Abstract
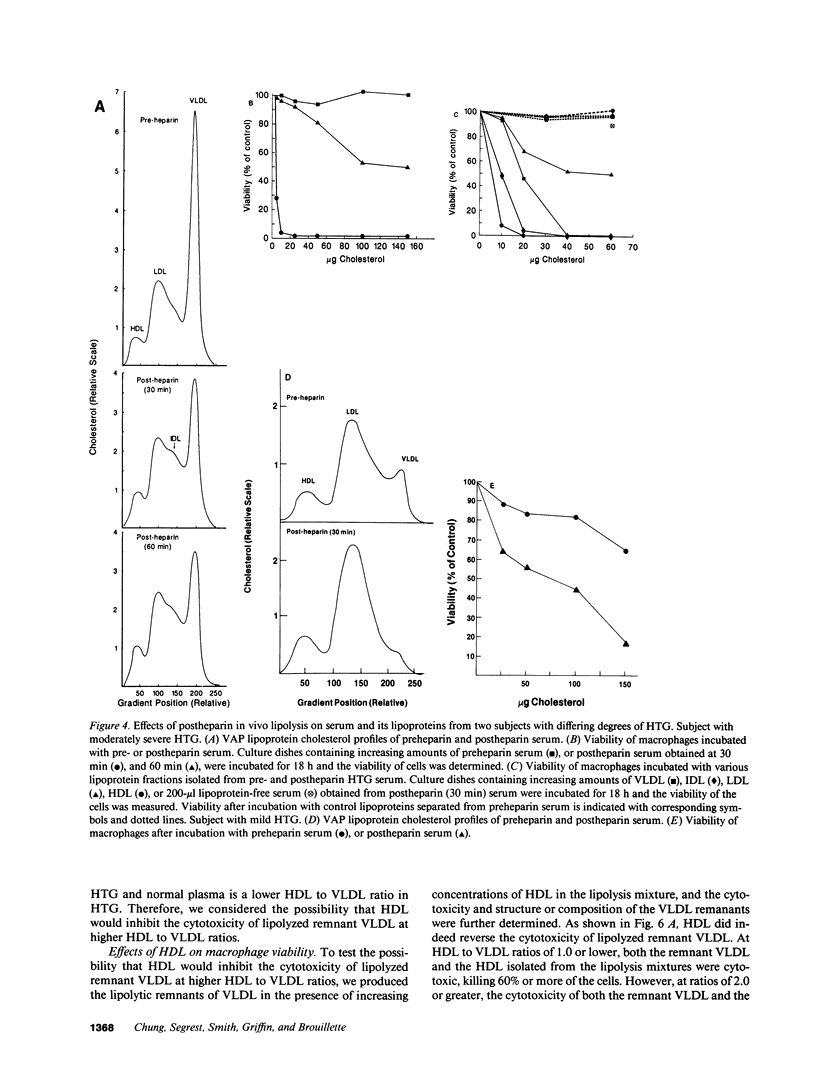
Hypertriglyceridemic (HTG) serum, lipolyzed in vitro by purified bovine milk lipoprotein lipase, was found to be cytotoxic to cultured macrophages. Surviving macrophages contained numerous lipid inclusions similar to those found in foam cells. Individual lipoprotein fractions isolated from the lipolyzed HTG serum, including HDL, were also cytotoxic. Lipolysis of isolated lipoprotein fractions (either HTG or normal) allowed localization of cytotoxicity to postlipolysis remnant VLDL and chylomicron particles. The presence of a critical concentration of HDL in either the lipolysis mixture or the culture dishes inhibited the cytotoxicity. Below this critical concentration HDL itself became cytotoxic, producing lipid inclusions in surviving macrophages. The lipid fraction of the cytotoxic remnants contained the cytotoxic factor(s); neither FFA nor lysolecithin alone could account for this cytotoxicity. Postprandial lipemic sera from subjects with a brisk chylomicron response, when lipolyzed in vitro, were cytotoxic to cultured macrophages; neither fasted sera from these subjects, nor postprandial sera from normolipidemic subjects with a normal chylomicron response, were cytotoxic. Postheparin (in vivo lipolyzed) serum and its isolated lipoprotein fractions obtained 30 min after heparin injection in subjects with HTG were shown to be cytotoxic to macrophages; by 60 min most of the cytotoxicity had disappeared. The postprandial and postheparin observations support an in vivo significance for remnant-associated cytotoxicity. We hypothesize that cytotoxic remnants of lipolyzed VLDL and chylomicrons may be one of the major atherogenic lipoproteins. Further, we suggest that inhibition of the cytotoxicity of these remnants may be one important way that HDL prevents atherosclerosis.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbogast B. W., Gill L. R., Schwertner H. A. A new protective factor in coronary artery disease. Very low density lipoprotein toxicity-preventing activity. Atherosclerosis. 1985 Oct;57(1):75–86. doi: 10.1016/0021-9150(85)90139-x. [DOI] [PubMed] [Google Scholar]
- Bennett D. R. Letter: Women in medical school - 1878. N Engl J Med. 1976 Jul 29;295(5):295–296. doi: 10.1056/NEJM197607292950531. [DOI] [PubMed] [Google Scholar]
- Chait A., Brunzell J. D., Albers J. J., Hazzard W. R. Type-III Hyperlipoproteinaemia ("remnant removal disease"). Insight into the pathogenetic mechanism. Lancet. 1977 Jun 4;1(8023):1176–1178. doi: 10.1016/s0140-6736(77)92717-9. [DOI] [PubMed] [Google Scholar]
- Chung B. H., Segrest J. P., Cone J. T., Pfau J., Geer J. C., Duncan L. A. High resolution plasma lipoprotein cholesterol profiles by a rapid, high volume semi-automated method. J Lipid Res. 1981 Aug;22(6):1003–1014. [PubMed] [Google Scholar]
- Chung B. H., Wilkinson T., Geer J. C., Segrest J. P. Preparative and quantitative isolation of plasma lipoproteins: rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor. J Lipid Res. 1980 Mar;21(3):284–291. [PubMed] [Google Scholar]
- GEER J. C. FINE STRUCTURE OF CANINE EXPERIMENTAL ATHEROSCLEROSIS. Am J Pathol. 1965 Aug;47:241–269. [PMC free article] [PubMed] [Google Scholar]
