Abstract
To provide a behavior-based estimate of odor similarity in larval Drosophila, we use 4 recognition-type experiments: 1) We train larvae to associate an odor with food and then test whether they would regard another odor as the same as the trained one. 2) We train larvae to associate an odor with food and test whether they prefer the trained odor against a novel nontrained one. 3) We train larvae differentially to associate one odor with food, but not the other one, and test whether they prefer the rewarded against the nonrewarded odor. 4) In an experiment like (3), we test the larvae after a 30-min break. This yields a combined task-independent estimate of perceived difference between odor pairs. Comparing these perceived differences to published measures of physicochemical difference reveals a weak correlation. A notable exception are 3-octanol and benzaldehyde, which are distinct in published accounts of chemical similarity and in terms of their published sensory representation but nevertheless are consistently regarded as the most similar of the 10 odor pairs employed. It thus appears as if at least some aspects of olfactory perception are “computed” in postreceptor circuits on the basis of sensory signals rather than being immediately given by them.
Keywords: discrimination, generalization, gustation, learning, olfaction, perception, sensory physiology, similarity
Introduction
The discoveries of the gene families coding for olfactory receptors in rodents (OR receptors: Buck and Axel 1991; V1R receptors: Dulac and Axel 1995; V2R receptors: Herrada and Dulac 1997; Matsunami and Buck 1997; Ryba and Tirindelli 1997; TAAR receptors: Liberles and Buck 2006) and later also in Drosophila (Or-gene family: Clyne et al. 1999; Vosshall et al. 1999; Ir-gene family: Benton et al. 2009) have led to a reasonably satisfying working hypothesis of how different odor substances evoke different patterns of activity along the olfactory pathways (concerning Drosophila see Hallem and Carlson 2006; Vosshall and Stocker 2007; Benton 2009; Gerber et al. 2009; Masse et al. 2009). Still, olfactory coding is far from being understood: It remains challenging to understand how the temporal characteristics of neuronal activity contribute to olfactory coding (Laurent 2002), whether and at which detail information about the physicochemical properties of odor substances is available to the olfactory subject in these patterns of activity (Schmuker et al. 2007; Haddad et al. 2008; Schmuker and Schneider 2007), and, even more embarrassingly we believe, it remains largely unclear which aspects of these different patterns of activity, and at which sites along the sensorimotor loop, underlie olfactory perceptions. Obviously, addressing such questions in animals requires developing an operational handle on perception in terms of well-defined behavioral tasks. Here, we take a step in this direction, using olfactory recognition experiments after odor–food associative learning in larval Drosophila.
In principle, the architecture of the olfactory pathways in larval Drosophila is the same as in adult flies and in mammals—but at a numerically much reduced level (reviewed in Gerber and Stocker 2007; Stocker 2008; Gerber et al. 2009): The larva has only 21 olfactory sensory neurons, organized in the so-called dorsal organ, each expressing but one member of the Or-gene family (plus the co-receptor Or83b) with its respective ligand profile. The olfactory sensory neurons then innervate the antennal lobe (the functional analogue of the olfactory bulb), where they synapse onto both local interneurons (regarding adults: Wilson 2008) and projection neurons (the functional analogue of the mitral cells). These connections are organized into glomeruli such that one anatomically identifiable antennal lobe glomerulus contains input from but one genetically defined olfactory sensory neuron, gives rise to output of but one projection neuron, and harbors the lateral connections toward and from the local interneurons (Ramaekers et al. 2005). Notably, the projection neurons have 2 target areas: First, they innervate the so-called lateral horn in an amazingly stereotyped way (Marin et al. 2002; Wong et al. 2002). The lateral horn in turn has access to premotor circuitry. It is arguably via this direct route that reflexive innate olfactory behavior is organized (regarding adult Drosophila: Heimbeck et al. 2001). Second, the projection neurons target the calyx of the so-called mushroom bodies (Ramaekers et al. 2005; Masuda-Nakagawa et al. 2005, 2009). In the larva, this structure is organized into approximately 40 anatomically identifiable glomeruli such that one projection neuron provides input to typically but one of these calyx glomeruli; consequently, most of the projection neurons can be individually identified based on the stereotyped combination of antennal input glomerulus and calycal output glomerulus (Ramaekers et al. 2005; Masuda-Nakagawa et al. 2009). In any event, the postsynaptic partners of the projection neurons in the mushroom bodies are the Kenyon cells. Each of the approximately 600 mature Kenyon cells receives input from an apparently random selection of 1–6 glomeruli (Masuda-Nakagawa et al. 2005, 2009). This entails a dense network of divergence–convergence connections in the calyx, reminiscent of olfactory cortex (Davis 2004; Tomer et al. 2010), and suitable for combinatorial coding. The mushroom bodies further receive input from aminergic reinforcement neurons such that within the mushroom bodies the association of odor-evoked activity with salient rewarding or punishing events can take place (regarding adult Drosophila: Schwaerzel et al. 2003; Riemensperger et al. 2005; Busch et al. 2009; Tomchik and Davis 2009; Busch and Tanimoto 2010; Gervasi et al. 2010; regarding larval Drosophila Schroll et al. 2006; Selcho et al. 2009). The Kenyon cells in turn synapse onto remarkably few (based on findings in adult flies; Aso et al. 2009; Ito et al. 1998) output neurons that entertain connections toward premotor centers. It is arguably by this detour via the mushroom body that learned olfactory behavior is organized (see discussions in Gerber et al. 2004, 2009; Heisenberg and Gerber 2008). Given that, in addition to this fairly detailed account of the connectivity of the circuit, the ligand profiles of all larval-expressed Or gene products are at least partially described (using a panel of 26 odors: Kreher et al. 2005, 2008) (ligand profiles of the larval-expressed Ir-gene family receptors [Benton et al. 2009] are not yet known), it has been attempted to predict the combinatorial, yet not temporal, patterns of odor-evoked activity along the olfactory pathways of the larva (Masuda-Nakagawa et al. 2009). Still, how larvae actually perceive odors remains unknown. Here, we make an attempt in this direction. Paramount to our approach is to not directly ask how the larvae perceive a given odor (because we did not expect an answer) but rather to ask whether the larvae perceive 2 given odors as different from each other.
