Preface
B cells are essential for humoral immunity, but the role that B cells have in regulating CD4+ T cell responses remains controversial. However, new data showing that transient depletion of B cells potently influences the induction, maintenance and reactivation of CD4+ T cells and the recent results identifying antibody-independent functions of B cells have reinvigorated interest in the many roles of B cells in both infectious and autoimmune diseases. In this Review, we discuss recent data showing how effector and regulatory B cells modulate CD4+ T cell responses to pathogens and autoantigens.
Immunity to pathogens often requires both B cell-dependent humoral immune responses and T cell-dependent cellular immune responses, which cooperate to clear infectious organisms. Although CD4+ T cells clearly participate in humoral immune responses by providing help to B cells and can enhance cellular immunity by producing cytokines, the converse possibility, that B cells participate in both types of immune response, is still not widely accepted. Some early studies of B cell-deficient mice indicated that the absence of B cells adversely affected both CD4+ T cell1–4 and CD8+ T cell responses5, 6. However, other studies showed that B cells were dispensable for the generation and maintenance of antigen-specific T cell responses7–10. These conflicting results were further clouded by data showing that mice lacking B cells during embryonic development exhibit immunological abnormalities, including defects in Peyer’s patch organogenesis11, loss of follicular dendritic cells (FDCs)12, 13 and gp38-expressing stromal cells in the spleen14, alterations in splenic dendritic cell (DC) homeostasis15 and decreased T cell numbers in the thymus16 and spleen14. Given that many of the developmental and architectural defects observed in B cell-deficient mice are likely to influence T cell responses, it has been difficult to unambiguously assign a role for B cells in regulating cellular immune responses to either pathogens or autoantigens.
The question of whether B cells have a role in cellular immune responses is now receiving renewed interest with the emergence of clinical data showing that B cell depletion is an effective treatment for several T cell-mediated autoimmune diseases Multiple Sclerosis (MS)17, Type 1 Diabetes (T1D)18 Rheumatoid Arthritis (RA)19 and others20, 21. Indeed, studies in both humans and mice show that the clinical efficacy of B cell depletion therapy does not necessarily correlate with changes in the levels of circulating autoantibody, suggesting that B cells may contribute to autoimmunity independently of autoantibody production22, 23. Importantly, transient B cell depletion studies that distinguish the role of B cells during development from their roles during the course of an immune response have provided convincing evidence that B cells do regulate T cell-mediated immune responses. Furthermore, new mouse models, in which B cells are present but cannot secrete antibody, show that B cells can regulate T cell-mediated immune responses via antibody-independent mechanisms.
In this Review, we focus on emerging data from patients and mouse models showing that B cells modulate CD4+ T cell responses. Specifically, we discuss the roles that B cells have in regulating the development, proliferation and maintenance of CD4+ effector and memory T cells and the data suggesting that B cells also modulate the number of regulatory T cells. We review experiments showing that recently described “effector” and “regulatory” B cell subsets modulate the function of T cells by presenting antigen, by providing co-stimulation and by producing cytokines that direct the proliferation and effector functions of responding T cells. Collectively, these data show that B cells are not simply the passive recipients of T cell help, but actively participate in cellular immune responses by directing the magnitude and quality of the T cell response to foreign and self-antigens.
Effects of B cell depletion on T cells in autoimmunity
Decreased CD4+ T cell effector responses
Rituximab, a mouse/human chimeric antibody that binds to human CD20, induces B cell depletion via FcR-mediated antibody dependent cell cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC) mechanisms24, 25. Rituximab treatment depletes naïve and memory B cells from peripheral blood26. However, it is less effective in depleting tissue residing marginal zone and germinal center B cells24, 27 and does not affect CD20neg long-lived plasma cells26.
