Abstract
Prostate and breast cancer are hormone dependent malignancies of the aging male and female and require the local production of androgens and estrogens to stimulate cell proliferation. Aldo-keto reductases (AKR) play key roles in this process. In the prostate, AKR1C3 (type 5 17β-HSD) reduces Δ4-androstene-3,17-dione to yield testosterone while AKR1C2 (type 3 3α-HSD) eliminates 5α-dihydrotestosterone (5α-DHT), and AKR1C1 forms 3β-androstanediol (a ligand for ERβ). In the breast, AKR1C3 forms testosterone which is converted to 17β-estradiol by aromatase or reduces estrone to 17β-estradiol directly. AKR1C3 also acts as a prostaglandin (PG) F synthase and forms PGF2α and 11β-PGF2α, which stimulate the FP receptor, and prevent the activation of PPARγ by PGJ2 ligands. This pro-proliferative signaling may stimulate the growth of hormone dependent and independent prostate and breast cancer
Keywords: aldo-keto reductase, hydroxysteroid dehydrogenase, nonsteroidal anti-inflammatory drugs, prostaglandin F, nuclear receptors
Introduction
Cancer of the prostate and breast are the leading hormone dependent malignancies in the aging male and female.1,2 Both cancers occur as the production of gonadal steroids wanes, which suggests that the local production of androgens and estrogens in the prostate and breast, respectively, drive cell proliferation. These diseases are initially responsive to hormone ablative therapy (receptor antagonists, and inhibitors of steroid hormone biosynthesis), but both can become refractory to this therapy, leading to the clonal expansion of hormone insensitive malignant cells.3–6 The identity of enzymes involved in the local production of androgens and estrogens and changes that occur in their expression in hormone and hormone-insensitive disease states is thus of significant interest. The focus of this article is on the 3-, 17- and 20-ketosteroid reductases of the aldo-keto reductase (AKR) superfamily that regulate the local production of androgens, estrogens and progestins, which bind to their respective nuclear receptors in the prostate and breast (see www.med.upenn.edu/akr).7,8
Properties of Steroid Hormone Transforming Aldo-Keto Reductases
AKR enzymes are soluble 37 kDa, monomeric, NAD(P)(H) dependent oxidoreductases that interconvert carbonyl groups with alcohols. These enzymes share a common protein fold, an (α/β8)-barrel also known as a triose-phosphate isomerase (TIM)-barrel, and contain a conserved catalytic tetrad of Tyr 55, Asp 50, Lys 84 and His 1177,8. In humans, 13 isoforms exist, four of which (AKR1C1-AKR1C4) reduce ketosteroids to hydroxysteroids,9 Table 1. Each of the AKR1C genes are located on chromosome 10p15–10p14 and share >86% amino acid sequence identity, yet they show distinct preference for the positions of the steroid nucleus and side-chain on which they work. AKR1C1 shows preference for 20-ketosteroid reduction and inactivates progesterone by forming 20α-hydroxyprogesterone.10 AKR1C2 shows preference for 3-ketosteroid reduction and inactivates 5α-dihydrotestosterone (5α-DHT) by forming 3α-androstanediol (3α-diol).11 AKR1C3 shows preference for 17-ketosteroid reduction and converts Δ4-androstene-3,17-dione to testosterone and to a lesser extent estrone to 17β-estradiol.12,13. AKR1C4 also converts 3-ketosteroids to 3α-hydroxysteroids.
Table 1.
Human Aldo-Keto Reductases involved in steroid hormone metabolism
| Gene | Protein | Favored Reactions | % Sequence Identity |
Chromosomal Localization |
|---|---|---|---|---|
| AKR1C1 | 20α(3β)-hydroxysteroid dehydrogenase (AKR1C1) |
Progesterone → 20α-hydroxyprogesterone 5α-dihydrotestosterone → 3β-androstanediol |
100% | 10p15-10p14 |
| AKR1C2 | Type 2 3α-hydroxysteroid dehydrogenase (AKR1C2) |
5α-dihydrotestosterone → 3α-androstanediol | 98% | 10p15-10p14 |
| AKR1C3 | Type 5 17β-hydroxysteroid dehydrogenase Prostaglandin (PG) F synthase (AKR1C3) |
Δ4-androstene-3,17-dione → testosterone PGH2→ PGF2α PGD2→ 11β-PGF2 |
86% | 10p15-10p14 |
| AKR1C4 | Type 1 3α-hydroxysteroid dehydrogenase (AKR1C4) |
3-ketosteroid → 3α-hydroxysteroid | 86% | 10p15-10p14 |
| AKR1D1 | Steroid 5β-reductase (AKR1D1) | Δ4-3-ketosteroid → 5β-dihydrosteroid | 57% | 7q32-7q33 |
Differences in AKR1C substrate preference can be explained by existing crystal structures. Using rat AKR1C9 [3α-hydroxysteroid dehydrogenase (HSD)]•NADP+•testosterone complex as a template,14 it is apparent that the binding of NADP(H) is strictly conserved so that the 4-pro-R-hydride is always transferred to the acceptor ketosteroid substrate. Thus differences in positional and stereospecificity can only be accounted for if the orientation of steroid substrate is altered. 17-Ketosteroid substrates must bind backwards and upside down (angular methyl groups inverted) relative to testosterone in the AKR1C9 structure for 17β-hydroxysteroids to form. Similarly, 20-ketosteroid substrates must bind backwards relative to testosterone in the AKR1C9 structure for 20α-hydroxysteroids to form. Evidence for these different modes of steroid binding are seen by comparing the AKR1C9 structure to the ternary complex structure AKR1C2•NADP+•Ursodeoxycholate,15 where the bile acid, a potent competitive inhibitor, is bound both backwards and upside down relative to the position of the steroid in the AKR1C9•NADP+•testosterone ternary complex structure.
