weight percentage of amino acid grafts (wt%) and the Mn of poly(l-lysine iso-phthalamide) determined by GPC.
2.3. Haemolysis assay
Membrane-lytic activity of the polymers was examined by haemolysis assay [38]. Phosphate buffers (100 mM) in the pH range of 5.0–7.4 and citrate buffers (100 mM) in the pH range of 4.0–5.0 were prepared to be isosmotic to the inside of an RBC and caused negligible haemolysis. Polymer solutions were prepared at required concentrations in the buffers at specific pHs and allowed to equilibrate for 48 h before use. Sheep RBCs were washed three times with NaCl aqueous solution (150 mM) and resuspended in the polymer solutions to achieve a final RBC concentration within the range of 1–2 × 108 RBCs mL−1. Two controls were prepared by resuspending RBCs either in buffer alone (negative control) or in deionised water (positive control). Each test was performed in triplicate. The samples were incubated in a waterbath at 37°C for various periods up to 2 h, and then centrifuged at 4000 rpm for 4 min. The absorbance of the supernatant from each sample was measured at 541 nm using a Spectronic UV1 spectrophotometer (Thermo Fisher Scientific, USA), and the percentages of haemolysis were determined. It has been demonstrated that the absorbance of haemoglobin solution was proportional to the number of lysed RBCs up to a concentration of 2.64 × 108 RBCs mL−1 (data not shown).
2.4. Cell culture
HeLa adherent epithelial cells (human cervical cancer cells) were grown in RPMI-1640 medium supplemented with 10% (v/v) FBS, 2 mM l-glutamine, 100 U mL−1 penicillin and 100 µg mL−1 streptomycin unless specified otherwise. The HeLa cells were trypsinised using trypsin-EDTA and maintained in a humidified incubator with 5% CO2 at 37°C.
2.5. Propidium iodide fluorescence assay
The cytotoxic effect of the polymers was evaluated using a propidium iodide fluorescence assay [41]. 0.5 mL of HeLa cells in DMEM supplemented with 10% (v/v) FBS, 100 U mL−1 penicillin and 100 µg mL−1 streptomycin (6 × 104 cells mL−1) were seeded in each well of a 12-well plate (Corning, USA). After incubation for 24 h in an incubator at 37°C, the spent medium was replaced with 0.5 mL of fresh serum free DMEM containing 0.22 µm filter-sterilised polymer solution at the described concentration. These solutions were diluted from a stock polymer solution in D-PBS (20 mg mL−1). After 48 h, the polymer containing medium was removed, and the cells were rinsed with D-PBS. Subsequently, trypsin-EDTA was added at 0.5 mL per well to detach cells from the growth surface followed by dilution with 0.5 mL of complete DMEM, and the cells were centrifuged to pellet. Supernatant was aspirated and the cells were resuspended in 0.5 mL of D-PBS containing 1.0 µg mL−1 propidium iodide. The stained cells were incubated on ice for 30 min, and then analysed with a Vantage SE flow cytometer and CellQuest software (BD Biosciences, USA). Each test was performed in triplicate.
2.6. Subcelluar localisation of FITC-labelled polymers
2 mL of HeLa cells (1–2 × 105 cells mL−1) were seeded into a 35 mm glass-bottom culture dish (MatTek, USA) and cultured for 24 h in an incubator at 37°C. The spent medium was removed and replaced with 2 mL of serum and phenol red free medium containing 0.22 µm filter-sterilised FITC-labelled polymer solution at the described concentration. The polymer containing medium was removed after 30 min of incubation. The dish was washed three times with D-PBS, and replenished with serum and phenol red free medium. The cells were then returned to the incubator for another 3.5 h. The images of the cells were obtained within 15 min using a FluoView™ FV300 inverted laser scanning confocal microscope (Olympus, Japan). The excitation and emission wavelengths were 480 and 530 nm respectively.
2.7. Release of calcein from intracellular vesicles
Calcein (a membrane-impermeable fluorophore) was used as a tracer molecule to monitor the effect of the polymers on internalised vesicles. 2 mL of HeLa cells (1–2 × 105 cells mL−1) were cultured for 24 h in a 35 mm glass-bottom culture dish (MatTek, USA), followed by the treatment with 2 mL of serum and phenol red free medium containing 0.22 µm filter-sterilised 2.0 mg mL−1 calcein in the absence or presence of the polymer (these solutions were titrated to pH 7.4 using 0.1 M NaOH). After being incubated for 30 min at 37°C, the cells were washed three times with D-PBS and then incubated with serum and phenol red free medium for 3.5 h at 37°C. The distribution of calcein within the cells was examined within 15 min using a FluoView™ FV300 inverted laser scanning confocal microscope (Olympus, Japan). The excitation and emission wavelengths were 488 and 535 nm respectively.
3. Results and discussion
3.1. Cell membrane disruption
3.1.1. pH-dependent haemolysis
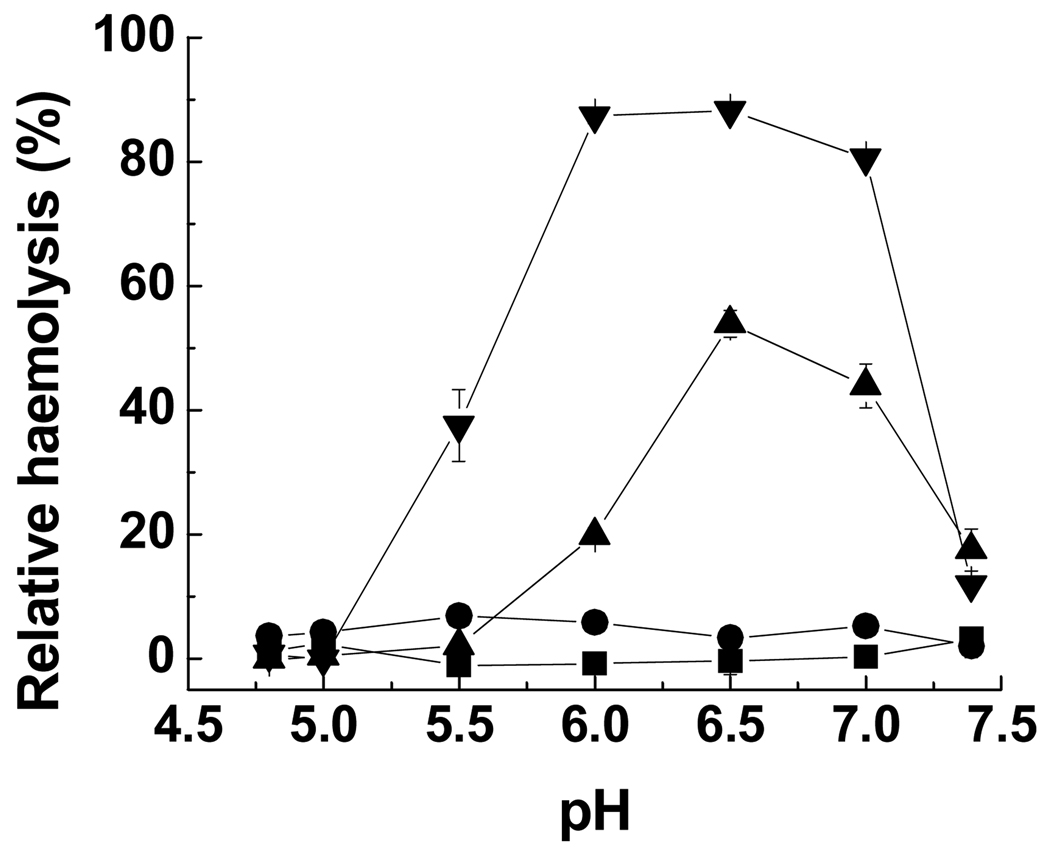
The effect of grafting poly(l-lysine iso-phthalamide) with hydrophobic amino acids l-valine, l-leucine and l-phenylalanine on the abilities of the grafted polymers to disrupt lipid bilayer membranes was investigated using a haemolysis model. RBC membranes have been selected as a model for endosomal membranes [8,17,38]. The relative haemolytic activity of poly(l-lysine iso-phthalamide), PV-75, PL-75 and PP-75 was examined as a function of pH at the low concentration of 0.025 mg mL−1 (Fig. 2). The pH range of 7.4–4.6 mimics the environment encountered during intracellular trafficking through early endosomes, late endosomes and lysosomes [4,5]. As shown in Fig. 2, in the presence of poly(l-lysine iso-phthalamide) no haemolytic activity was detected within the pH range studied. PV-75 showed a marginal increase in haemolysis compared to the unmodified polymer. PL-75 and PP-75 were almost non-haemolytic at pH 7.4, but their haemolytic activity increased considerably with decreasing pH, reaching maximum degrees of haemolysis of 54 and 90% respectively at pH 6.5, within the pH range characteristic of early endosomes (pH 6.0–6.8) [4,5].