- Geer J. C., Catsulis C., McGill H. C., Jr, Stron J. P. Fine structure of the baboon aortic fatty streak. Am J Pathol. 1968 Feb;52(2):265–286. [PMC free article] [PubMed] [Google Scholar]
- Geer J. C. Fine structure of human aortic intimal thickening and fatty streaks. Lab Invest. 1965 Oct;14(10):1764–1783. [PubMed] [Google Scholar]
- Gianturco S. H., Eskin S. G., Navarro L. T., Lahart C. J., Smith L. C., Gotto A. M., Jr Abnormal effects of hypertriacylglycerolemic very low-density lipoproteins on 3-hydroxy-3-methylglutaryl-CoA reductase activity and viability of cultured bovine aortic endothelial cells. Biochim Biophys Acta. 1980 Apr 18;618(1):143–152. doi: 10.1016/0005-2760(80)90061-2. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Brown M. S., Innerarity T. L., Mahley R. W. Cholesteryl ester accumulation in macrophages resulting from receptor-mediated uptake and degradation of hypercholesterolemic canine beta-very low density lipoproteins. J Biol Chem. 1980 Mar 10;255(5):1839–1848. [PubMed] [Google Scholar]
- Iverius P. H., Ostlund-Lindqvist A. M. Lipoprotein lipase from bovine milk. Isolation procedure, chemical characterization, and molecular weight analysis. J Biol Chem. 1976 Dec 25;251(24):7791–7795. [PubMed] [Google Scholar]
- Kannel W. B., Castelli W. P., Gordon T. Cholesterol in the prediction of atherosclerotic disease. New perspectives based on the Framingham study. Ann Intern Med. 1979 Jan;90(1):85–91. doi: 10.7326/0003-4819-90-1-85. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., Castelli W. P., Gordon T., McNamara P. M. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann Intern Med. 1971 Jan;74(1):1–12. doi: 10.7326/0003-4819-74-1-1. [DOI] [PubMed] [Google Scholar]
- Kostner G. M., Avogaro P., Cazzolato G., Marth E., Bittolo-Bon G., Qunici G. B. Lipoprotein Lp(a) and the risk for myocardial infarction. Atherosclerosis. 1981 Jan-Feb;38(1-2):51–61. doi: 10.1016/0021-9150(81)90103-9. [DOI] [PubMed] [Google Scholar]
- Kruth H. S., Fry D. L. Histochemical detection and differentiation of free and esterified cholesterol in swine atherosclerosis using filipin. Exp Mol Pathol. 1984 Jun;40(3):288–294. doi: 10.1016/0014-4800(84)90046-7. [DOI] [PubMed] [Google Scholar]
- Kruth H. S. Subendothelial accumulation of unesterified cholesterol. An early event in atherosclerotic lesion development. Atherosclerosis. 1985 Nov;57(2-3):337–341. doi: 10.1016/0021-9150(85)90045-0. [DOI] [PubMed] [Google Scholar]
- Ku D. N., Giddens D. P., Zarins C. K., Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985 May-Jun;5(3):293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Brown M. S., Ho Y. K., Goldstein J. L. Cholesteryl ester synthesis in macrophages: stimulation by beta-very low density lipoproteins from cholesterol-fed animals of several species. J Lipid Res. 1980 Nov;21(8):970–980. [PubMed] [Google Scholar]
- Morel D. W., Hessler J. R., Chisolm G. M. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983 Aug;24(8):1070–1076. [PubMed] [Google Scholar]