Although AKR1C enzymes can oxidize hydroxysteroids in vitro, the accumulated evidence indicates that they work primarily as ketosteroid reductases in vivo. The evidence is as follows. First, the Kd values for NADPH and NAD+ are 120 nM and 200µM, respectively and overwhelming favor the binding of the major reductive cofactor at concentrations that prevail within the cell. 16,17 Second, measurement of the Keq or calculation of the kinetic Haldane shows that the equilibrium constant of these reactions favors ketosteroid reduction; Keqs are of the order of 8- 22.17,18 Third, in transfection studies in COS-1 cells where the enzymes are forced to use the prevailing concentrations of cofactor the enzymes only perform ketosteroid reduction.11 Fourth, in vitro NAD+-dependent driven hydroxysteroid oxidation is potently inhibited by 1– 10 µM NADPH.11 In vitro, hydroxysteroid oxidation only goes to completion when mM NAD+ and pH 9–10 are used to force the reaction. Thus under normal physiologic conditions the reduction reaction predominates.
Whether ketosteroid reduction catalyzed by AKRs will have an impact on local steroid hormone biosynthesis is governed by their tissue specific expression patterns. Using an isoform specific semi-quantitative RT-PCR protocol, the expression of AKR1C1–AKR1C4 was measured across nine human tissues.9 All four isoforms were expressed in liver. However, AKR1C4 was liver specific suggesting that its dominant role is in hepatic steroid metabolism. By contrast AKR1C1, AKR1C2 and AKR1C3 were highly expressed in prostate and mammary gland.
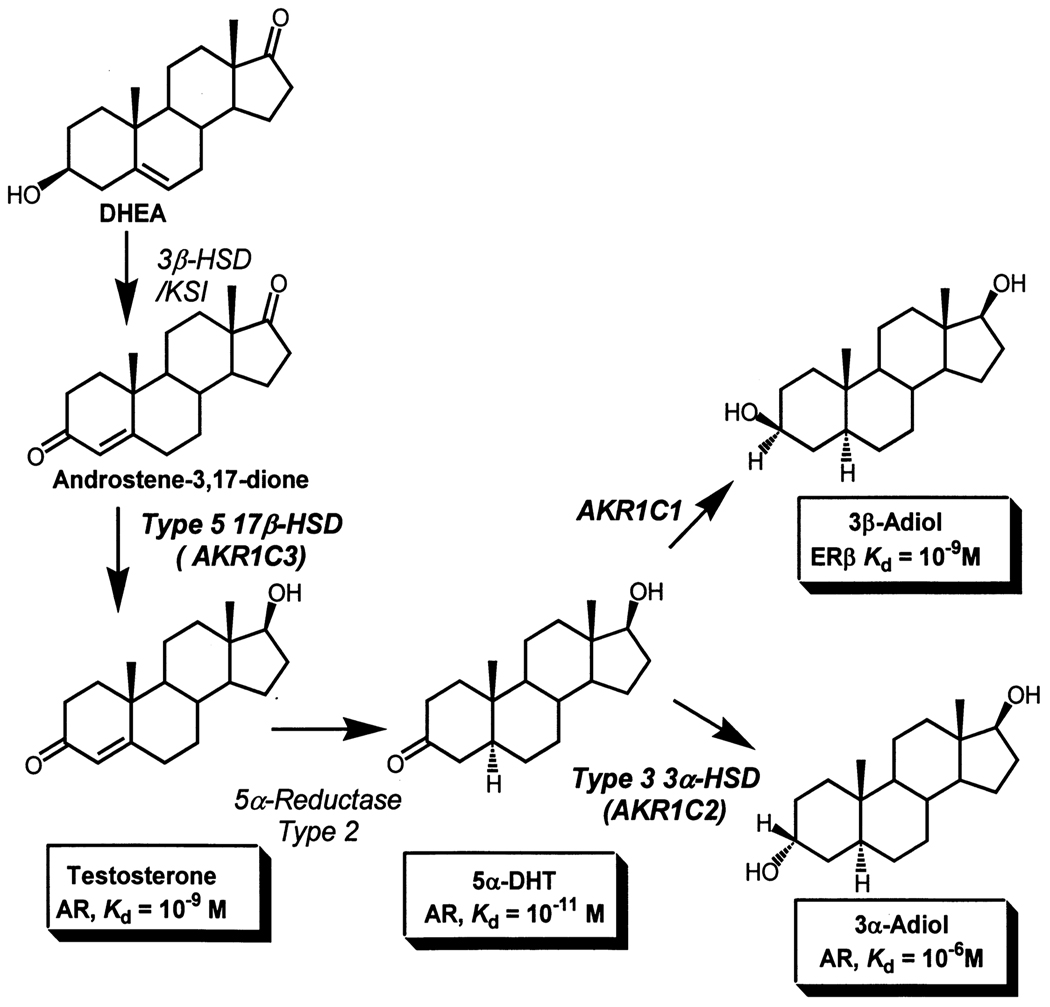
Roles of AKR1C1, AKR1C2 and AKR1C3 in Prostate
In elaborating the roles of AKR1C1-AKR1C3 in prostate, Steckelbroeck and co-workers used discriminating TLC systems and made the unexpected discovery that AKR1C1, AKR1C2 and AKR1C3 have both 3α- and 3β-HSD activities.19 As a result they reduced 5α-DHT (a potent androgen) to yield either 3α-andostanediol (3α-diol; an inactive androgen) or 3β-androstanediol (3β-diol; a pro-apoptotic ligand for ERβ) [20]. Examination of the ratio of specific activities of 3α-HSD : 3β-HSD at saturating 5α-DHT concentrations gave the following ratios in parenthesis: AKR1C1 (0.23); AKR1C2 (17.0); AKR1C3 (1.4), Table 2. Based on these ratios, the absolute specific activity values, and expression in the prostate, it was concluded that AKR1C1 would be the source of 3β-androstanediol, and AKR1C2 would inactivate the potent androgen 5α-DHT, Figure 1. The ability of AKR1C2 to inactivate 5α-DHT was also supported by transfection studies of AKR1C2 into COS-1 cells and human prostate PC-3 cells that showed that the conversion of 5α-DHT to 3α-androstanediol occurred smoothly.11
Table 2.