Figure 2.
Relative haemolysis of sheep RBCs as a function of pH in the presence of poly(l-lysine iso-phthalamide) (■), PV-75 (●), PL-75 (▲) and PP-75 (▼) at 0.025 mg mL−1. Error bars represent standard deviations of three samples.
In the early endosomal pH range the ranking of haemolytic activity amongst the polymers was poly(l-lysine iso-phthalamide) < PV-75 < PL-75 < PP-75. This ranking correlates with the relative hydrophobicity and structures of l-valine, l-leucine and l-phenylalanine grafts. Normalised to a scale of 0 for glycine and 100 for the most hydrophobic amino acid among the 20 natural amino acids, the hydrophobicity indices of valine, leucine and phenylalanine at pH 7.0 are 76, 97 and 100 respectively [42], which are close to those at acidic pH 2.0 (79, 100 and 92 respectively) [43]. This supports the observation that upon reduction of pH PL-75 and PP-75 display significant hydrophobic association and form more hydrophobic and compact structures than the parent polymer and PV-75 (data not shown). PL-75 and PP-75 with higher hydrophobicity could cause enhanced polymer-membrane interactions and subsequent higher levels of membrane disruption. This is consistent with the report that increased peptide hydrophobicity results in stronger haemolytic activity [44]. Also, others have shown that the membrane-lytic activity of poly(α-alkylacrylic acid)s can be modulated by changing the alkyl chain length of monomer units [45]. With an additional methylene unit, poly(α-propylacrylic acid) was 15-fold more lytic than poly(α-ethylacrylic acid) on a molar basis [3,17]. PP-75 was more haemolytic than PL-75 although l-phenylalanine has a similar hydrophobicity index to l-leucine, which might reflect enhanced hydrophobic association resulting from π-π interactions between aromatic rings on the polymer backbone and those on the l-phenylalanine grafts, and between l-phenylalanine moieties. This is in agreement with the report that the aromatic residues in peptide structures can cause a dramatic increase in membrane-disruptive ability [8].
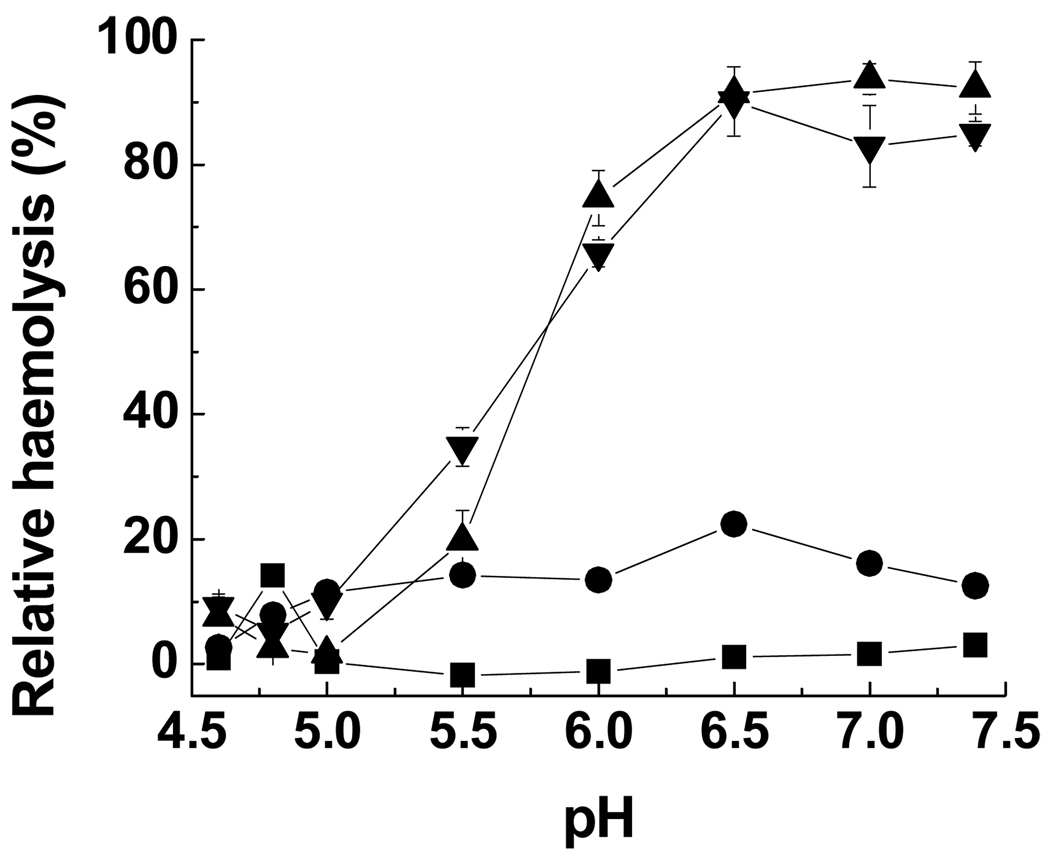
As shown in Fig. 3, at 1.0 mg mL−1, PV-75 showed slightly increased haemolytic activity (23%) compared to the parent poly(l-lysine iso-phthalamide) (14%), with a concomitant marked increase in the pH corresponding to the maximum degree of haemolysis from 4.8 to 6.5 due to grafting with hydrophobic l-valine. PP-75 and PL-75 had a similar haemolytic profile, displaying maximum degrees of haemolysis (83–94%) over the pH range of 6.5–7.4. It has been similarly reported by Thomas et al. [22] that the higher concentration of poly(α-ethylacrylic acid) causes disruption of lipid bilayer membranes at a higher pH and sufficiently high polymer concentrations lead to complete membrane solubilisation at physiological pH. With further acidification, a sharp decrease in haemolytic activity occurred due to precipitation of PL-75 and PP-75. A similar effect has been noted for the haemolytic activity of poly(α-propylacrylic acid) which decreased at pH 5.0–5.4 [45].
Figure 3.