Given the relative effectiveness of B cell depletion by Rituximab, the drug has been tested in a wide variety of diseases. It is approved to treat non-Hodgkin’s lymphoma and RA in patients with disease that is refractory to anti-tumour necrosis factor (TNF) therapy. Rituximab is also being evaluated for the treatment of other autoimmune diseases, including systemic lupus erythamatosus (SLE), type 1 diabetes, idiopathic thrombocytopenic purpura (ITP), pemphigus vulgaris (PV), mixed cryoglobulinemia vasculitis (MCV), T1D, MS and others20. Most of the clinical studies to date have focused on the extent of B cell depletion and clinical correlates of disease remission. Interestingly, autoantibody titres to some self-antigens declined following B cell depletion while others did not22, 23, suggesting that some autoantibody responses are continuously initiated by new cohorts of CD20-expressing B cells while others are likely being maintained by long-lived CD20neg plasma cells. Regardless, in many studies B cell depletion was clinically effective even in patients that did not have decreased autoantibody titres22, 23, indicating that B cells must also contribute to pathology by other mechanisms. Given that a number of T cell-mediated autoimmune diseases are being effectively treated with B cell depletion, investigators have begun examining whether Rituximab treatment alters the subset composition, activation or function of T cells (Tables 1 and 2). For example, T cell responses were monitored in Rituximab-treated patients with pemphigus vulgaris (PV)28. Although B cell depletion therapy did not change the proportion of total T cells in these patients, the frequency of desmoglein-specific (autoreactive) T cells in peripheral blood that produced interferon-γ (IFNγ) or interleukin-4 (IL-4) declined significantly following B cell depletion28. By contrast, Rituximab treatment did not affect the number of IFNγ-producing tetanus toxoid specific T cells28. Patients who remained in complete remission for more than one year exhibited the greatest decline in desmoglein-reactive T cells, whereas autoreactive T cells reemerged in patients who relapsed28. Interestingly, the titres of autoantibodies specific for desmoglein correlated with the frequency of autoreactive T cells; Rituximab-treated patients with lower numbers of desmoglein-reactive T cells also had lower desmoglein-specific antibody titres, whereas relapsed patients whose autoreactive T cell numbers increased also showed a rebound in desmoglein autoantibodies28. These data suggested that B cells contribute to PV both directly through autoantibody production and indirectly through regulation of autoreactive T cells.
Table 1.
Impact of B cell depletion therapy on Effector and Regulatory T cells (humans)
| Disease (Human or Mouse) |
Treatment protocol |
AutoAb titres |
T cell activation |
T cell prolifera- tion |
T cell and systemic cytokines |
Regulatory T cells |
Effect on disease |
Reference |
|---|---|---|---|---|---|---|---|---|
| pemphigus vulgaris (human) | Rituximab | Decreased in all patients | ND | ND | Decreased in responder patients | ND | 8/11 patients complete remission | 28 |
| idiopathic thrombocy-topenic purpura (human) | Rituximab | ND | ND | Altered T cell repertoire | Altered Th1/Th2 ratio | ND | Reduced in ~50% of patients | 29 |
| mixed cryoglobuli-nemia vasculitis (human) | Rituximab | Decreased | DecreasedHLA-DR+ CD8+ T cells | ND | Decreased systemic IL-12 and IFNγ | Increased FOXP3+ CD25+ CD4+ T cells | Complete remission in 14/21 of patients | 36 |
| SLE (human) | Rituximab | No effect on anti-dsDNA | Decreased CD40L+ CD4+ T cells | ND | ND | Increased FOXP3, GITR, CTLA4, CD25 and TGFβ1 mRNA in PBL | 80% with complete or partial remission | 31, 32 |
| SLE (human) | Rituximab | anti-dsDNA and anti-ANA titres decreased in some patients | Decreased CD69+ CD4 T cells | ND | Altered Th1/Th2 ratio | ND | Remission in 8/9 patients | 96 |
| SLE (human) | Rituximab | decreased anti-dsDNA no change in anti-Ro or anti-La | Increased CD25+ CD4+ T cells | ND | ND | Increased CD25bright Foxp3+ CD4+ T cells | Improvement in 100% of patients | 34 |
| SLE (human) | Rituximab | decreased anti-dsDNA, anti-ANA and anti-phospholipid Abs in subset of patients | ND | Increased apoptosis | ND | Increased CD25bright and TGFβ1+ CD4+ T cells Increased suppressor activity in vitro | Disease remission in 90% of patients | 33 |
Table 2.