Specific activities for the reduction of 5α-dihydrotestosterone into 3α/3β-androstanediol catalyzed by AKR1C1-AKR1C2
| AKR1C1 | AKR1C2 | AKR1C3 | AKR1C4 | |
|---|---|---|---|---|
| Products | Specific activity (nmoles product min−1 mg−1) | |||
| 3α-androstanediol | 2.8 | 75.6 | 2.7 | 102.0 |
| 3β-androstanediol | 12.4 | 4.4 | 2.0 | 29.8 |
| Ratio specific activities 3α-HSD : 3β-HSD |
0.22 | 17.2 | 1.35 | 3.4 |
Figure 1.
Central role of human AKR1C enzymes in androgen metabolism in the prostate; AR, androgen receptor; ERβ, estrogen receptor.
In the adult aging male, the main source of prostatic androgens is adrenal dehydroepiandrosterone (DHEA).1 Local conversion of DHEA to testosterone within the prostate requires the action of 3β-HSD/ketosteroid isomerase to yield Δ4-androstene-3,17-dione, and the action of a reductive 17β-HSD to produce testosterone. As 17β-HSD type 3 is mainly a Leydig cell specific enzyme, 21,22 the candidate enzyme to perform this reaction in prostate is AKR1C3, which was originally cloned from a human prostatic cDNA library.12 The combined action of AKR1C1, AKR1C2 and AKR1C3 in prostate androgen metabolism prompted detailed expression analysis in this gland.
Using a monoclonal, monospecific anti-human AKR1C3 antibody, AKR1C3 expression was seen in stromal cells, endothelial cells, trans-uroepithelial cells and adenocarcinoma cells in the prostate.23–25 Bauman et al, also conducted transcript profiling using primary cultures of epithelial and stromal cells from normal prostate, prostate cancer and BPH and compared expression levels of AKR1C isoforms with nuclear receptors (AR, ERα and ERβ).26 Normal prostate epithelial cells (n = 14) had higher levels of AKR1C1 (10-fold, p< 0.001), AKR1C2 (115-fold, p< 0.001) and AKR1C3 (6-fold, p < 0.001) than normal stromal cells, suggesting that reductive androgen metabolism occurs preferentially in the epithelial cells. All three enzymes showed an increase in expression in epithelial cells in prostate cancer and BPH that did not reach significance. In BPH, prostate stromal cells (n =21) showed uniform higher levels of expression of AKR1C1 (4-fold p < 0.001); AKR1C2 (10-fold p< 0.001) and AKR1C3 (4-fold, p < 0.001) than normal stromal cells, showing that reductive androgen metabolism had increased. But even with these increases, the levels were still significantly lower than those found in normal epithelial cells.
The ratio of AKR1C1:AKR1C2 mRNA expression was also examined since this would predict the ability to produce either 3β-diol or 3α-diol in each cell type. In whole prostate this ratio is 13, but in prostate epithelial cells this ratio was 1.9 and this decreased ratio was unchanged in epithelial cells from prostate cancer and BPH. In stromal cells the AKR1C1 : AKR1C2 mRNA ratio was 22 and declined to 13 in prostate cancer and 9.0 in BPH. These studies show that prostate epithelial cells contain less AKR1C1 transcript than stromal cells. Moreover, stromal cells from diseased prostate make less 3β-diol which could act as pro-apoptotic siganl in adjacent epithelial cells
The ratio of AR : ERβ was also examined. In normal prostate epithelial cells the ratio is 8, while in prostate stromal cells this ratio was 280. The difference in this ratio between the two cell types was maintained in prostate cancer and BPH. The low levels of AKR1C1 expression and high levels of ERβ expression in prostate epithelial cells suggest that in these proliferative cells, ERβ is deprived of its ligand. Thus a place exists for a SERM that selectively activates ERβ in prostate cancer.
The ratio of AR :ERα in normal prostate was also measured since ERα often has opposing actions to ERβ. In normal prostate epithelial cells this ratio was 6 and increased to 30 in prostate cancer and was 6 in BPH. In normal stromal cells the AR : ERα ratio was 50, and increased to 60 and 180 in prostate cancer and BPH, respectively. These represent significant differences in the balance between AR and ERa expression in stromal cells and suggests that BPH will be responsive to AR receptor antagonists.