Relative haemolysis of sheep RBCs as a function of pH in the presence of poly(l-lysine iso-phthalamide) (■), PV-75 (●), PL-75 (▲) and PP-75 (▼) at 1.0 mg mL−1. Error bars represent standard deviations of three samples.
3.1.2. Concentration-dependent haemolysis
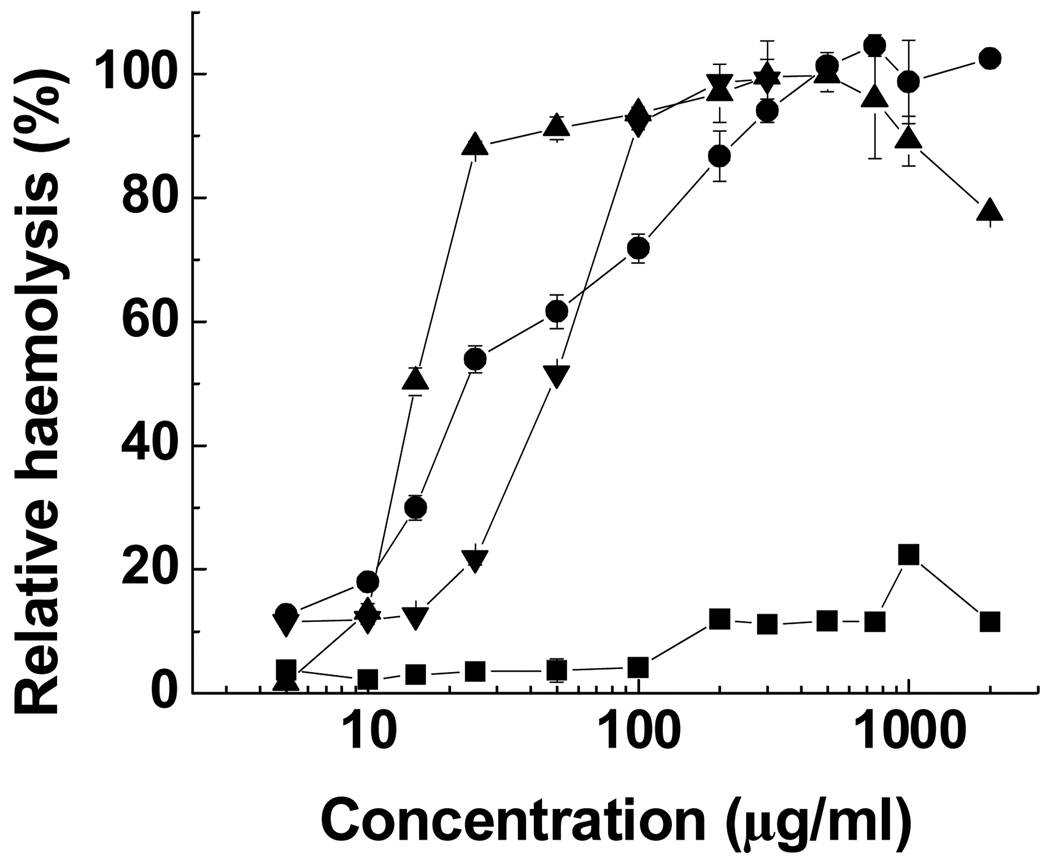
The relative haemolytic activity of PV-75, PL-75 and PP-75 at pH 6.5 (a very early stage of endosomal acidification) was determined as a function of concentration in comparison to melittin, a highly membrane-lytic bee venom peptide [46]. As shown in Fig. 4, PV-75 displayed low levels of haemolysis throughout the concentration range tested, with a maximum of 23% at 1.0 mg mL−1. The haemolytic activity of PP-75, PL-75 and melittin was strongly influenced by concentration. PP-75 had no haemolytic effect at 5 µg mL−1, but exhibited a rapid increase in the degree of haemolysis to 90% at 25 µg mL−1. Complete disruption of erythrocyte membranes was achieved gradually by PP-75 with further increasing concentration to 200–750 µg mL−1. PL-75 and melittin caused a low level of haemolysis of 12% at 5 µg mL−1, and reached the degree of haemolysis of 90% at about 250 and 100 µg mL−1 respectively. Comparatively, PP-75 was 11-fold more efficient than PL-75 and 35-fold more efficient than melittin at membrane disruption on a molar basis. Thomas et al. [22] has reported a similar observation for the membrane-lytic activity of poly(α-ethylacrylic acid) and argued that increasing polymer concentration can enhance the driving force for migration of polymer molecules to lipid bilayer membranes, resulting in an increase in membrane destabilisation.
Figure 4.
Concentration-dependent relative haemolysis of sheep RBCs by PV-75 (■), PL-75 (●), PP-75 (▲) and melittin (▼) at pH 6.5. Error bars represent standard deviations of three samples.
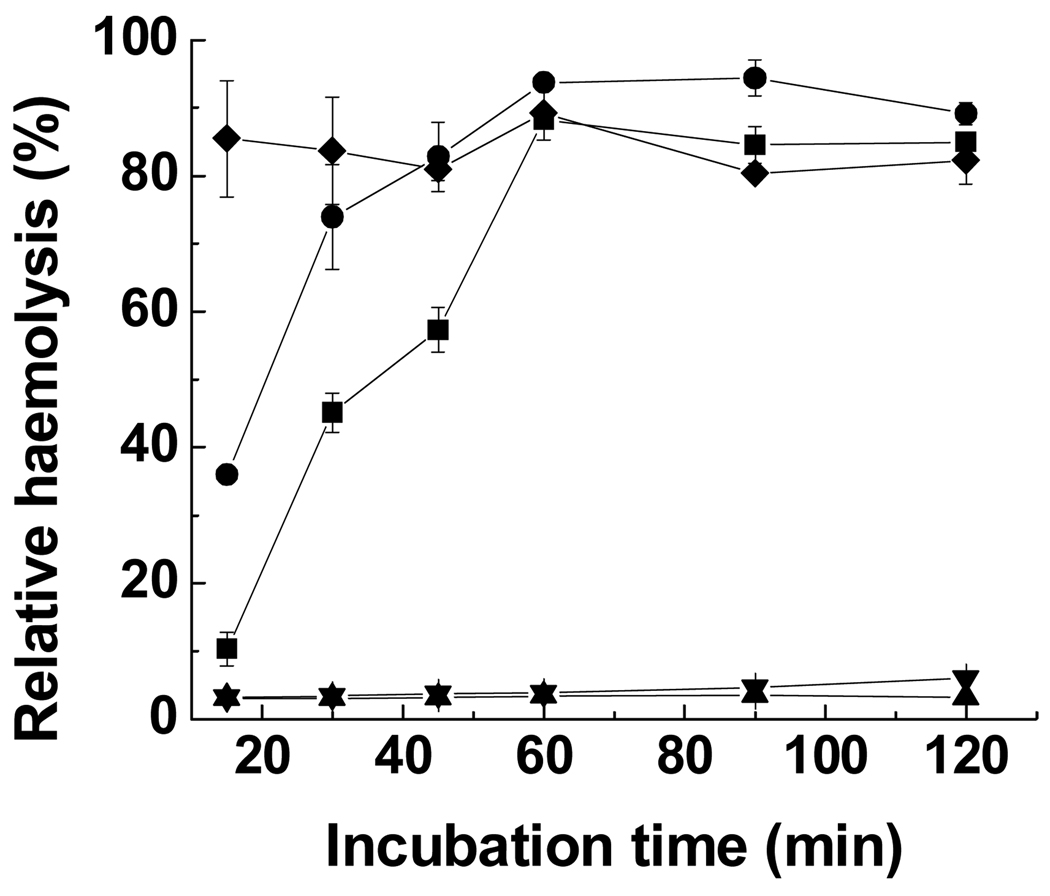
3.1.3. Time-dependent haemolysis
The kinetics of haemolytic activity of poly(l-lysine iso-phthalamide) and PP-75 were determined at pH 6.5. As seen in Fig. 5, only a low level of haemolysis (7%) was detected after incubation of RBCs with 1.0 mg mL−1 poly(l-lysine iso-phthalamide) for 120 min. The hydrophobically modified polymer PP-75 displayed not only greater membrane disruption but also faster kinetics of haemolytic activity. At 0.025 mg mL−1, PP-75 showed haemolysis of 11% after 15 min, and then its haemolytic activity increased significantly to a maximum of 90% after 60 min. With increasing concentration to 0.1 mg mL−1, PP-75 haemolysed 36% sheep RBCs after 15 min, followed by an increase to a maximum degree of haemolysis of 94% after 60 min. The presence of PP-75 at 1.0 mg mL−1 resulted in a high level of haemolysis of 86% after 15 min and almost no further haemolysis occurred thereafter. It has been noted similarly by Jones et al. [45] that poly(α-propylacrylic acid) showed faster and enhanced disruption of vesicular membranes than poly(α-methylacrylic acid) due to a higher degree of hydrophobicity. Endocytosed macromolecules have been reported to be trafficked from early endosomes to lysosomes within several hours [14]. The significantly improved kinetics of haemolytic activity of PP-75 enables the polymer to disrupt endosomal membranes before the vesicular evolution from endsomes to lysosomes, a feature critical for potential drug delivery applications.