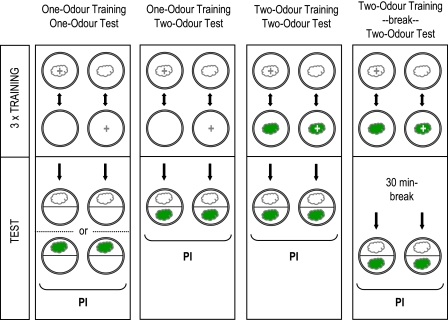
Using 4 kinds of recognition task (Figure 1), we seek to come up with one task-independent estimate of perceived difference between 10 odor pairs. The tasks are (i) we train larvae to associate an odor with a food reward and then test whether, in a subsequent test, they would regard another odor as the same as the trained one; (ii) we train larvae to associate an odor with a food reward and then test in a choice situation whether they can tell the trained odor from a novel nontrained odor; (iii) we train larvae differentially to associate one, but not another odor with a food reward, and then test in a choice situation whether they can tell the previously rewarded from the previously nonrewarded odor; (iv) in an experiment alike (iii), we test the larvae after an additional 30-min break.
Figure 1.
The rationale of the learning tasks. In task (i), larvae are trained to associate an odor with a sugar reward and are tested for their approach to either that trained odor or a novel, not previously trained odor afterward. In task (ii), animals are trained to associate an odor with a sugar reward and are tested for their choice between that trained odor versus a novel odor. In task (iii), larvae are trained differentially and tested for their choice between the previously rewarded versus the previously nonrewarded odor; the same procedure is employed in task (iv), except that an additional retention period of 30 min is introduced. This figure appears in color in the online version of Chemical Senses.
A distinguishing feature of our approach (as compared with Cobb and Domain 2000; Boyle and Cobb 2005; Guerrieri et al. 2005; Kreher et al. 2008) is that we choose odor dilutions on a behavioral, rather than physical, basis. That is, we were adjusting odor dilutions for equal learnability (Figure 2A) rather than using the same dilution for all odors. Why would this be important? Suppose we would use odor dilutions in task (i) such that a given odor A would be learnt well, whereas odor B would be less well learnable if the same dilution is used. Thus, after training with A, we may find strong learnt attraction to B because A and B are to some extent similar and because the memory for A is strong. In turn, after training with B, learnt attraction to A may be low simply because the memory for B is weak and although A and B actually are regarded as similar by the larvae. This would entail an apparent asymmetry of similarity judgments, which as we argue here complicates interpretation of previous approaches toward odor similarity (Cobb and Domain 2000; Boyle and Cobb 2005; Guerrieri et al. 2005; Kreher et al. 2008). Symmetry is an essential property for a metric in the mathematical sense (the distance between X and Y must be equal to the distance between Y and X). Odor similarity metrics based on physicochemical properties of the odorant molecules or on odor-evoked physiological activity patterns fulfil this criterion. Thus, in order to be comparable with such metrics, symmetric measures of perceptual similarity are indispensable.
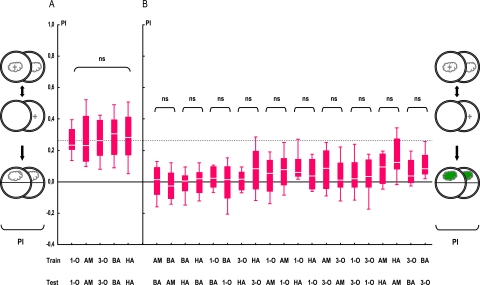
Figure 2.
Symmetry of perceived distances. Associative PIs are presented depending on the combination of TRAINing versus TESTing odor (1-O, AM, 3-O, BA, and HA). (A) Larvae are tested with the trained odor, yielding the same level of PIs across the 5 odors used. Sample sizes are from left to right: 28, 28, 28, 44, 48. ns: KW test, P> 0.05. (B) Larvae are tested with a novel, not previously trained odor, yielding PIs generally below the stippled line, that is, below the median of the pooled data from (A). Note that PIs are symmetrical in all cases: Scores are equal when, for example, AM is trained and BA is tested as in the case when BA is trained and AM is tested. Sample sizes are from left to right: 12, 12, 12, 12, 12, 12, 12, 12, 28, 28, 12, 16, 28, 28, 28, 28, 12, 12, 12, 12. ns: MW tests, P> 0.05/ 10 (Bonferroni correction). For the underlying preference data, see Figure S1. Data are presented as box plots with the middle line as the median, box boundaries and whiskers as 25/75% and 10/90% quantiles, respectively. This figure appears in color in the online version of Chemical Senses.
In any event, using a recognition-based approach obviously relies on the faculty of the larvae to learn and remember odors and their association with food reward. Given that odor–food memory traces are arguably established in the mushroom bodies (Gerber et al. 2004, 2009; Heisenberg and Gerber 2008), our approach therefore probes for behaviorally-relevant central brain aspects of olfactory perception (this approach had been pioneered by Pavlov [1927; loc. cit. chapter VII], who had attempted to describe the discrimination powers of the “cortical analyzers” by means of discrimination–generalization experiments in the dog). We will then discuss whether these aspects of olfactory perception are correlated to physicochemical properties of the odors.
Materials and methods
We use feeding-stage third-instar larvae of the wild-type Canton-S strain (stock collection, Universität Würzburg), aged 5 days after egg laying. Larvae are maintained in mass culture on standard medium at 25 °C, 60–70% relative humidity, and a 14:10 h light:dark cycle. All experiments are performed under a fume hood in a regularly lit room at 21–26 °C room temperature.
Prior to the learning experiments, the odor stimuli are prepared by adding 10 μL of odor substance into custom-made Teflon containers of 5 mm diameter that are closed by a perforated lid (7 holes, 0.5 mm diameter). As stimuli we use 1-octanol (1-O, Sigma-Aldrich, CAS: 111-87-5), n-amyl acetate (synonymous for n-pentyl acetate) (AM, Merck, CAS: 628-63-7), 3-octanol (3-O, Merck, CAS: 589-98-0), benzaldehyde (BA, Fluka, CAS: 100-52-7), hexyl acetate (HA, Sigma-Aldrich, CAS: 142-92-7), or an odor container without any odor applied (empty: EM). Odorants are used diluted in paraffin oil (1-O: 1:100; AM: 1:3333; 3-O: 1:105; BA: 1:100; HA: 1:100, unless mentioned otherwise; paraffin oil: CAS: 8012-95-1; Merck). The choice of these dilutions is based on a comprehensive description of the dose-dependent learnability of these odors (Mishra D, Chen Y-C, Yarali A and Gerber B, in preparation): We chose dilutions such that learnability is equal for all odors and as near as possible to the lowest intensity that supports asymptotic associative performance. Paraffin oil is behaviorally ineffective (Saumweber et al. 2010).