Impact of B cell depletion therapy on Effector and Regulatory T cells (mice)
| Disease (Human or Mouse) |
Treatment protocol |
AutoAb titres |
T cell activation |
T cell proliferation |
T cell and systemic cytokines |
Regulatory T cells |
Effect on clinical disease |
Reference |
|---|---|---|---|---|---|---|---|---|
| SLE (Human CD20 transgenic mice) | Rituximab | Decreased | Decreased memory T cells | ND | ND | ND | In animals with good B depletion see reduced disease severity | 27 |
| Diabetes (human CD20 transgenic mice) | Rituximab Before or after development of insulitis. after establishment of clinical disease | Decreased | ND | ND | Decreased IFNγ and IL-17 | Increased CD25+Foxp3+ CD4+ splenic T cells | Prevents or delays onset when given early, ameliorates disease in ~40% of animals when given after disease onset | 37 |
| Diabetes (mice) | Anti-mouse CD20 Before development of insulitis | ND | No effect | Decreased following anti-CD3 + CD28 | ND | No effect | Prevents or delays onset depending on timing of anti-CD20 administration | 97 |
| Diabetes (mice) | BCMA-Fc at the time of insulitis | ND | Decreased CD40+ CD4+ and CD8+ T cells | Decreased systemic IL-7, IL-15 and IL-17 | Increased CD25+Foxp3+ CD4+ T cells | Prevents development of disease | 38 | |
| Diabetes (mice) | Anti-CD22 At the onset of insulitis After onset of clinical disease | ND | ND | ND | Decreased IFNγ producing T cells and reduced systemic inflammatory cytokines | Increased CD25+Foxp3+ CD4+ T cells | Prevents onset when given early and reverses disease when given after onset | 39 |
| EAE (mice) | Anti-mCD20 Deplete before disease | No AutoAb | Increased CNS infiltrating MOG-specific CD4+ T cells | Increased | Increased IFNγ and IL-17 producing CD4+ T cells | No effect | Disease is worse | 57 |
| EAE (mice) | Anti-mCD20 deplete after initiation of disease | Decreased | Decreased CNS infiltrating MOG-specific CD4+ T cells | Decreased | Decreased IFNγ and IL-17 producing CD4+ T cells | No effect | Disease improved | 57 |
| proteoglycan induced arthritis (mice) | Anti-mCD20 deplete after disease onset (after 2 immunizations with autoantigens) | Decreased | ND | Decreased | Decreased IFNγ and IL-17 producing CD4+ T cells | ND | Suppression of histopathology and T cells unable to transfer disease | 73 |
| Collagen induced arthritis (mice) | Anti-mCD20 deplete before priming | No AutoAb | ND | Decreased | ND | ND | significant delay and suppression of disease | 40 98 |
| Collagen induced arthritis (mice) | Anti-mCD20 deplete after prime + challenge with Collagen | Variable effects depending on isotype | Decreased | ND | Decreased (data not shown) | ND | No suppression of clinical disease | 98 |
| Autoimmune thyroditis (mice) | Anti-CD20 Deplete before initiation of disease | ND | ND | ND | Reduced # of T cells in target organ | ND | In animals with good B depletion see reduced disease severity | 99 |
ND- not determined
Similar results were observed in patients with idiopathic thrombocytopenic purpura (ITP)29. Although autoantibodies do contribute to the pathogenesis of ITP, patients with ITP often present with monoclonal or pauciclonal repertoires of T cells, which are preferentially skewed toward the production of T helper 1 (TH1)-type cytokines such as IFNγ30. In addition, T cells from patients with ITP express higher levels of the anti-apoptotic protein BCL-2, and lower levels of the pro-apoptotic protein BAX and are more resistant to apoptosis than T cells from healthy controls30. Each of these T cell abnormalities was reversed in ITP patients who responded positively to B cell depletion therapy, but was unaffected in non-responders29, suggesting that the efficacy of the B cell depletion therapy in ITP was associated with changes in T cell repertoire and function. Thus, B cells from patients with PV and ITP, as well as other human and mouse autoimmune diseases (see Tables 1 and 2), appear to enhance autoreactive T cell responses.