AKR1C expression was also measured by microarray analysis in metastatic prostate cancer that was insensitive to androgen ablative therapy.3 It was found that there was coordinated increase in the expression of AK1R1C3 (5.3-fold), AKR1C2 (3.4 fold), AKR1C1 (3.1 fold) and 5α-reductase type 2 (2.1 fold) over that seen in primary prostate tumors, which was validated by real time quantitative PCR. These data support the hypothesis that androgen independent prostate cancer undergoes an adaptive response to androgen ablative therapy by increasing local androgen production, which cannot be effectively surmounted by a 5α-reductase type 2 inhibitor or androgen receptor antagonist. Thus the disease is insensitive to androgen ablation even though its is still androgen dependent. This conclusion is supported by the success of a recent clinical trial of the cytochrome P450 (CYP) 17 inhibitor abiraterone acetate, which confirms that castration-resistant prostate cancer commonly remains hormone driven.27
Roles of AKR1C1, AKR1C2 and AKR1C3 in Breast
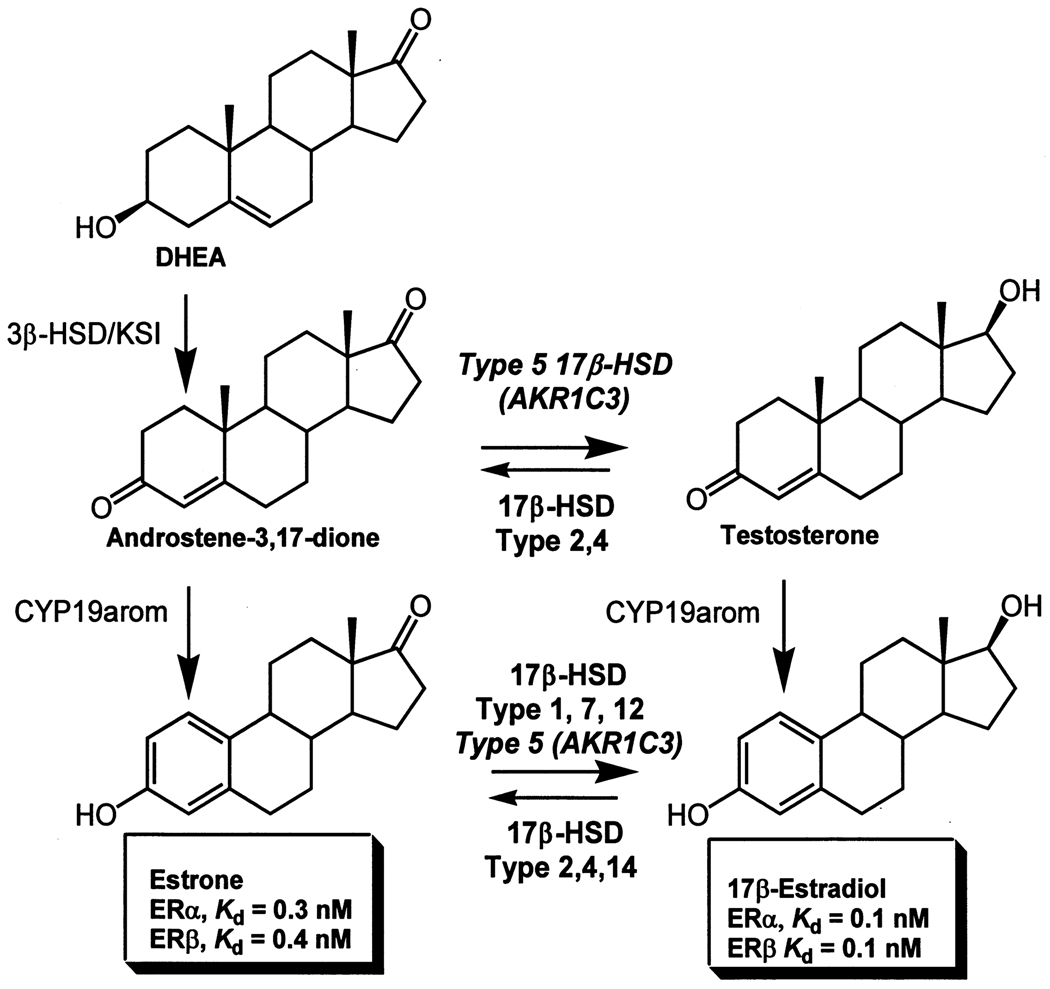
The 17-ketosteroid reductase activity of AKR1C3 is believed to create a pro-estrogenic state in the breast.9 Conversion of Δ4-androstene-3,17-dione to testosterone by AKR1C3, followed by aromatization by CYP19 aromatase provides a route to 17β-estradiol independent of the reductive type 1 17β-HSD, Figure 2. In addition, conversion of progesterone (active progestin) to 20α-hydroxyprogesterone (inactive progestin) provides a route to alter the estrogen : progesterone ratio in the breast. AKR1C3 has been shown to be consistently over-expressed by immunohistochemistry in ductal carcinoma in situ in the breast, and invasive ductal carcinoma.24,28,29 In addition, high expression of AKR1C3 in breast cancer is significantly associated with a poor prognosis30 and increased rate of occurrence beyond 5 years.29
Figure 2.
Role of AKR1C3 in the local production of 17β-estradiol in the breast.