Petri dishes (Sarstedt) of 85 mm diameter are filled either with only 1% agarose (electrophoresis grade; Roth) or with agarose containing the sugar reward in addition (+; 2 mol/L fructose, purity 99%, Roth) which is added to agarose 10 min after boiling. After solidification, petri dishes are covered with their lids and left untreated at room temperature until the following day. Before starting experiments, we replace the regular lids of the petri dishes with lids perforated in the center by fifteen 1-mm holes to improve aeration.
General procedure of the learning experiments
A spoonful of food medium containing larvae is taken from the food vial, 30 animals are collected, briefly rinsed in distilled water, and used as a group for the experiment. In all experiments, we train larvae using either of 2 reciprocal training regimen: for one regimen, animals receive stimulus X with a positive reinforcer (+) and stimulus Y without a reinforcer (Train: X+ // Y; the chemical identity of X and Y as 1-O, AM, 3-O, BA, HA, or EM is mentioned along the Results); for the second regimen, animals are trained reciprocally (Train: X // Y+). Afterward, animals are tested for their choice between stimulus X versus stimulus Y (please note that in half of the cases, we start with stimulus X [i.e., X+ // Y and X // Y+], whereas in the other half of the cases, we start with stimulus Y [Y // X+ and Y+ // X]). Associative learning is indicated by systematic differences in test performance between the reciprocally trained conditions; these differences are quantified by the performance index (PI; see following paragraph). This conclusion is compelling as during training animals from both training regimen have identical exposure to both odors and the reward—what differs between them is solely the contingency between these stimuli.
Immediately before a trial, 2 containers loaded with the same stimulus are placed on opposite sites of the petri dish, which may or may not contain the sugar reward; animals are transferred to the petri dish and the lid is closed. After 5 min, animals are transferred to a fresh petri dish with the alternative stimulus–substrate combination. This training cycle is repeated 3 times. Fresh petri dishes are used for each trial. After such training, animals are tested for their choice between 2 testing stimuli. They are placed in a 7-mm middle stripe of a testing petri dish; this testing petri dish does not contain the sugar reward. On either side of the petri dish, we place one odor container, 7 mm from the edge, each loaded with a different stimulus to create a choice situation. For example, in the simplest case (deviations are mentioned in the tasks below and along the Results), the containers are loaded with stimulus X on one side and stimulus Y on the other side (Test: X–Y). After 3 min, the number of animals on the X-side, the Y-side, and the middle stripe is determined. We then calculate a preference score (PREF) by subtracting the number of animals observed on the Y-side from the number of animals observed on the X-side, divided by the total number of animals (larvae which remain in the 7-mm middle stripe are included in that total) (PREF scores for all experiments are documented in the Supplementary Material):
Then, another group of 30 animals is trained in a reciprocal manner, and the PREF score is determined as
To determine whether preferences are different depending on training regimen, we calculate a performance index (PI) from these 2 reciprocally trained groups ranging from –1 to 1 as
Positive PIs thus indicate conditioned approach, negative PIs represent conditioned avoidance. Data from experimental conditions to be compared statistically are obtained in parallel. Larvae are trained and tested only once.
Features of the learning tasks
According to this general principle, a series of generalization–discrimination types of learning task is performed:
(i) In a 5 × 5 generalization type of task, larvae are trained with any one of the 5 odor stimuli against EM. Afterward, they are tested either for their choice between the trained odor versus EM or for any one of the 4 remaining nontrained odors versus EM. An abbreviated form for this task may thus read as
Train: X // EM,
Test: X–EM (or Y–EM).
Thus, the larger the perceptual distance between X and Y is, the less conditioned behavior toward Y we should observe (i.e., the smaller PI scores for Y should be). Note that this logic is valid only if odor intensities are adjusted for equal learnability (the same caveat also applies to the tasks ii–iv below).
(ii) Larvae are trained as in the previous task but are tested in a 2-odor choice situation between the trained versus any of the 4 nontrained odors:
Train: X // EM,
Test: X–Y.
Thus, the larger perceptual distance between X and Y is, the more conditioned behavior toward X we should observe (i.e., the larger PI scores should be).
(iii) In a discrimination type of task, larvae are trained differentially between 2 odors and then are tested for their choice between them in a 2-odor choice situation:
Train: X // Y,
Test: X–Y.
Thus, the larger perceptual distance between X and Y is, the more conditioned behavior we should obtain (i.e., the larger PI scores should be).
(iv) Larvae are trained and tested as in (iii) but testing takes place only after an additional 30-min break during which larvae are kept with few drops of water in an otherwise blank petri dish:
Train: X // Y,
30-min break,
Test: X–Y.
Again, the larger perceptual distance between X and Y is, the more conditioned behavior we should obtain (i.e., the larger PI scores should be).
Data acquisition and statistics
Data are presented as box plots with the bold line showing the median, the 25/75% and the 10/90% quantiles as box boundaries and whiskers, respectively. Sample sizes are represented within the figure legends.
In a conservative approach, nonparametric analyses are performed; for multiple-group comparisons, we use Kruskal–Wallis (KW) tests, and for 2-group comparisons Mann–Whitney U (MW) tests are performed. Significance of differences is assigned if P < 0.05. When multiple tests are performed within one experiment, we correct the significance level by dividing the P value of 0.05 according to the number of comparisons made (Bonferroni-correction) to maintain an experiment-wide error rate at 5%; if, for example, 3 such comparisons are made, P < 0.05/3 is applied.
Spearman's rank correlation provides a distribution test of dependence between behavioral and chemical odor similarities.
All statistical analyses are performed with Statistica 7.0 (Statsoft).
Experimenters are blind with respect to treatment condition (reward status of the petri dishes).
Results
The rationale of the experiments is to ask whether larvae perceive a test odor as the same as a previously trained odor. For this purpose, we first present the results of 4 independent recognition tasks and then combine these results into one comprehensive task-independent score of perceived odor distance.