Increased regulatory T cell development
T cells were also phenotypically altered in Rituximab-treated patients with SLE. In one study, Rituximab treatment reduced the number of CD40L-expressing CD4+ T cells in the peripheral blood of SLE patients but did not alter the expression of other activation markers like HLA-DR, CD69 or CD2531. In a follow up study, the investigators showed that Rituximab treatment decreased CD40L mRNA expression in T cells from the responder patients with SLE32. Interestingly, investigators analyzing two separate cohorts of patients with SLE reported that the frequency of CD4+CD25hi T cells in peripheral blood was significantly increased after B cell depletion33, 34 and these T cells expressed FOXP334. Furthermore, the proportion of CD4+ T cells spontaneously producing IL-10 or transforming growth factor-β (TGFβ) was increased in the Rituximab-responsive patients33. Increased expression levels of mRNAs associated with regulatory T cells, including CD25, FOXP3, cytotoxic T lymphocyte antigen 4 (CTLA4) and glucocorticoid induced TNF-receptor related protein (GITR) were observed in the Rituximab-responsive patients with SLE32. Moreover, FOXP3 levels remained low in patients who did not respond to therapy and declined to baseline levels in patients who relapsed32. Together, these data are consistent with the idea that successful Rituximab treatment not only curtails T cell effector responses but also promotes the expansion of regulatory T cell populations.
In support of this hypothesis, Rituximab treatment of patients with mixed cryoglobulinemia vasculitis (MCV) also resulted in changes to effector and regulatory T cell populations. Patients with MCV normally present with increased systemic levels of TH1 cytokines and greater numbers of activated T cells and activated, clonally expanded memory B cells35. After Rituximab treatment, both B and T cell abnormalities were reversed in a subset of MCV patients36. The clinical efficacy of the B cell depletion correlated best with reductions in systemic levels of IFNγ and IL-2 and a decrease in the size of the clonally-expanded B cell population36. In addition, investigators observed a notable increase in the frequency of CD4+CD25+FOXP3+ regulatory T cells in Rituximab-responsive MCV patients, but not in non-responder patients or patients who relapsed in less than one year36. These data suggested that elimination of the clonal (and presumably autoreactive) B cells reduced inflammation and favoured the development or proliferation of regulatory T cells.
Similar increases in regulatory T cells were reported in a B cell-depleted autoimmune mouse model. Early Rituximab treatment (before the onset of clinical disease) of non-obese diabetic (NOD) mice expressing a human CD20 transgene27 led to less insulitis and delayed the onset of diabetes32. Moreover, Rituximab treatment after the onset of hyperglycemia, combined with therapeutic administration of insulin, led to long-term remission in 36% of the animals and ultimately ameliorated the requirement for daily insulin injections37. Mice that were B cell depleted before disease onset had increased numbers of Foxp3+ T cells and CD4+ T cells from these animals suppressed diabetes in adoptive transfer experiments37. In addition, the B cells that repopulated Rituximab-treated animals were able to suppress diabetes development when transferred with autoreactive T cells into NOD/severe combined immunodeficient (SCID) hosts37. This result suggests that depletion of the pathogenic B cell population facilitated the establishment of both B cell and T cell regulatory populations and indicated that at least some B cells can suppress autoimmune disease.
An increase in the number of regulatory T cells was also observed in NOD mice treated with B cell maturation antigen (BCMA)-Fc to block B cell-activating factor (BAFF)- and a proliferation-inducing ligand (APRIL)-mediated survival signals for B cells38. B cell depletion with BCMA-Fc in 9-week-old NOD mice (after the onset of insulitis) prevented hyperglycemia and islet destruction, even though the BCMA-Fc treatment protocol did not effectively deplete all of the B cells in the pancreas and diabetogenic T cells were still present and sufficient to cause disease when transferred into NOD/SCID recipients38. Thus, the remaining lymphocytes appeared to be held in check in the BCMA-Fc treated animals. Indeed, T cells from spleen and pancreatic lymph nodes of BCMA-Fc treated mice were less activated as measured by reduced CD40 expression on T cells and decreased systemic levels of IL-1738. Furthermore, BCMA-Fc treatment also promoted a gradual increase in the number of CD4+CD25+Foxp3+ regulatory T cells in the spleen and pancreatic LN38. These regulatory T cells seemed to be important for ameliorating disease, as CD25 blockade after B cell depletion resulted in recurrence of diabetes38.
Likewise, another group reported that the frequency of Foxp3+ regulatory T cells in the pancreatic lymph nodes and islets increased in NOD mice after treatment with CD22-specific monoclonal antibodies to deplete B cells39. Similar to the results of Hu et al37, the B cells that repopulated the CD22-specific antibody-treated mice prevented the development of diabetes when transferred with diabetogenic T cells into NOD/SCID hosts39. Transcriptome analysis of these “protective” B cells revealed significant changes in gene expression with almost 200 genes downregulated in the newly emerging B cells compared to B cells from normoglycemic 10-week-old NOD mice39. The B cells that repopulated the hosts were also less effective in presenting antigen to T cells and inducing inflammatory cytokine production by the T cells39.