Figure 5.
Relative haemolysis of sheep RBCs at pH 6.5 as a function of incubation time in the absence of polymer (▲) and in the presence of poly(l-lysine iso-phthalamide) (▼) at 1.0 mg mL−1 and PP-75 at 0.025 (■), 0.1 (●) and 1.0 mg mL−1 (♦). Error bars represent standard deviations of three samples.
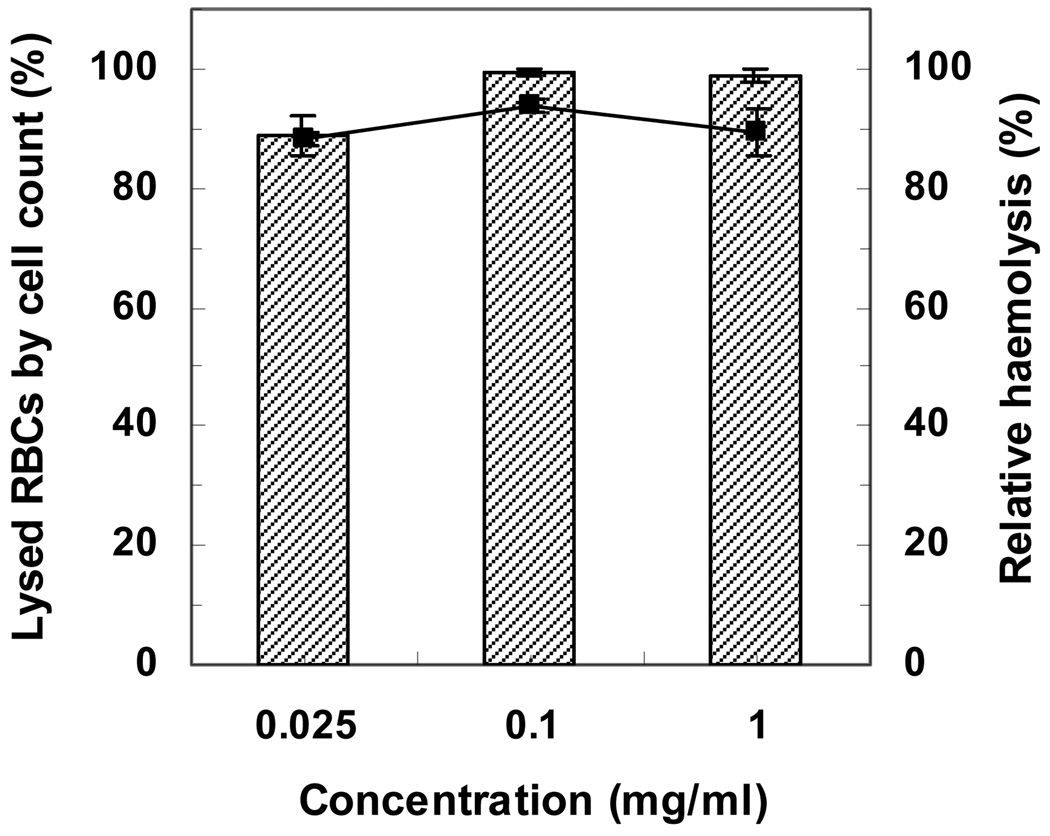
3.1.4. Mechanism of cell membrane disruption
The sheep RBC count was performed using a haemocytometer to investigate the mechanism of haemolysis by the hydrophobically modified polymers. The percentages of lysed RBCs by PP-75 (0.025, 0.1 and 1.0 mg mL−1) relative to the total RBC number in buffer alone were determined after 1 h of incubation at pH 6.5. As seen in Fig. 6, the percentages of lysed RBCs are consistent with the haemolysis results, indicating that haemoglobin was released into the buffer after rupture of cell membranes by PP-75. This is different from the mechanism of haemolysis by poly(l-lysine iso-phthalamide) grafted with 17.4 wt% hydrophilic methoxy polyethylene glycol (mPEG), which could release haemoglobin into the extracelluar medium without lysing membranes [47].
Figure 6.
Comparison between the percentage of lysed RBCs (column) and relative haemolysis (line) by PP-75 at 0.025, 0.1 and 1.0 mg mL−1. The sheep RBC count by a haemocytometer and haemolysis assay were performed following 1 h of incubation at pH 6.5 in a 37°C waterbath. Error bar represent standard deviations of three samples.