Task (i)
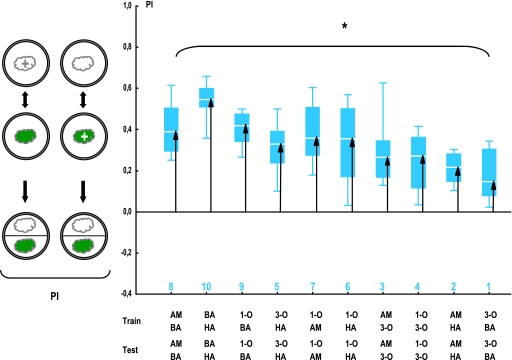
Larvae are trained to associate an odor with a sugar reward and are tested for their approach either to that trained odor or to a novel, previously nontrained odor (see sketches in Figures 1 and 2). Importantly, all 5 employed odors are equally learnable, yielding associative performance indices of about 0.3 (stippled line in Figure 2A; KW test: H = 1.07, P= 0.90; N = 28, 28, 28, 44, 48). When nontrained odors are used for testing, performance indices are generally lower (Figure 2B); for example, if AM is trained and BA is tested, performance indices are indistinguishable from zero, arguing that AM and BA are perceptually distinct to the larvae. Notably, these measures of perceptual distance are in all cases symmetrical: for instance, the performance indices of larvae trained with AM and tested with BA is as low as when BA is trained and AM is tested (Figure 2B; MW test: U = 63, P = 0.60; N = 12, 12); the same result we find for all other odor pairs as well (Figure 2B). Therefore, we pool these respective subgroups (Figure 3A). It turns out that performance indices differ among odor pairs, meaning that perceived distances (black arrows in Figure 3A) are different among odor pairs (Figure 3A; KW test: H = 20.68, P< 0.05; N = 24, 24, 24, 24, 56, 28, 56, 56, 24, 24). In a conservative approach, we assign ranks to the perceived distances thus obtained (see Table 1); we note that odor pair AM–BA yields the highest perceptual distance and odor pair AM–HA the lowest perceptual distance for the larvae—with respect of this kind of learning task.
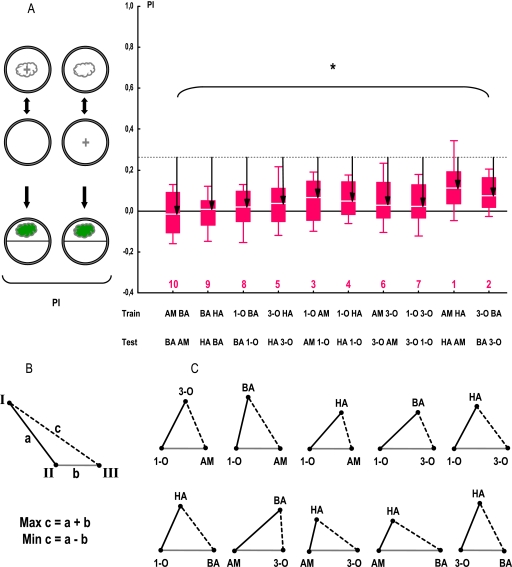
Figure 3.
Odor pairs differ in perceived distances in an one-odor training, one-odor test task. (A) Re-presenting the pooled data from Figure 2. The stippled gray line shows the level of PIs when TRAINing and TESTing odor are actually the same (pooled from Figure 2A). The more different larvae regard the TESTing odor from the TRAINing odor, the smaller PIs should be observed; this is quantified by the “distance” arrows. Note that PIs differ among odor pairs, indicating that perceived distances are different among odor pairs. Sample sizes are from left to right: 24, 24, 24, 24, 56, 28, 56, 56, 24, 24. *: KW test: P < 0.05. The numbers below the plots refer to the distance rank of the respective odor pair (see Table 1). Other details are described in Figure 2. (B) Sketch to describe the minimal–maximal range of distances between odors I and III in relation to known distances between odors I and II, as well as odors II–III. (C) For all 10 sets of 3 odors, the distances from (A) can be represented as triangles, arguing that the consistency-criterion in (B) is met. The same holds true for 29 of the 30 additional cases using the data from Figures 4–6 (exception being the triplet AM-3O-BA in task ii). This figure appears in color in the online version of Chemical Senses.
Table 1.
Ranks of perceived distance
| Odor pair | Task (i) PD | Rank | Task (ii) PD | Rank | Task (iii) PD | Rank | Task (iv) PD | Rank | Median rank |
| AM versus BA | 0.279 | 10 | 0.171 | 10 | 0.389 | 8 | 0.225 | 4 | 9 |
| BA versus HA | 0.254 | 9 | 0.148 | 9 | 0.545 | 10 | 0.261 | 7 | 9 |
| 1-OCT versus BA | 0.244 | 8 | 0.132a | 6 | 0.417 | 9 | 0.240 | 5 | 7 |
| 3-OCT versus HA | 0.227 | 5 | 0.144 | 8 | 0.327 | 5 | 0.263 | 8 | 6.5 |
| 1-OCT versus AM | 0.196 | 3 | 0.132b | 5 | 0.359 | 7 | 0.280 | 10 | 6 |
| 1-OCT versus HA | 0.215 | 4 | 0.143 | 7 | 0.355 | 6 | 0.222 | 3 | 5 |
| AM versus 3-OCT | 0.233 | 6 | 0.102 | 2 | 0.263 | 3 | 0.264 | 9 | 4.5 |
| 1-OCT versus 3-OCT | 0.240 | 7 | 0.120 | 4 | 0.272 | 4 | 0.221 | 2 | 4 |
| AM versus HA | 0.151 | 1 | 0.116 | 3 | 0.215 | 2 | 0.246 | 6 | 2.5 |
| 3-OCT versus BA | 0.188 | 2 | 0.027 | 1 | 0.146 | 1 | 0.139 | 1 | 1 |
For each of the 4 tasks, we assign the indicated odor pair a rank based on perceived distance (PD) (arrows in Figures 3–6); the right-most column presents the median of the obtained ranks for the respective odor pair.
0.1327.
0.1326.
Considering perceived distances among 3, rather than 2 odors, our results allow us to consistently describe distances for odor triplets (Figure 3B). That is, for cases of known distance between odors I–II and odors II–III, the maximal distance between odor I and odor III is given by the sum of the I–II plus the II–III distance, whereas the minimal distance between I and III is given by the difference between the I–II distance minus the II–III distance. This is indeed the case for all 10 triplets (Figure 3C), arguing for the internal consistency of the obtained perceptual distances. Similar analyses of the data from tasks ii, iii, and iv yield the same conclusion for 29/30 cases (not shown; the exception is the AM-3O-BA triplet in task ii).