- Morganroth J., Levy R. I., Fredrickson D. S. The biochemical, clinical, and genetic features of type III hyperlipoproteinemia. Ann Intern Med. 1975 Feb;82(2):158–174. doi: 10.7326/0003-4819-82-2-158. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Fidge N. H., Tan M. H. Increased lipoprotein-remnant formation in chronic renal failure. N Engl J Med. 1982 Aug 5;307(6):329–333. doi: 10.1056/NEJM198208053070601. [DOI] [PubMed] [Google Scholar]
- Nilsson-Ehle P., Garfinkel A. S., Schotz M. C. Lipolytic enzymes and plasma lipoprotein metabolism. Annu Rev Biochem. 1980;49:667–693. doi: 10.1146/annurev.bi.49.070180.003315. [DOI] [PubMed] [Google Scholar]
- O'Connell P. B., Brady C. J. Polyacrylamide gels with modified cross-linkages. Anal Biochem. 1976 Nov;76(50):63–73. doi: 10.1016/0003-2697(76)90264-5. [DOI] [PubMed] [Google Scholar]
- Ostlund-Lindqvist A. M., Gustafson S., Lindqvist P., Witztum J. L., Little J. A. Uptake and degradation of human chylomicrons by macrophages in culture. Role of lipoprotein lipase. Arteriosclerosis. 1983 Sep-Oct;3(5):433–440. doi: 10.1161/01.atv.3.5.433. [DOI] [PubMed] [Google Scholar]
- Patsch J. R., Gotto A. M., Jr, Olivercrona T., Eisenberg S. Formation of high density lipoprotein2-like particles during lipolysis of very low density lipoproteins in vitro. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4519–4523. doi: 10.1073/pnas.75.9.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalde H. R. A simple method of obtaining monocytes in suspension. J Immunol Methods. 1984 Apr 13;69(1):71–77. doi: 10.1016/0022-1759(84)90278-3. [DOI] [PubMed] [Google Scholar]
- Scow R. O., Blanchette-Mackie E. J., Smith L. C. Role of capillary endothelium in the clearance of chylomicrons. A model for lipid transport from blood by lateral diffusion in cell membranes. Circ Res. 1976 Aug;39(2):149–162. doi: 10.1161/01.res.39.2.149. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Vasile E., Lupu F., Popescu G., Simionescu M. Prelesional events in atherogenesis. Accumulation of extracellular cholesterol-rich liposomes in the arterial intima and cardiac valves of the hyperlipidemic rabbit. Am J Pathol. 1986 Apr;123(1):109–125. [PMC free article] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. C. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980 May 1;104(1):10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- Tatami R., Mabuchi H., Ueda K., Ueda R., Haba T., Kametani T., Ito S., Koizumi J., Ohta M., Miyamoto S. Intermediate-density lipoprotein and cholesterol-rich very low density lipoprotein in angiographically determined coronary artery disease. Circulation. 1981 Dec;64(6):1174–1184. doi: 10.1161/01.cir.64.6.1174. [DOI] [PubMed] [Google Scholar]
- Van Lenten B. J., Fogelman A. M., Jackson R. L., Shapiro S., Haberland M. E., Edwards P. A. Receptor-mediated uptake of remnant lipoproteins by cholesterol-loaded human monocyte-macrophages. J Biol Chem. 1985 Jul 25;260(15):8783–8788. [PubMed] [Google Scholar]
- Zilversmit D. B. A proposal linking atherogenesis to the interaction of endothelial lipoprotein lipase with triglyceride-rich lipoproteins. Circ Res. 1973 Dec;33(6):633–638. doi: 10.1161/01.res.33.6.633. [DOI] [PubMed] [Google Scholar]