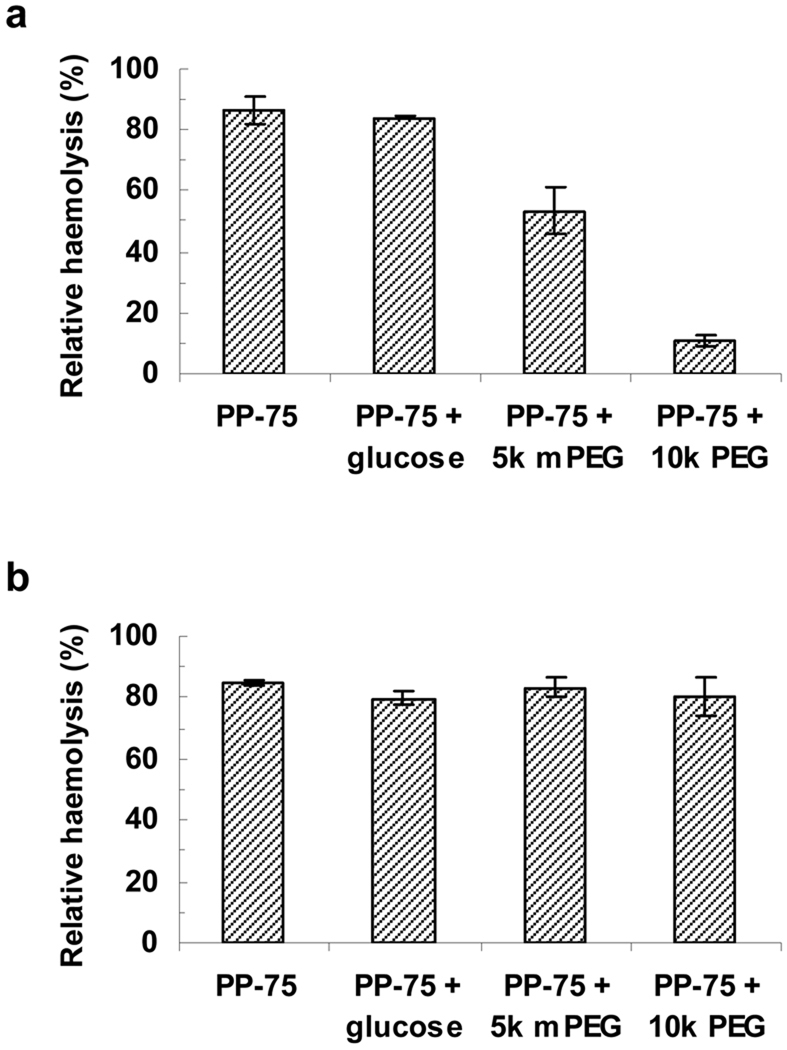
Further insight into the mechanism of cell membrane disruption was obtained by examining the effect of osmolytes with different molecular weights on the haemolytic activity of PP-75 (0.025 and 1.0 mg mL−1) at pH 6.5 (Fig. 7). d-glucose (180 Da), mPEG-5000 (5k Da) and PEG-10000 (10k Da) were added separately to a buffered suspension of RBC at the concentration of 10 mM, below the range at which these solutes alone could induce haemolysis due to osmotic pressures. As shown in Fig. 7a, both PEG-10000 and mPEG-5000 inhibited haemolysis by 0.025 mg mL−1 PP-75 substantially, indicating that defects such as pores or channels could be formed in RBC membranes and haemolysis occurred as a result of osmotic swelling and rupture of the membranes. Low molecular weight solutes in the RBC cytosol can diffuse out of the RBC, whilst haemoglobin is too large to permeate through the pores or channels formed by PP-75. The resulting osmotic imbalance can be balanced by addition of high molecular weight PEG-10000 and mPEG-5000 which are unable to permeate through the pores or channels, leading to a marked decrease in haemolysis. The presence of low molecular weight d-glucose had no effect on the osmotic imbalance caused by the haemoglobin within the RBC cytoplasm, so no inhibition of the haemolytic activity was observed. It has been noted that poly(α-ethylacrylic acid) [24] and GALA (an endosomolytic synthetic peptide) [8] can also create defects such as pores or channels in lipid bilayer membranes and lyse the membranes due to collapse of transmembrane potential and osmotic swelling [6,8,17]. By contrast, d-glucose, mPEG-5000 and PEG-10000 all had no effect on haemolysis by PP-75 at 1.0 mg mL−1 (Fig. 7b), suggesting that membrane lysis occurred in the absence of osmotic cell swelling. It has been reported that poly(α-alkylacrylic acid)s at sufficiently high concentrations can cause complete membrane solubilisation through micellisation due to extensive polymer adsorption [19,21,22].
Figure 7.
The effect of osmolytes on the relative haemolysis of sheep RBCs by PP-75 in PBS buffer at pH 6.5. (a) Relative haemolysis by 0.025 mg mL−1 PP-75 in the absence and presence of 10 mM d-glucose (180 Da), mPEG (5k Da) and PEG-10000 (10k Da); (b) relative haemolysis by 1.0 mg mL−1 PP-75 in the absence and presence of 10 mM d-glucose (180 Da), mPEG (5k Da) and PEG-10000 (10k Da). Error bars represent standard deviations of three samples.
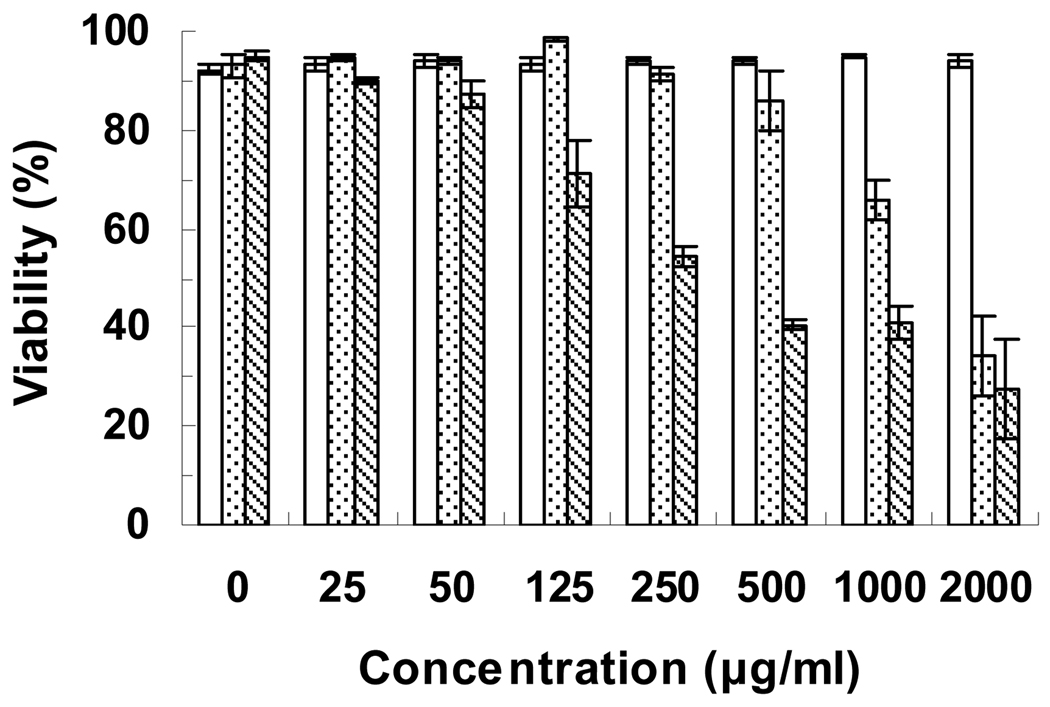
3.2. In-vitro cytotoxicity
The membrane-impermeant dye, propidium iodide, can enter cells with compromised cellular membranes, a late sign of cell death, and binds to DNA by intercalation, resulting in strong red fluorescence [41]. The cytotoxic effect of poly(l-lysine iso-phthalamide), PL-75 and PP-75 towards HeLa cells was evaluated by propidium iodide fluorescence assay. As shown in Fig. 8, HeLa cells well tolerated poly(l-lysine iso-phthalamide), displaying viabilities of ~94% within the polymer concentration range of 0–2000 µg mL−1. PL-75 was cytotoxic at the concentration above 500 µg mL−1, with the IC50 (defined as the concentration at which 50% of cell growth is inhibited relative to non-treated control cells) between 1000 and 2000 µg mL−1. PP-75 almost had no cytotoxic effect at the concentration ≤ 50 µg mL−1. With increasing concentration up to 2000 µg mL−1, PP-75 progressively decreased the viability of HeLa cells to 28%, with the IC50 between 250 and 500 µg mL−1. A similar concentration effect of poly(α-ethylacrylic acid) has shown that the higher concentrations can drive more polymer molecules to the membrane surface regardless of the electrostatic repulsion effect at physiological pH and cause destabilisation of lipid bilayer membranes due to reorgnisation and expansion of the membranes [22]. The ranking of IC50 amongst the polymers was poly(l-lysine iso-phthalamide) > PL-75 > PP-75, indicating that at the same degree of grafting, l-phenylalanine derivatisation results in a more cytotoxic polymer.