Task (ii)
We train larvae to associate an odor with a sugar reward and test their choice between that trained odor versus a novel odor (see sketches in Figures 1 and 4). If larvae regard these 2 odors as similar, that is, if perceived distance is low, they should distribute equally between both odors in the test situation, resulting in low PIs. We note that also for this experiment, PIs are symmetrical such that, for example, the PI in the case when choice between AM and BA is tested after AM training is as high as in the case when the same choice is offered after BA training (Figure S2A; MW test: U = 57, P= 0.39; N = 12, 12); the same is found for all other odor pairs as well (Figure S2A). Therefore, we can pool these respective subgroups; we find that PIs are different among odor pairs (Figure 4; KW test: H = 17.19, P< 0.05; N = 24 in all cases), arguing that perceived distances also differ between odor pairs in this task. For example, the odor pair AM–BA yields the highest PIs, and hence the largest perceived distance, whereas for the odor pair 3-O and BA, we find the smallest perceptual distance (black arrows in Figure 4). Again, we assign ranks to the odor pairs according to these perceived distances (Table 1).
Figure 4.
Odor pairs differ in perceived distances in an one-odor training, 1-odor test task. Larvae are trained to associate one given odor and then are offered a choice between this trained odor versus a novel odor. The more different larvae regard both odors, the larger PIs would be observed; perceived distances can thus be estimated as indicated by the arrows. *: KW test: P < 0.05; N = 24 in all cases. For the Pls of the subgroups and the underlying preference data, see Figure S2A and S2B, respectively. The numbers below the plots refer to the distance rank of the respective odor pair (see Table 1). Other details are described in Figure 2. This figure appears in color in the online version of Chemical Senses.
Task (iii)
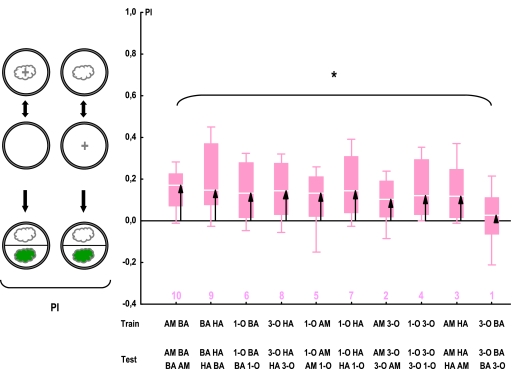
Larvae are trained to discriminate 2 odors such that one odor is paired with a sugar reward, whereas the other odor is presented without reward; at test, larvae are given the choice between these 2 odors (see sketches in Figures 1 and 5). If the 2 odors are similar to the larvae, we expect low PIs. We find that PIs differ among odor pairs (Figure 5; KW test: H = 55.71, P< 0.05; N = 16 in all cases), once more arguing that perceived distances differ among odor pairs. For example, in this task, BA and HA appear as the most distinct pair to the larvae, whereas 3-O and BA appear to be similar to them. In Table 1, we present the ranks of perceived distances (black arrows in Figure 5) thus obtained.
Figure 5.
Odor pairs differ in perceived distances in a 2-odor training, 2-odor test task. Larvae are trained differentially by rewarding one but not the respective other odor and are then offered a choice between the previously rewarded versus the previously nonrewarded odor. The more distinct both odors are, the higher PIs we should observe; perceived distance can thus be approximated as indicated by the arrows. *: KW test: P < 0.05; N = 16 in all cases. For the underlying preference data, see Figure S3. The numbers below the plots refer to the distance rank of the respective odor pair (see Table 1). Other details are described in Figure 2. This figure appears in color in the online version of Chemical Senses.
Task (iv)
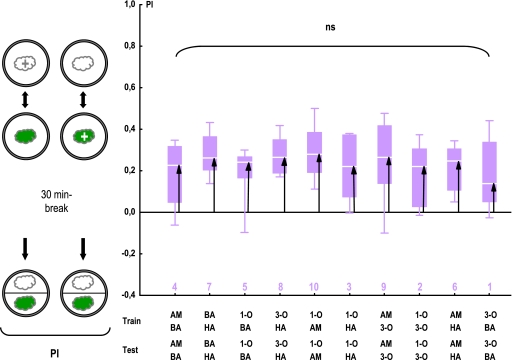
The procedure of this task is exactly the same as in task (iii), only that between training and test, an additional retention period of 30 min is introduced (see sketches in Figures 1 and 6). Notably, in this case, PIs do not formally differ among groups (Figure 6; KW test: H = 6.03, P= 0.74; N = 12 in all cases); in other words, perceived distances (black arrows in Figure 6) in this task do not differ between odor pairs. We note that PIs for some odor pairs apparently decrease from immediate testing to testing after a 30-min retention period; for the odor pair BA and HA as an example, PIs at 30 min are only about half as compared with immediate testing. For other odor pairs, such as AM and HA, in contrast, levels of PIs are stable over time (see Smith 1991 for similar dynamics in bees). In any event, regarding task (iv) as well, we present the ranks of the obtained perceived distances (black arrows in Figure 6) in Table 1.
Figure 6.
Perceived distances after a 30-min retention period. Larvae are trained and tested differentially in the same way as in the experiment displayed in Figure 5; however, testing is performed only after an additional 30-min retention period. The arrows indicate perceptual distances; apparently, after this retention period, there are no significant differences among odor pairs in terms of their perceived distances. ns: KW test: P > 0.05; N =12 in all cases. For the underlying preference data, see Fig. S4. The numbers below the plots refer to the distance rank of the respective odor pair (see Table 1). Other details are described in Figure 2. This figure appears in color in the online version of Chemical Senses.