To further examine the role of AKR1C3 in a breast cancer cell model, ectopic expression of AKR1C3 in MCF-7 cells was performed using a retroviral pLNCX-AKR1C3 vector. The MCF7-AKR1C3 transfectants expressed AKR1C3 at levels of 0.01% to 0.1% of the soluble protein and these values were similar to those observed for HeLa cell expression. These results suggest that levels of expression attained were not supra-physiologic. Metabolism studies of radiolabeled Δ4-androstene-3,17-dione, progesterone and estrone were performed in one of these cell lines using 0.1 µM and 5.0 µM steroid (Byrns and Penning, unpublished data). As expected, reduction of Δ4-androstene-3,17-dione to testosterone and progesterone to 20α-hydroxyprogesterone was observed. Further conversion of testosterone to 17β–estradiol was not observed since these cells poorly express CYP19 aromatase.31 Unexpectedly, AKR1C3 catalyzed rapid reduction of 0.1 µM estrone to 17β-estradiol, and this reaction was preferred over the other substrates tested at this low concentration. By contrast, the reduction of estrone to 17β-estradiol at 5 µM was not significantly different from the rate seen in the parental cells. These data suggest that AKR1C3 is a high affinity, low capacity reductive 17β-HSD that will synthesize 17β-estradiol when challenged with physiological concentrations of estrone.
Hormone dependent proliferation of MCF-7 AKR1C3 transfectants was also examined. Cell proliferation by picomolar concentrations of estrone was enhanced in the AKR1C3 transfectants over that seen in the parental cells, and a similar result was observed with 17β-estradiol. Both effects can be explained if the equilibrium between endogenous reductive and oxidative 17β-HSDs has been disturbed to favor higher steady state concentrations of 17β-estradiol. Estrogen dependent proliferation of MCF-7 cells was blocked by 4-hydroxytamoxifen indicating that the effects seen were ERα dependent.
AKR1C3 and Prostaglandin F Synthase Activity
Examination of the steady-state kinetic parameters of AKR1C3 in vitro showed that its abilities to reduce prostaglandin (PG) H2 to PGF2α, and PGD2 to 11β-PGF2 (prostaglandin F synthase activity) were its preferred reactions based on catalytic efficiency,32–34 Table 3. These reactions could lead to a cell-proliferation phenotype in prostate and breast cancer irrespective of whether the disease is hormone sensitive or insensitive. Formation of these PGF2 epimers could lead to activation of the FP-receptor, MAPK, and phosphorylation of transcription factors, e.g. PPARγ (leading to inactivation) or phosphorylation of NFkB (leading to its activation). These events would lead to cell proliferation. In addition, reduction of PGD2 prevents its dehydration and subsequent isomerization to produce PGJ2 and 15-deoxy-Δ12,14-PGJ2 (15-dPGJ2), which are putative ligands for PPARγ [35]. The reactive cyclopentenone of 15-dPGJ2 can perform selective covalent modification of nuclear receptors including, PPARγ (activation) and NFkB (inactivation), leading to cellular differentiation and an anti-proliferative phenotype.
Table 3.
Steady-state kinetic parameters for reactions catalyzed by AKR1C3
| Substrate | Km(µM) | kcat (min−1) | kcat/Km (min−1 mM−1) |
|---|---|---|---|
| 5α-Dihydrotestosterone (R) | 20 | 0.26 | 13 |
| Δ4-Androstene-3,17-dione (F) | 5.3 | 0.31 | 58 |
| Progesterone (F) | 20 | 0.60 | 30 |
| Prostaglandin D2 (F) | 1.1 | 1.30 | 1200 |
R = radiometric determination; F = fluorimetric determination
We examined the ability of AKR1C3 expression to reduce PGD2 in MCF-7 cells. Radiometric PGD2 spontaneously dehydrated to form PGJ2 and 15dPGJ2 in cell-free media and parental MCF-7 cells. In the presence of cells, much of the radioactivity remained in the aqueous fraction, possibly the result of reaction of the J2 products with cellular nucleophiles. The expression of AKR1C3 resulted in formation of a PGF2 product, with a corresponding increase in the disappearance of the PGD2 substrate. In order to definitively identify this product, we performed chiral-phase liquid chromatography-electron capture atmospheric pressure chemical ionization/multiple reaction monitoring/mass spectrometry of pentafluorobenzyl derivatives.36 With this method, the product formed in the AKR1C3 expressing cells was shown to be 11β-PGF2. A growth phenotype has yet to be associated with this PG reaction, but its absence could be related to the lack of the FP receptor in MCF-7 cells.
AKR1C3 Inhibitors in Prostate and Breast Cancer
The potential role of AKR1C3 in the proliferation of hormone dependent and independent malignancies of the prostate and breast suggests that selective AKR1C3 inhibitors would be desirable. Nonsteroid based scaffolds are preferred to reduce the potential to inhibit other steroid transforming enzymes or to act as antagonists of nuclear receptors. Twenty-five years ago nonsteroidal anti-inflammatory drugs (NSAIDs) were shown to be potent competitive inhibitors of AKR1C9,37 a finding that also applies to the human AKR1C1-AKR1C3 isoforms.38 Advances have been made to develop NSAID analogs that are selective for AKR1C3 yet do not inhibit COX-1 or COX-2. Two scaffolds have been used to achieve this objective, the N-phenylanthranilates and the indoleacetic acids.38,39 In the former case substitution of the A-ring was sufficient to abolish COX-1 and COX-2 inhibition but maintain AKR1C inhibition. However, only marginal selectivity between the various AKR1C isoforms was established. In another approach, the finding that indomethacin is a selective inhibitor of AKR1C3 was exploited and led to the development of 4-chlorobenzoyl melatonin. This compound exhibited a Ki = 8.0 µM for competitive inhibition of Δ4-androstene-3,17-dione reduction and minimal inhibition of AKR1C1 and AKR1C2 (IC50 > 100 µM) was observed.