Figure 8.
Concentration-dependent viabilities of HeLa cells treated with poly(l-lysine iso-phthalamide) (blank), PL-75 (stippled) and PP-75 (striped) over 48 h as determined by propidium iodide fluorescence assay. Error bars represent standard deviations of three samples.
3.3. Release of endocytosed materials from intracellular vesicles
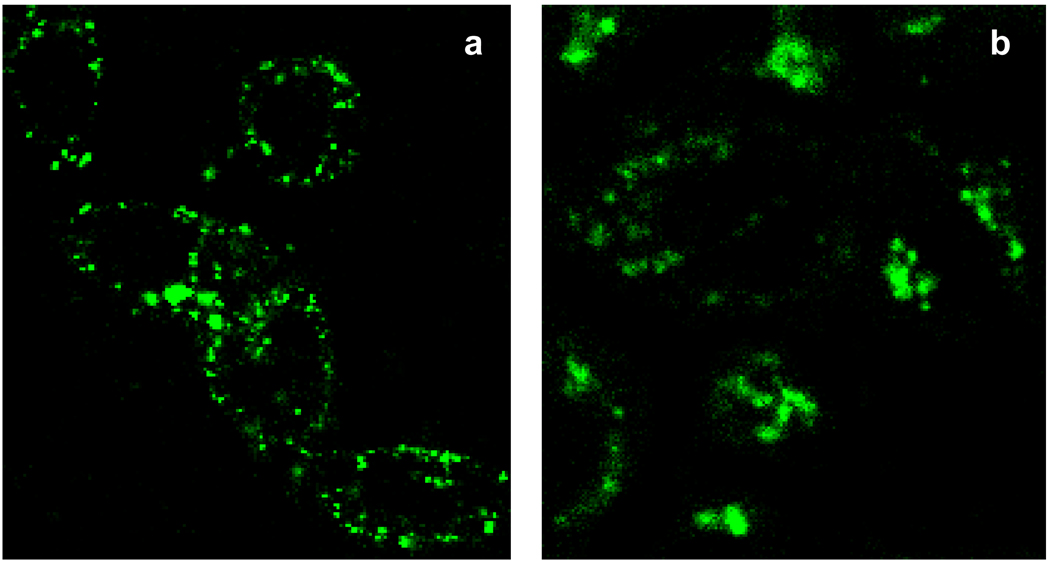
The high efficiency of the hydrophobically modified polymers in disrupting erythrocyte membranes within the pH range characteristic of early endosomes has prompted an evaluation of the ability of PP-75 to release endocytosed materials into the cytoplasm of HeLa cells. Fig. 9 shows the subcellular localisations of the FITC-labelled poly(l-lysine iso-phthalamide) and PP-75 in HeLa cells which were incubated for 3.5 h after 30 min of endocytic uptake of the polymers. When HeLa cells were treated with 1.0 mg mL−1 FITC-labelled poly(l-lysine iso-phthalamide), a large number of punctate spots were observed in the cells together with a very limited amount of diffuse staining (Fig. 9a). This indicates that most of the parent polymer molecules were trapped within intracellular vesicles after being endocytosed [47]. Treatment of HeLa cells with 0.025 mg mL−1 FITC-labelled PP-75 led to a considerable amount of diffuse staining resulting from homogenous dispersion of the polymer in the cytoplasm after disrupting the endosomal/lysosomal membrane (Fig. 9b). Almost no punctate staining is shown in Fig. 9b, suggesting that all the endocytosed materials were released into the cytoplasm by PP-75 at a 40-fold lower concentration than the parent polymer.
Figure 9.
Confocal microscopy images of HeLa cells incubated for 30 min with (a) 1.0 mg mL−1 FITC-labelled poly(l-lysine iso-phthalamide) and (b) 0.025 mg mL−1 FITC-labelled PP-75. Images were acquired with an Olympus FluoView™ FV300 confocal unit (×60 magnification) at 3.5 h after uptake, and show the subcellular distribution of FITC fluorescence.
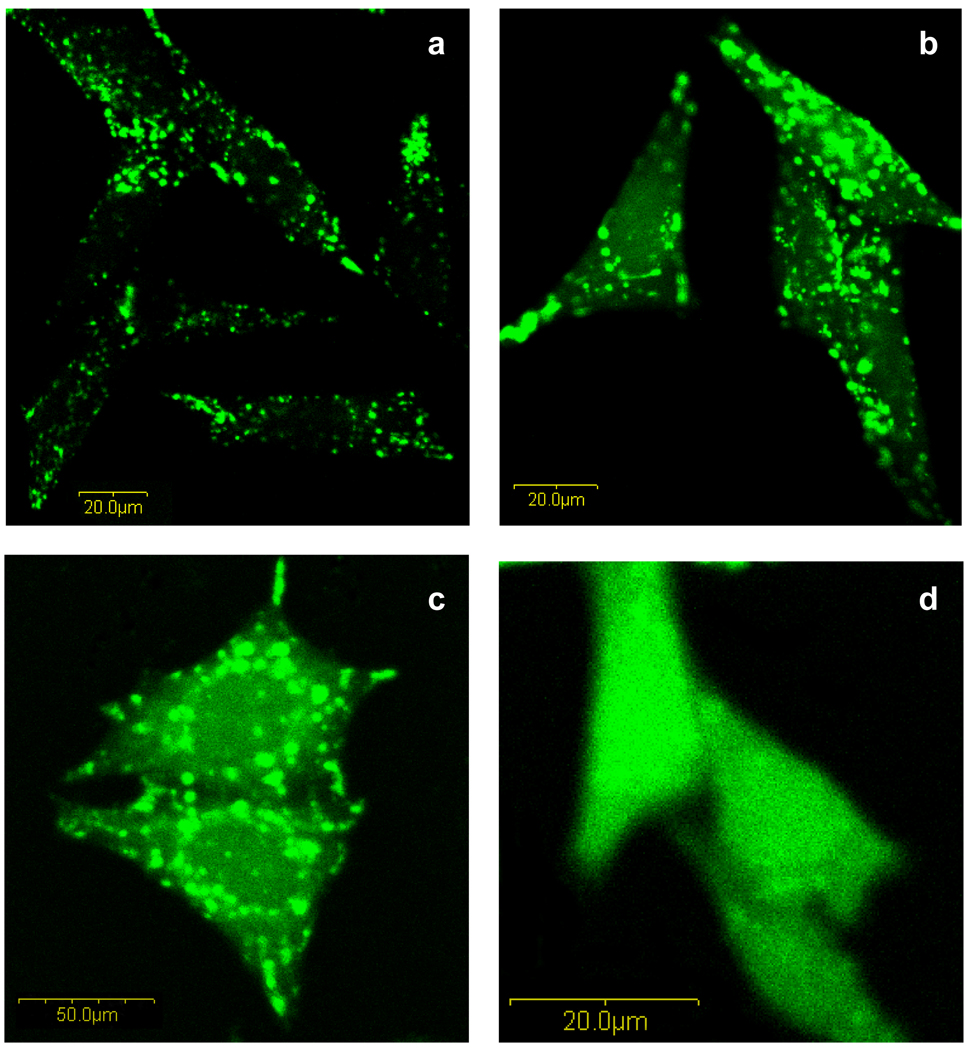
Fig. 10 shows the effect of poly(l-lysine iso-phthalamide) and PP-75 on the release of non-conjugated calcein into the cytoplasm of HeLa cells after 30 min of treatment and 3.5 h of further incubation. As seen in Fig. 10a, in the absence of polymer, the membrane-impermeable fluorophore was restricted within intracellular vesicles appearing as bright punctate structures, consistent with constitutive endocytosis of the external medium. When the cells were treated with 1.0 mg mL−1 poly(l-lysine iso-phthalamide), a small amount of green fluorescence was released into the cytoplasm, whilst a large number of bright intact vesicles still existed (Fig. 10b) [47]. Uptake of 0.025 mg mL−1 PP-75 resulted in a similar calcein-release profile to the parent polymer at 1.0 mg mL−1, but caused stronger diffuse staining (Fig. 10c). As the concentration of PP-75 was increased to 0.1 mg mL−1, uniform fluorescence throughout the cells was observed, with disappearance of bright punctate vesicles (Fig. 10d). The significantly efficient release of endocytosed materials into the cytoplasm through PP-75-mediated vesicular membrane disruption is consistent with the haemolysis results and indicates the potential applications of the hydrophobically modified polymers in cytoplasmic drug delivery.