Ranking perceived distances
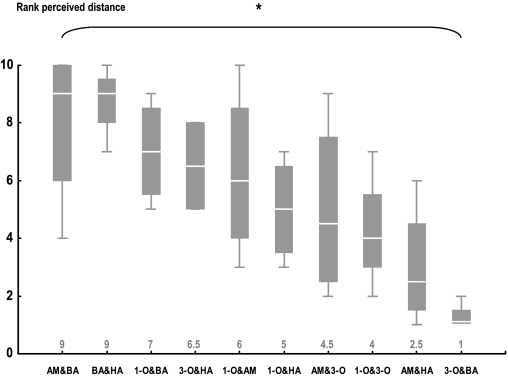
Given that the pattern of perceptual distances we find appears fairly concordant across these 4 tasks, we combine all the data to come up with one task-independent estimate of perceived distance. For this purpose, we take a conservative approach and use the ranked perceived distance scores from all tasks (Table 1) and present a summary of these ranks as a box plot in Figure 7. It turns out that these ranks differ among odor pairs (Figure 7; KW test: H = 22.22, P< 0.05; N = 4 in all cases), arguing that, irrespective of the task used, odor pairs are reliably different in their pairwise perceived distances.
Figure 7.
Estimating task-independent perceptual distances among odors. On the basis of the ranks in perceptual distances (Table 1), each box plot represents 4 combined perceptual distance ranks for each odor pair. *: KW test: P < 0.05. N = 4 in all cases.
Discussion
Task independence of odor distance
This study used 4 independent associative recognition tasks (Figure 1) (Figures 3–6) in an attempt to provide a task-independent measure of perceived distance for 10 odor pairs. We find that, for example, 3-O and BA consistently turn out as least distinct (i.e., most similar) in behavior: Considering the 10 odor pairs and all 4 tasks, there is a significant difference in perceived distances between odor pairs (Figure 7), meaning that our approach indeed could reveal consistent perceived distances between the 10 odor pairs across all 4 tasks. This conclusion is in line with data from Niewalda T, Völler T, Eschbach C, Ehmer J, Chou W-C, Timme M, Fiala A, Gerber B (in preparation) using recognition experiments after odor–shock training in adult flies.
As a drastic exception to this rule of task independence, we have recently found (Mishra et al. 2010) that 3-O can be discriminated well from 1-octen-3-ol if larvae had been trained discriminatively, that is, by rewarding one but not the other odor (task iii). On the other hand, no odor specificity is observed after nondiscriminative training (task i). That is, for this odor pair, there is both strong discrimination and full generalization. If the test involves a choice between these 2 odors, larvae show conditioned preference for the rewarded odor if training had been performed discriminatively (task iii) but not if training had not been performed discriminatively (task ii). In other words, for 3-O and 1-octen-3-ol, only discrimination training confers an odor specific memory trace, whereas one-odor training does not. This means that, at least for 3-O and 1-octen-3-ol which have strongly overlapping electrophysiological activation profiles (Kreher et al. 2008), there is a degree of freedom in the olfactory system that allows enhancing or ignoring differences between odors flexibly, depending on the task.
Obviously, however, there is no perfect concordance among tasks. When we probe for correlations between tasks in ranks of perceived distance, we find a significant correlation only between task ii and task iii (Spearman's rank correlation: R = 0.82, P< 0.05/6; N = 10); this suggests that both the nature of the test situation (one-odor test/2-odor test), and the training-to-test interval (immediate/30-min break) can modify the larvae’s odor distance “landscape” to some extent. Regarding the training-to-test interval, we note that statistically speaking 30 min after training all odor pairs appear equally distant to the larvae; thus, in addition to an overall decrease in associative scores between immediate testing and testing after a 30-min break, it seems that memory is losing specificity over time. Interestingly, the data of Niewalda T, Völler T, Eschbach C, Ehmer J, Chou W-c, Timme M, Fiala A, Gerber B (in preparation) suggest similar effects of the training-to-test interval for odor–shock associations in adult Drosophila. Although in particular this loss of specificity is an interesting phenomenon from a mnemonic perspective, this practically means that longer term memory assays should rather be avoided in future attempts to characterize the odor space in Drosophila.
Taken together, as a rule, associative odor recognition seems to draw upon a given stable representation of the odors such that the features of the behavioral regimen are of little influence. Still, given that there are obvious and drastic exceptions to this rule, as mentioned above for 3-O and 1-octen-3-ol, and given some variance between the results obtained by different tasks, we do not believe there is any one best solution to estimate perceived distance from behavioral experiments. Rather, we believe it is wise to use more than one behavioral task to “distil” the stable perceptual distances between odor pairs. Clearly, the labor invested in using multiple behavioral tasks then has to be traded off with the number of odor pairs one can include in the analysis.
Physicochemical distances
Given the fair concordance of perceived distances across tasks, we wonder whether the physicochemical properties of the odors might be a determinant for these perceived distances. To this end, we follow the approaches by Schmuker et al. (2007), Schmuker and Schneider (2007) and Haddad et al. (2008) (Table 2). In the Schmuker and Schneider (2007) approach, a set of 184 physicochemical descriptors is calculated using the MOE software (Chemical Computing Group). Descriptors are normalized to zero mean and unit variance. Distances are calculated using the sum of absolute coordinate differences (Manhattan or city-block metric) and are reported in Table 2. In the Haddad et al. (2008) approach, each odor structure is obtained from PubChem (http://pubchem.ncbi.nlm.nih.goc) and entered into the Dragon software (http://www.talete.mi.it/products/dragon_description.htm). Then, each odor is represented as a vector of 1664 molecular descriptor values. For the respective odor pairs, we obtain the distance values as displayed in Table 2.
Table 2.
Physicochemical distances between odors
| Odor pair | Distance Schmuker et al. | Rank | Distance Haddad et al. | Rank |
| AM versus BA | 169.66 | 7 | 34.17 | 7 |
| BA versus HA | 186.01 | 8 | 38.80 | 9 |
| 1-OCT versus BA | 202.40 | 10 | 41.71 | 10 |
| 3-OCT versus HA | 88.76 | 4 | 18.31 | 3 |
| 1-OCT versus AM | 94.33 | 5 | 22.02 | 6 |
| 1-OCT versus HA | 80.16 | 3 | 19.35 | 4 |
| AM versus 3-OCT | 94.61 | 6 | 16.50 | 2 |
| 1-OCT versus 3-OCT | 28.74 | 2 | 19.65 | 5 |
| AM versus HA | 25.74 | 1 | 11.99 | 1 |
| 3-OCT versus BA | 197.45 | 9 | 37.08 | 8 |
Physicochemical distance values for odor pairs are determined according to Schmuker and Schneider (2007) and Haddad et al. (2008), respectively. Within each approach, odor pairs are assigned ranks according to the respective values obtained.