Conclusions and Future Directions
AKR1C1 is a dominant 20α-HSD involved in the elimination of progesterone. Together with AKR1C3 these enzymes likely control the estradiol : progesterone ratios formed locally in the endometrium and mammary gland. AKR1C2 is the dominant 3α-HSD involved in the elimination of 5α-DHT from the prostate, while AKR1C1 is the 3β-HSD that would make 3β-diol. While one report indicates that AKR1C2 expression is reduced in prostate cancer,40 these studies were not confirmed in discrete cells isolated by laser capture microdissection where increased expression was associated with androgen resistance.3 Further studies are required to elucidate this point.
Numerous reports have validated the over expression of AKR1C3 in prostate and breast cancer.3,13,25,28–30,41 However, the question arises as to whether it is the major reductive 17β-HSD capable for forming testosterone in both tissues, and whether it has a direct role in 17β-estradiol production in the breast. Until recently, AKR1C3 was considered the only peripheral 17β-HSD responsible for significant testosterone production. However, a recent study also detected 17β-HSD type 3 in castration-resistant prostate cancer.42 This was an unexpected finding since this enzyme was thought to be Leydig cell specific.21,22 By contrast 17β-HSD type 1 has been considered to be the major reductive enzyme responsible for the production of 17β-estradiol in the breast.43 But studies on pathological specimens and on breast cancer cell lines suggest that different expression patterns exist for the 17β-HSD reductive isoforms (type 1, 5, 7 and 12) and that each may contribute in a given setting28–30,43 Taken together these findings suggest that molecular pathology of prostate and breast cancer biopsies should extent beyond nuclear receptor status and report the expression profile of 17β-HSD isoforms so that hormone ablative therapy targeting these isoforms can be individualized to the tumor.
The fact that AKR1C3 is implicated in hormone dependent and independent malignancies and that it is potently inhibited by an approved drug, e.g. indomethacin, suggests that this is an opportunity too good to pass by. NSAIDs block aromatase expression through both COX-2 dependent and COX-independent mechanisms.44,45 NSAID inhibition of AKR1C3 now represents an additional non-COX target for the therapy of breast cancer, which could perhaps be extended to prostate cancer. To advance this prospect, androgen dependent prostate cancer cells transfected with AKR1C3 (LNCaP-AKR1C3) and estrogen dependent breast cancer cells transfected with AKR1C3 should be shown to be growth stimulated with Δ4-androstene-3,17-dione in cell culture and in xeno-transplants, and the growth proliferation phenotype should be attenuated with indomethacin and NSAID analogs that do not inhibit COX-1 or COX-2.
References
- 1.Labrie FA, Belanger, Simard J. Intracrinology: Autonomy and freedom of peripheral tissues. Annuals Endocrinology. 1995;56:23–29. [PubMed] [Google Scholar]
- 2.Labrie F, Luu-The V, Lin SX, et al. Intracrinology: role of the family of 17β-hydroxysteroid dehydrogenases in human physiology and disease. J. Mol. Endocrinol. 2000;25:1–16. doi: 10.1677/jme.0.0250001. [DOI] [PubMed] [Google Scholar]
- 3.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 4.Fujimoto N, Miyamoto H, Mizokami A, et al. Prostate cancer cells increase androgen sensitivity by increase in nuclear androgen receptor and androgen receptor co-activators: a possible mechanism of hormone-resistance of prostate cancer cells. Cancer Invest. 2007;25:32–37. doi: 10.1080/07357900601130698. [DOI] [PubMed] [Google Scholar]
- 5.Jordan CV, Leows-Wambi J, Kim H, et al. Exploiting the apoptotic actions of oestrogens to reverse antihormonal drug resistance in oestrogen receptor positive breast cancer patients. Breast. 2007;16 Suppl2:S105–S113. doi: 10.1016/j.breast.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sengupta S, Jordan VC. Selective estrogen modulators as an anticancer tool: mechanism of efficiency and resistance. Adv. Exp. Med. Biol. 2008;630:206–219. doi: 10.1007/978-0-387-78818-0_13. [DOI] [PubMed] [Google Scholar]
- 7.Jez JM, Bennett MJ, Schlegel BP, et al. Comparitive anatomy of the aldo-keto reductase superfamily. Biochem. J. 1997;326:625–636. doi: 10.1042/bj3260625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jez JM, Penning TM. The aldo-keto reductase (AKR) superfamily: an update. Chemico-Biological Interactions. 2001;130–132:499–527. doi: 10.1016/s0009-2797(00)00295-7. [DOI] [PubMed] [Google Scholar]