Figure 10.
Confocal microscopy images of HeLa cells showing the subcellular distribution of calcein fluorescence. The cells were treated with (a) 2.0 mg mL−1 calcein, (b) both 2.0 mg mL−1 calcein and 1.0 mg mL−1 poly(l-lysine iso-phthalamide), (c) both 2.0 mg mL−1 calcein and 0.025 mg mL−1 PP-75, and (d) both 2.0 mg mL−1 calcein and 0.1 mg mL−1 PP-75. Images were acquired with an Olympus FluoView™ FV300 confocal unit (×60 magnification) at 3.5 h after 30 min of uptake.
4. Conclusions
pH-responsive biomimetic polymers have been synthesised by grafting hydrophobic amino acids l-valine, l-leucine and l-phenylalanine onto the pendant carboxylic acid groups of poly(l-lysine iso-phthalamide) to have a range of membrane-lytic profiles. PV-75 (62.1 mol% l-valine) caused only a marginal increase in haemolytic activity compared to the parent poly(l-lysine iso-phthalamide). PL-75 (61.2 mol% l-leucine) showed significantly higher haemolytic efficiency than PV-75. PP-75 (63.2 mol% l-phenylalanine) was 11-fold more efficient than PL-75 and 35-fold more efficient than melittin at membrane disruption on a molar basis. Haemolysis induced by PP-75 and PL-75 was pH-dependent at 0.025 mg mL−1, being non-haemolytic at pH 7.4 but mediating considerable membrane disruption (90%) after 60 min at early endosomal pH by the colloid osmotic mechanism. Sufficiently high concentrations (e.g. 1.0 mg mL−1) led to marked membrane destabilisation at pH 7.4. The grafted polymers were well tolerated by HeLa cells at low concentrations, but displayed increasing cytotoxic effects as the concentration increased. l-phenylalanine derivatisation resulted in a more cytotoxic polymer. The considerably efficient release of endocytosed materials into the cytoplasm of HeLa cells by PP-75 suggests its potential applications in cytoplasmic drug delivery.
Acknowledgements
Rongjun Chen wishes to thank the Gates Cambridge Trust and the Universities UK organisations for the financial support that enabled this work to be carried out. Sariah Khormaee acknowledges the support of the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health and the Marshall Aid Commemoration Commission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dinçer S, Turk M, Piskin E. Intelligent polymers as nonviral vectors. Gene Ther. 2005;12:S139–S145. doi: 10.1038/sj.gt.3302628. [DOI] [PubMed] [Google Scholar]
- 2.Pouton CW, Seymour LW. Key issues in non-viral gene delivery. Adv Drug Deliv Rev. 2001;46:187–203. doi: 10.1016/s0169-409x(00)00133-2. [DOI] [PubMed] [Google Scholar]
- 3.Stayton PS, Hoffman AS, Murthy N, Lackey C, Cheung C, Tan P, et al. Molecular engineering of proteins and polymers for targeting and intracellular delivery of therapeutics. J Control Release. 2000;65:203–220. doi: 10.1016/s0168-3659(99)00236-9. [DOI] [PubMed] [Google Scholar]
- 4.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 6.Plank C, Zauner W, Wagner E. Application of membrane-active peptides for drug and gene delivery across cellular membranes. Adv Drug Deliv Rev. 1998;34:21–35. doi: 10.1016/s0169-409x(98)00005-2. [DOI] [PubMed] [Google Scholar]
- 7.Wagner E, Plank C, Zatloukal K, Cotten M, Birnstiel ML. Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin- polylysine-DNA complexes: Toward a synthetic virus-like gene-transfer vehicle. Proc Nat Acad Sci USA. 1992;89:7934–7938. doi: 10.1073/pnas.89.17.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plank C, Oberhauser B, Mechtler K, Koch C, Wagner E. The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems. J Biol Chem. 1994;269:12918–12924. [PubMed] [Google Scholar]
- 9.McTaggart S, Al-Rubeai M. Retroviral vectors for human gene delivery. Biotechnol Adv. 2002;20:1–31. doi: 10.1016/s0734-9750(01)00087-8. [DOI] [PubMed] [Google Scholar]
- 10.Lam JKM, Ma Y, Armes SP, Lewis AL, Baldwin T, Stolnik S. Phosphorylcholine-polycation diblock copolymers as synthetic vectors for gene therapy. J Control Release. 2004;100:293–312. doi: 10.1016/j.jconrel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Boussif O, Lezoualch F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Nat Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Wetering P, Moret EE, Schuurmans-Nieuwenbroek NME, van Steenbergen MJ, Hennink WE. Structure-activity relationships of water-soluble cationic methacrylate/methacrylamide polymers for nonviral gene delivery. Bioconjugate Chem. 1999;10:589–597. doi: 10.1021/bc980148w. [DOI] [PubMed] [Google Scholar]
- 13.Haensler J, Szoka FC., Jr Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjugate Chem. 1993;4:372–379. doi: 10.1021/bc00023a012. [DOI] [PubMed] [Google Scholar]
- 14.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 15.Fischer D, Li YX, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121–1131. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 16.van de Wetering P, Cherng JY, Talsma H, Crommelin DJA, Hennink WE. 2-(dimethylamino)ethyl methacrylate based (co)polymers as gene transfer agents. J Control Release. 1998;53:145–153. doi: 10.1016/s0168-3659(97)00248-4. [DOI] [PubMed] [Google Scholar]
- 17.Murthy N, Robichaud JR, Tirrell DA, Stayton PS, Hoffman AS. The design and synthesis of polymers for eukaryotic membrane disruption. J Control Release. 1999;61:137–143. doi: 10.1016/s0168-3659(99)00114-5. [DOI] [PubMed] [Google Scholar]
- 18.Tonge SR, Tighe BJ. Responsive hydrophobically associating polymers: a review of structure and properties. Adv Drug Deliv Rev. 2001;53:109–122. doi: 10.1016/s0169-409x(01)00223-x. [DOI] [PubMed] [Google Scholar]
- 19.Seki K, Tirrell DA. pH-dependent complexation of poly (acrylic acid) derivatives with phospholipid vesicle membrane. Macromolecules. 1984;17:1692–1698. [Google Scholar]
- 20.Tirrell DA, Takigawa DY, Seki K. Interactions of synthetic-polymers with cell-membranes and model membrane systems. 7. pH-sensitization of phospholipid vesicles via complexation with synthetic poly(carboxylic acid)s. Ann NY Acad Sci. 1985;446:237–248. doi: 10.1111/j.1749-6632.1985.tb18404.x. [DOI] [PubMed] [Google Scholar]