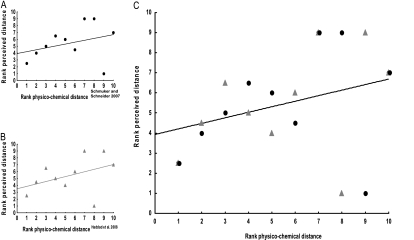
We then assign ranks to the odor pairs according to the respective physicochemical distance values obtained (Table 2). In Figure 8, we can thus plot the ranks of perceived distance versus the ranks of physicochemical distance. When considering the combined data set, that is, when treating the results of the Schmuker and Schneider (2007) and the Haddad et al. (2008) approaches as independent approaches, we find a just-significant correlation between physicochemical and perceived distance (Figure 8C; Spearman's rank correlation: R = 0.45, P= 0.04; N = 20) (within each of these 2 approaches, only trends for such correlations are observed [Figure 8A; Spearman's rank correlation: R = 0.41, P= 0.24; N = 10; Figure 8B; Spearman's rank correlation: R = 0.48, P= 0.16; N = 10]). This suggests that, as a rule, small differences in the physicochemical properties of odors entail small differences in perception and that associative memory trace formation and associative recognition draw upon these task-invariant percepts. Still, we should note that both Schmuker and Schneider (2007) and Haddad et al. (2008) implicitly assume odor intensity, which can be a profound determinant of olfactory perception, to be equal. However, meeting this assumption in behavioral experiments is not trivial and requires experimental scrutiny to adjust odor dilutions for equal effectiveness in the respective behavioral task.
Figure 8.
Comparing perceived distances to physicochemical distances. (A,B) According to 2 independent odor–distance metrics (based on Schmuker and Schneider (2007) and Haddad et al. (2008), respectively) (Table 2), we rank odor pairs according to physicochemical distance. The plot presents perceived distance ranks on the y axis and physicochemical distance ranks on the x axis. Spearman's rank correlation: R = 0.41, 0.48, P = 0.24, 0.16; N = 10, 10 for (A) and (B) respectively. (C) The combined data from (A) and (B) suggest a just-significant correlation between physiochemical and perceived odor distances. Spearman's rank correlation: R = 0.45, P= 0.048; N = 20.
Physiology
Again, it seems important to draw attention to the exception (see also the discussion in Sell (2006)): The pair 3-O and BA is regarded as most similar by the larvae, across all tasks (Figures 3–7); however, both the Schmuker and Schneider (2007) and the Haddad et al. (2008) approach agrees that these odors are relatively different in their physicochemical features (Figure 8C). Interestingly, from an electrophysiological perspective, 3-O and BA appear clearly distinct, too: 3-O activates Or85c-expressing olfactory sensory neurons, whereas BA activates Or45b-expressing cells (Kreher et al. 2008), a distinctiveness that is maintained even at relatively higher odor concentrations (3-O: Or13a, Or35a, Or45a, Or47a, Or85c; BA: Or7a, Or24a, Or30a, Or45b, Or67b) and also with regard to inhibition (relatively high concentration, 3-O: Or22c, Or24a, Or33b; BA: Or13a, Or42b, Or82a; relatively low concentration, 3-O: Or33b; BA: Or33b, Or85c). Unfortunately, a comprehensive comparison of our behavioral data to the physiology of Or-expressing neurons is not possible because the odor set used by Kreher et al. (2008) does not include data for all odor pairs employed here. In any event, although 3-O and BA are distinct chemically as well as in terms of their sensory representation, the larvae still regard them as the most similar of all the 10 odor pairs employed in our study. This suggests a step of “merging” of both odors at a point between the first-order sensory layer and behavioral control (see Niewalda T, Völler T, Eschbach C, Ehmer J, Chou W-C, Timme M, Fiala A, Gerber B, in preparation, for a similar suggestion on the basis of a combined behavioral and optical imaging approach in adult flies). It therefore appears as if, similar to the case of color vision, for example, relevant aspects of the olfactory percept need to be “computed” in postreceptor circuits on the basis of the sensory signals rather than being immediately given by the sensory signals.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (IRTG 1156 Synaptic and behavioural plasticity, SPP 1392 Integrative analyses of olfaction); German Federal Ministry of Science and Technology (BMBF) (Bernstein Focus Insect-inspired robotics).
Acknowledgments
Thanks to K. Tschirner and K. Gerber for help with the experiments and to M. Heisenberg, E. Buchner, B. Michels, A. Yarali, and T. Saumweber for discussion and support. Experiments comply with applicable law. B.G. is a Heisenberg Fellow of the Deutsche Forschungsgemeinschaft.