- 9.Penning TM, Burczynski ME, Jez JM, et al. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem. J. 2000;351:67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizner T, Smuce T, Rupreht R, et al. AKR1C1 and AKR1C3 may determine progesterone and estrogen action in endometrial cancer. Mol. Cell. Endcorinol. 2006;248:126–135. doi: 10.1016/j.mce.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Rizner T, Lin H-K, Peehl DM, et al. Human type 3 3α-hydroxysteroid dehydrogenase (AKR1C2) and androgen metabolism in prostate cells. Endocrinology. 2003;144:2922–2932. doi: 10.1210/en.2002-0032. [DOI] [PubMed] [Google Scholar]
- 12.Lin H-K, Jez JM, Schlegel BP, et al. Expression and characterization of recombinant type 2 3α-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3α/17β-HSD activity and cellular distribution. Mol. Endocrinol. 1997;11:971–984. doi: 10.1210/mend.11.13.0026. [DOI] [PubMed] [Google Scholar]
- 13.Penning TM, Steckelbroeck S, Bauman DR, et al. Aldo-keto reductase (AKR) 1C3: Role in prostate disease and the development of specific inhibitors. Mol. Cell. Endocrinol. 2006;248:182–191. doi: 10.1016/j.mce.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Bennett MJ, Albert RH, Jez JM, et al. Steroid recognition and regulation of hormone action: crystal structure of testosterone and NADP+ bound to 3α-hydroxysteroid/dihydrodiol dehydrogenase. Structure. 1997;5:799–812. doi: 10.1016/s0969-2126(97)00234-7. [DOI] [PubMed] [Google Scholar]
- 15.Jin Y, Stayrook SE, Albert RH, et al. Crystal structure of human type 3 3α-hydroxysteroid dehydrogenase/bile acid binding protein complexed with NADP+ and ursodeoxycholate. Biochemistry. 2001;40:10161–101688. doi: 10.1021/bi010919a. [DOI] [PubMed] [Google Scholar]
- 16.Ma H, Ratnam K, Penning TM. Mutation of nicotinamide pocket residues in rat liver 3α-hydroxysteroid dehydrogenase reveals different modes of cofactor binding. Biochemistry. 2000;39:102–109. doi: 10.1021/bi991659o. [DOI] [PubMed] [Google Scholar]
- 17.Jin Y, Penning TM. Multiple steps determine the overall rate of the reduction of 5α-dihydrotestosterone catalyzed by human type 3 3α-hydroxysteroid dehydrogenase: implications for the elimination of androgens. Biochemistry. 2006;45:13054–13063. doi: 10.1021/bi060591r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper WC, Jin Y, Penning TM. Elucidation of a complete kinetic mechanism for a mammalian hydroxysteroid dehdyrogenase (HSD) and identification of all enzyme forms on the reaction coordinate: The example of rat liver 3 -HSD (AKR1C9) J. Biol. Chem. 2007;282:33484–33493. doi: 10.1074/jbc.M703414200. [DOI] [PubMed] [Google Scholar]
- 19.Steckelbroeck S, Jin Y, Gopishetty S, et al. Human cytosolic 3α-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3β-hydroxysteroid dehydrogenase activity: Implications for steroid hormone metabolism and action. J. Biol. Chem. 2003;279:10784–10795. doi: 10.1074/jbc.M313308200. [DOI] [PubMed] [Google Scholar]
- 20.Guerini V, Sau D, Scaccianoce E, et al. The androgen derivative 5α-androstane-3β,17β-diol inhibits prostate cancer cell migration through activation of the estrogen receptor beta subtype. Cancer Res. 2005;65:5445–5453. doi: 10.1158/0008-5472.CAN-04-1941. [DOI] [PubMed] [Google Scholar]
- 21.Andersson S, Geissler WM, Patel S, et al. The molecular biology of androgenic 17β-hydroxysteroid dehydrogenases. J Steroid Biochem Mol Biol. 1995;53:37–39. doi: 10.1016/0960-0760(95)00039-3. [DOI] [PubMed] [Google Scholar]
- 22.Andersson S, Geissler WM, Wu L, et al. Molecular genetics and pathophysiology of 17β-hydroxysteroid dehydrogenase 3 deficiency. J Clin Endocrinol Metab. 1996;81:130–136. doi: 10.1210/jcem.81.1.8550739. [DOI] [PubMed] [Google Scholar]
- 23.Pelletier G, Luu-The V, Ei-Alfy M, et al. Immunoelectron microscopic localization of 3b-hydroxysteroid dehydrogenase and type 5 17β-hydroxysteroid dehydrogenase in the human prostate and mammary gland. J. Mol. Endocrinol. 2001;26:11–19. doi: 10.1677/jme.0.0260011. [DOI] [PubMed] [Google Scholar]
- 24.Lin H-K, Steckelbroeck S, Fung KM, et al. Characterization of a monoclonal antibody for human aldo-keto reductase AKR1C3 (type 2 3α-hydroxysteroid dehydrogenase/type 5 17β-hydroxysteroid dehydrogenase); immunohistochemical detection in breast and prostate. Steroids. 2004;69:795–801. doi: 10.1016/j.steroids.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Fung K, Samara EN, Wong C, et al. Increased expression of type 2 3alpha-hydroxysteroid dehydrogenase/type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) and its relationship with androgen receptor in prostate carcinoma. Endocrin. Relat. Cancer. 2006;13:169–180. doi: 10.1677/erc.1.01048. [DOI] [PubMed] [Google Scholar]
- 26.Bauman DR, Steckelbroeck S, Peehl DM, et al. Transcript profiling of the androgen signal in normal prostate, benign prostatic hyperplasia, and prostate cancer. Endocrinology. 2006;147:5806–5816. doi: 10.1210/en.2006-0627. [DOI] [PubMed] [Google Scholar]
- 27.Attard G, Reid AHM, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone actetae, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clinical Oncology. 2008 doi: 10.1200/JCO.2007.15.9749. (Epub ahead of print, July 21, 2008] [DOI] [PubMed] [Google Scholar]