- 21.Thomas JL, Tirrell DA. Polyectrolyte-sensitized phospholipids-vesicles. Accounts Chem Res. 1992;25:336–342. [Google Scholar]
- 22.Thomas JL, Barton SW, Tirrell DA. Membrane solubilisation by a hydrophobic polyelectrolyte: surface activity and membrane binding. Biophys J. 1994;67:1101–1106. doi: 10.1016/S0006-3495(94)80575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yessine MA, Lafleur M, Meier C, Petereit HU, Leroux JC. Characterisation of the membrane-destabilization properties of different pH-sensitive methacrylic acid copolymers. Biochim Biophys Acta – Biomembranes. 2003;1613:28–38. doi: 10.1016/s0005-2736(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 24.Cheung JC, Gross DJ, Thomas JL, Tirrell DA, Opsahl-Ong LR. pH-sensitive, cation-selective channels formed by a simple synthetic polyelectrolyte in artificial bilayer membranes. Macromolecules. 1996;29:4636–4641. [Google Scholar]
- 25.Thomas JL, Tirrell DA. Polymer induced leakage of cations from dioleoyl phosphatidylcholine and phosphatidylglycerol liposomes. J Control Release. 2000;67:203–209. doi: 10.1016/s0168-3659(00)00209-1. [DOI] [PubMed] [Google Scholar]
- 26.Thomas JL, Devlin BP, Tirrell DA. Kinetics of membrane micellization by the hydrophobic polyelectrolyte poly(2-ethylacrylic acid) Biochim Biophys Acta – Biomembranes. 1996;1278:73–78. doi: 10.1016/0005-2736(95)00192-1. [DOI] [PubMed] [Google Scholar]
- 27.Yessine MA, Leroux JC. Membrane-destabilizing polyanions: interaction with lipid bilayers and endosomal escape of biomacromolecules. Adv Drug Deliv Rev. 2004;56:999–1021. doi: 10.1016/j.addr.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 28.Thomas JL, You H, Tirrell DA. Tuning the response of a pH-sensitive membrane switch. J Am Chem Soc. 1995;117:2949–2950. [Google Scholar]
- 29.Kusonwiriyawong C, van de Wetering P, Hubbell JA, Merkle HP, Walter E. Evaluation of pH-dependent membrane-disruptive properties of poly(acrylic acid) derived polymers. Eur J Pharm Biopharm. 2003;56:237–246. doi: 10.1016/s0939-6411(03)00093-6. [DOI] [PubMed] [Google Scholar]
- 30.Cheung CY, Murthy N, Stayton PS, Hoffman AS. A pH-sensitive polymer that enhances cationic lipid-mediated gene transfer. Bioconjugate Chem. 2001;12:906–910. doi: 10.1021/bc0100408. [DOI] [PubMed] [Google Scholar]
- 31.Lackey CA, Press OW, Hoffman AS, Stayton PS. A biomimetic pH-responsive polymer directs endosomal release and intracellular delivery of an endocytosed antibody complex. Bioconjugate Chem. 2002;13:996–1001. doi: 10.1021/bc010053l. [DOI] [PubMed] [Google Scholar]
- 32.Kiang T, Bright C, Cheung CY, Stayton PS, Hoffman AS, Leong KW. Formation of chitosan-DNA nanoparticles with poly(propyl acrylic acid) enhances gene expression. J Biomat Sci – Polym E. 2004;15:1405–1421. doi: 10.1163/1568562042368112. [DOI] [PubMed] [Google Scholar]
- 33.Kyriakides TR, Cheung CY, Murthy N, Bornstein P, Stayton PS, Hoffman AS. pH-sensitive polymers that enhance intracellular drug delivery in vivo. J Control Release. 2002;78:295–303. doi: 10.1016/s0168-3659(01)00504-1. [DOI] [PubMed] [Google Scholar]
- 34.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 35.Eccleston ME, Slater NKH, Tighe BJ. Synthetic routes to responsive polymers; co-polycondensation of tri-functional amino acids with diacylchlorides. React Funct Polym. 1999;42:147–161. [Google Scholar]
- 36.Eccleston ME, Kuiper M, Gilchrist FM, Slater NKH. pH-responsive pseudo-peptides for cell membrane disruption. J Control Release. 2000;69:297–307. doi: 10.1016/s0168-3659(00)00316-3. [DOI] [PubMed] [Google Scholar]
- 37.Eccleston ME, Slater NKH. Hypercoiling polymers and their use in cellular delivery. Patent No. WO2004052402, EP1567194 (A1), US2006172418 (A1), AU2003290222 (A1) 2004
- 38.Chen R, Yue Z, Eccleston ME, Williams S, Slater NKH. Modulation of cell membrane disruption by pH-responsive pseudo-peptides through grafting with hydrophilic side chains. J Control Release. 2005;108:63–72. doi: 10.1016/j.jconrel.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Haslam E. Recent developments in methods for the esterification and protection of the carboxylic acid group. Tetrahedron. 1980;36:2409–2433. [Google Scholar]
- 40.Okada Y. Synthesis of peptides by solution methods. Curr Org Chem. 2001;5:1–43. [Google Scholar]
- 41.Dengler WA, Schulte J, Berger DP, Mertelsmann R, Fiebig HH. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assays. Anti-Cancer Drugs. 1995;6:522–532. doi: 10.1097/00001813-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Monera OD, Sereda TJ, Zhou NE, Kay CM, Hodges RS. Relationship of sidechain hydrophobicity and α-helical propensity on the stability of the single-stranded amphiphilic α-helix. J Pept Sci. 1995;1:319–329. doi: 10.1002/psc.310010507. [DOI] [PubMed] [Google Scholar]
- 43.Sereda TJ, Mant CT, Sönnichsen FD, Hodges RS. Reversed-phase chromatography of synthetic amphipathic α-helical peptides as a model for ligand/receptor interactions Effect of changing hydrophobic environment on the relative hydrophilicity/hydrophobicity of amino acid side-chains. J Chromatogr A. 1994;676:139–153. doi: 10.1016/0021-9673(94)00371-8. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Mant CT, Farmar SW, Hancock REW, Vasil MW, Hodges RS. Rational design of α-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J Biol Chem. 2005;280:12316–12329. doi: 10.1074/jbc.M413406200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones RA, Cheung CY, Black FE, Zia JK, Stayton PS, Hoffman AS, et al. Poly (2-alkylacrylic acid) polymers deliver molecules to the cytosol by pH-sensitive disruption of endosomal vesicles. Biochem J. 2003;372:65–75. doi: 10.1042/BJ20021945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Habermann E. Bee wasp venoms. Science. 1972;177:314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 47.Chen R, Yue Z, Eccleston ME, Slater NKH. Aqueous solution behaviour and membrane disruptive activity of pH-responsive PEGylated pseudo-peptides and their intracellular distribution. Biomaterials. 2008;29:4333–4340. doi: 10.1016/j.biomaterials.2008.07.040. [DOI] [PubMed] [Google Scholar]