References
- Aso Y, Grubel K, Busch S, Friedrich AB, Siwanowicz I, Tanimoto H. The mushroom body of adult Drosophila characterized by GAL4 drivers. J Neurogenet. 2009;23:156–172. doi: 10.1080/01677060802471718. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Benton R. Molecular basis of odor detection in insects. Ann N Y Acad Sci. 2009;1170:478–481. doi: 10.1111/j.1749-6632.2009.03880.x. [DOI] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J, Cobb M. Olfactory coding in Drosophila larvae investigated by cross-adaptation. J Exp Biol. 2005;208:3483–3491. doi: 10.1242/jeb.01810. [DOI] [PubMed] [Google Scholar]
- Busch S, Selcho M, Ito K, Tanimoto H. A map of octopaminergic neurons in the Drosophila brain. J Comp Neurol. 2009;513:643–667. doi: 10.1002/cne.21966. [DOI] [PubMed] [Google Scholar]
- Busch S, Tanimoto H. Cellular configuration of single octopamine neurons in Drosophila. J Comp Neurol. 2010;518:2355–2364. doi: 10.1002/cne.22337. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim J, Carlson JR. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron. 1999;22:327–338. doi: 10.1016/s0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- Cobb M, Domain I. Olfactory coding in a simple nervous system: adaptation in Drosophila larvae. Proc R Soc Lond B Biol Sci. 2000;267:2119–2125. doi: 10.1098/rspb.2000.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. Olfactory learning. Neuron. 2004;44:31–48. doi: 10.1016/j.neuron.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell. 1995;83:195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Gerber B, Stocker RF. The Drosophila larva as a model for studying chemosensation and chemosensory learning: a review. Chem Senses. 2007;32:65–89. doi: 10.1093/chemse/bjl030. [DOI] [PubMed] [Google Scholar]
- Gerber B, Stocker RF, Tanimura T, Thum AS. Smelling, tasting, learning: Drosophila as a study case. Results Probl Cell Differ. 2009;47:139–185. doi: 10.1007/400_2008_9. [DOI] [PubMed] [Google Scholar]
- Gerber B, Tanimoto H, Heisenberg M. An engram found? Evaluating the evidence from fruit flies. Curr Opin Neurobiol. 2004;14:737–744. doi: 10.1016/j.conb.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Gervasi N, Tchenio P, Preat T. PKA dynamics in a Drosophila learning center: coincidence detection by rutabaga adenylyl cyclase and spatial regulation by dunce phosphodiesterase. Neuron. 2010;65:516–529. doi: 10.1016/j.neuron.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Guerrieri F, Schubert M, Sandoz JC, Giurfa M. Perceptual and neural olfactory similarity in honeybees. PLoS Biol. 2005;3:e60. doi: 10.1371/journal.pbio.0030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R, Khan R, Takahashi YK, Mori K, Harel D, Sobel N. A metric for odorant comparison. Nat Methods. 2008;5:425–429. doi: 10.1038/nmeth.1197. [DOI] [PubMed] [Google Scholar]
- Heimbeck G, Bugnon V, Gendre N, Keller A, Stocker RF. A central neural circuit for experience-independent olfactory and courtship behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2001;98:15336–15341. doi: 10.1073/pnas.011314898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M, Gerber B. Learning and memory in Drosophila. In: Menzel R, editor. Learning theory and behavior. Oxford: Elsevier; 2008. pp. 549–559. [Google Scholar]
- Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90:763–773. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- Ito K, Suzuki K, Estes P, Ramaswami M, Yamamoto D, Strausfeld NJ. The organization of extrinsic neurons and their implications in the functional roles of the mushroom bodies in Drosophila melanogaster Meigen. Learn Mem. 1998;5:52–77. [PMC free article] [PubMed] [Google Scholar]
- Kreher SA, Kwon JY, Carlson JR, Hallem EA, Ho MG. The molecular basis of odor coding in the Drosophila larva. Neuron. 2005;46:445–456. doi: 10.1016/j.neuron.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Kreher SA, Mathew D, Kim J, Carlson JR. Translation of sensory input into behavioral output via an olfactory system. Neuron. 2008;59:110–124. doi: 10.1016/j.neuron.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci. 2002;3:884–895. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–255. doi: 10.1016/s0092-8674(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Masse NY, Turner GC, Jefferis GS. Olfactory information processing in Drosophila. Curr Biol. 2009;19:R700–R713. doi: 10.1016/j.cub.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Masuda-Nakagawa LM, Gendre N, O'Kane CJ, Stocker RF. Localized olfactory representation in mushroom bodies of Drosophila larvae. Proc Natl Acad Sci U S A. 2009;106:10314–10319. doi: 10.1073/pnas.0900178106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Nakagawa LM, Tanaka NK, O'Kane CJ. Stereotypic and random patterns of connectivity in the larval mushroom body calyx of Drosophila. Proc Natl Acad Sci U S A. 2005;102:19027–19032. doi: 10.1073/pnas.0509643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami H, Buck LB. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell. 1997;90:775–784. doi: 10.1016/s0092-8674(00)80537-1. [DOI] [PubMed] [Google Scholar]
- Mishra D, Louis M, Gerber B Adaptive adjustment of the generalization-discrimination balance in larval Drosophila. J Neurogenet. 2010;24:168–175. doi: 10.3109/01677063.2010.498066. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes. Anrep GV, translator. Oxford: Oxford University Press; 1927. (translation by G.V. Anrep) [Google Scholar]
- Ramaekers A, Magnenat E, Marin E, Gendre N, Jefferis G, Luo L, Stocker R. Glomerular maps without cellular redundancy at successive levels of the Drosophila larval olfactory circuit. Curr Biol. 2005;15:982–992. doi: 10.1016/j.cub.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Riemensperger T, Voller T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Ryba NJ, Tirindelli R. A new multigene family of putative pheromone receptors. Neuron. 1997;19:371–379. doi: 10.1016/s0896-6273(00)80946-0. [DOI] [PubMed] [Google Scholar]
- Saumweber T, Husse J, Gerber B Forthcoming. Innate attractiveness and associative learnability of odours can be dissociated in larval Drosophila. Chem Senses. 2010;36:223–235. doi: 10.1093/chemse/bjq128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuker M, de Bruyne M, Hähnel M, Schneider G. Predicting olfactory receptor neuron responses from odorant structure. Chem Cent J. 2007;1:11. doi: 10.1186/1752-153X-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuker M, Schneider G. Processing and classification of chemical data inispired by insect olfaction. Proc Natl Acad Sci USA. 2007;104:20285–20289. doi: 10.1073/pnas.0705683104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcho M, Pauls D, Han KA, Stocker RF, Thum AS. The role of dopamine in Drosophila larval classical olfactory conditioning. PloS ONE. 2009;4:e5897. doi: 10.1371/journal.pone.0005897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell CS. On the unpredictability of odor. Angew Chem Int Ed Engl. 2006;45:6254–6261. doi: 10.1002/anie.200600782. [DOI] [PubMed] [Google Scholar]
- Smith BH. The olfactory memory of the honeybee Apis mellifera: I. Odorant modulation of short- and intermediate-term memory after single-trial conditioning. J Exp Biol. 1991;161:367–438. [Google Scholar]
- Stocker RF. Design of the larval chemosensory system. Adv Exp Med Biol. 2008;628:69–81. doi: 10.1007/978-0-387-78261-4_5. [DOI] [PubMed] [Google Scholar]
- Tomchik SM, Davis RL. Dynamics of learning-related cAMP signaling and stimulus integration in the Drosophila olfactory pathway. Neuron. 2009;64:510–521. doi: 10.1016/j.neuron.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R, Denes AS, Tessmar-Raible K, Arendt D. Profiling by image registration reveals common origin of annelid mushroom bodies and vertebrate pallium. Cell. 2010;142:800–809. doi: 10.1016/j.cell.2010.07.043. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Wilson RI. Neural and behavioral mechanisms of olfactory perception. Curr Opin Neurobiol. 2008;18:408–412. doi: 10.1016/j.conb.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.