- 28.Sasano H, Suzuki T, Miki Y, et al. Intracrinology of estrogens and androgens in breast carcinoma. J. Steroid. Biochem. Mol. Biol. 2008;108:181–185. doi: 10.1016/j.jsbmb.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Jansson AK, Gunnarsson C, Cohen M, et al. 17β-Hydroxysteroid dehydrogenase 14 affects estradiol levels in breast cancer cells and is a prognostic marker in estrogen receptor positive breast cancer. Cancer Res. 2006;66:11471–11477. doi: 10.1158/0008-5472.CAN-06-1448. [DOI] [PubMed] [Google Scholar]
- 30.Oduwole OO, Li Y, Isomaa VV, et al. 17β-hydroxysteroid dehydrogenase type 1 is an independent prognostic marker in breast cancer. Cancer Res. 2004;64:7604–7609. doi: 10.1158/0008-5472.CAN-04-0446. [DOI] [PubMed] [Google Scholar]
- 31.Santner SJ, Chen S, Zhou D, et al. Effect of androstenedione on growth of untransfected and aromatase-transfected MCF-7 cells in culture. J. Steroid Biochem. Mol. Biol. 1993;44:611–616. doi: 10.1016/0960-0760(93)90267-z. [DOI] [PubMed] [Google Scholar]
- 32.Matsuura K, Shirasishi H, Hara A, et al. Identification of a principal mRNA species for human 3α-hydroxysteroid dehydrogenase isoform (AKR1C3) that exhibits high prostaglandin D2 11-ketoreductase activity. J. Biochem. (Tokyo) 1998;124:940–946. doi: 10.1093/oxfordjournals.jbchem.a022211. [DOI] [PubMed] [Google Scholar]
- 33.Koda N, Tsutsui Y, Niwa H, et al. Synthesis of prostaglandin F ethanolamide by prostaglandin F synthase and identification of Bimatoprost as a potent inhibitor of the enzyme: new enzyme assay method using LC/ESI/MS. Arch. Biochem. & Biophys. 2004;424:128–136. doi: 10.1016/j.abb.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Komoto J, Yamada T, Watanabe K, et al. Crystal structure of human prostaglandin F synthase (AKR1C3) Biochemistry. 2004;43:2188–2198. doi: 10.1021/bi036046x. [DOI] [PubMed] [Google Scholar]
- 35.Desmond JC, Mountford JC, Drayson MT, et al. The aldo-keto reductase AKR1C3 is a novel suppressor of cell-differentiation that provides a plausible target for the non-cyclooxygenase-dependent antineoplastic actions of nonsteroidal anti-inflammatory drugs. Cancer Res. 2003;63:505–512. [PubMed] [Google Scholar]
- 36.Lee S-H, Blair IA. Targeted lipidomics analysis by liquid chromatography electron capture atmospheric pressure chemical ionization mass spectrometry (LC-ECAPCI/MS) Methods Enzymol. 2007;433:159–174. doi: 10.1016/S0076-6879(07)33009-7. [DOI] [PubMed] [Google Scholar]
- 37.Penning TM, Talalay P. Inhibition of a major NAD(P)+-linked oxidoreductase from rat liver cytosol by steroidal and nonsteroidal anti-inflammatory agents and by prostaglandins. Proc. Natl. Acad. Sci. USA. 1983;80:4504–4508. doi: 10.1073/pnas.80.14.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byrns MC, Steckelbroeck S, Penning TM. An indomethacin analogue, N-(4-chlorobenzoyl)-melatonin, is a selective inhibitor of aldo-keto reductase 1C3 (type 2 3α-HSD, type 5 17β -HSD, and prostaglandin F synthase), a potential target for the treatment of hormone dependent and hormone independent malignancies. Biochem. Pharmacol. 2008;75:484–493. doi: 10.1016/j.bcp.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauman DR, Rudnick S, Szewczuk LM, et al. Development of non-steroidal anti-inflammatory drug (NSAID) analogs and steroid carboxylates selective for human aldo-keto reductase isoforms: Potential antineoplastic agents that work independently of cyclooxygenase isozymes. Mol. Pharmacol. 2005;67:60–68. doi: 10.1124/mol.104.006569. [DOI] [PubMed] [Google Scholar]
- 40.Ji Q, Chang L, VanDenBreg D, et al. A, Selective reduction of AKR1C2 in prostate cancer and its role in DHT metabolism. Prostate. 2003;54:275–289. doi: 10.1002/pros.10192. [DOI] [PubMed] [Google Scholar]
- 41.Nakamuura Y, Suzuki T, Nakabayashi M, et al. In situ androgen producing enzymes in human prostate cancer. Endocrin. Relat. Cancer. 2005;12:101–107. doi: 10.1677/erc.1.00914. [DOI] [PubMed] [Google Scholar]
- 42.Montgomery RB, Mostgahel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gunnarsson C, Jerevall P-L, Hammar K, et al. Amplification of HSD17B1 has prognostic significance in postmenopausal bresat cancer. Breast Cancer Res. Treat. 2007 doi: 10.1007/s10549-007-9579-7. [DOI] [PubMed] [Google Scholar]
- 44.Brueggemeier R, Su B, Sugimoto Y, et al. Aromatase and COX in breast cancer: enzyme inhibitors and beyond. J. Steroid. Biochem. Mol. Biol. 2007;106:16–23. doi: 10.1016/j.jsbmb.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su B, Diaz-Cruz E-S, Landini S, et al. Suppression of aromatase in human breast cells by a cyclooxygenase-2 inhibitor and its analog involves multiple mechanisms independent of cyclooxygenase-2 inhibition. Steroids. 2008;73:104–111. doi: 10.1016/j.steroids.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]