Collectively, the data suggest a model (Fig. 1) in which a positive feedback loop between autoreactive effector B cells and T cells is established, leading to exacerbated inflammation and tissue damage. B cell depletion, when effective, blocks the further proliferation or maintenance of autoreactive T cells that may prevent the repopulating potentially autoreactive B cells from being selected and expanded in the periphery. Depletion of autoreactive B cells also prevents the formation of new plasmablasts, resulting in a decrease in autoantibody titres specific for some, but not all, autoantigens. Together, the reductions in autoreactive B and T cells lead to a decline in organ-specific and systemic inflammation, favoring the emergence of regulatory T and B cells that can prevent the reactivation of remaining autoreactive cells. In the next sections we will discuss experimental data that describe at a mechanistic level how B cells might regulate CD4+ T cell responses.
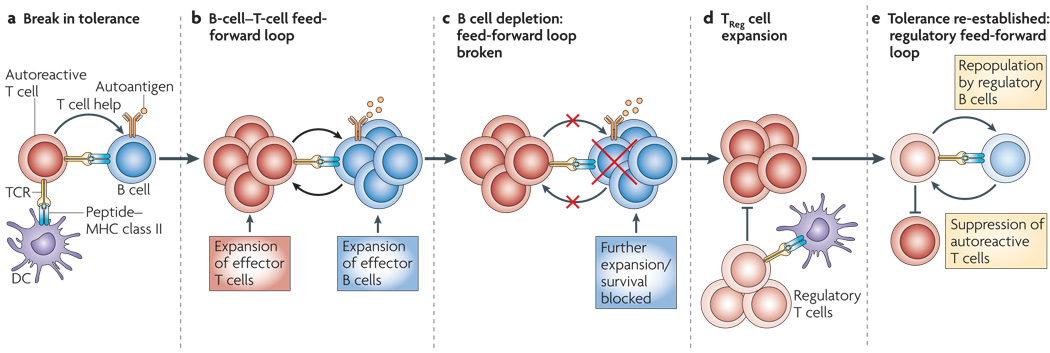
Figure 1. Resetting the effector and regulatory B and T cell networks after B cell depletion.
The mechanistic basis for the effectiveness of B cell depletion in treating autoimmune disease is not understood. Based on the data, we propose a model in which autoreactive effector B and T cells establish a feed-forward loop that destroys the interconnected effector and regulatory B and T cell networks. B cell depletion allows for re-establishment of the regulatory network and favors development of tolerance upon B cell repopulation. In this model, a break in tolerance of either the B or T cell occurs (panel a), allowing for the initiation of cognate interactions between autoreactive T and B cells and the establishment of a B and T cell dependent feed-forward loop (panel b). This antigen-driven feed-forward loop eventually dominates the response and is no longer susceptible to suppression by regulatory B and T cells. When B cells are depleted (panel c), the T/B feed-forward loop is broken and the number of responding T cells decreases. This results in decreased inflammatory cytokine production by the autoreactive T and B cells favors the generation, expansion and function of regulatory T cells (panel d) that can suppress remaining autoreactive effector T cells. Finally, the transitional and naïve B cells that initially repopulate the patient (panel e) are likely to have regulatory or tolerance inducing capacity, similar to the B cells found in neonates. These B cells can drive the induction or expansion of new cohorts of regulatory T cells, resetting the balance of regulatory and effector T cells to favor tolerance rather than responsiveness.
B cells regulate CD4+ T cell mediated immune responses
Not required for CD4+ T cell homeostasis
The clinical data evaluating T cell responses in Rituximab-treated patients are limited by the types of experiments that can be carried out with peripheral blood cells. However, mechanistic studies are possible when evaluating T cells in B cell-depleted mice. For example, a recent study used B cell depletion to show that B cells do not control normal T cell homeostasis40. In fact, T cells and T cell subsets were present in typical numbers in the thymus and secondary lymphoid tissues of B cell-depleted naïve mice40. In addition, the phenotypic and transcriptional profiles of the peripheral T cells in B cell-depleted naïve mice were identical to those of T cells from non-depleted mice40. Furthermore, the T cells from B cell-depleted naïve mice responded normally to polyclonal stimuli such as treatment with combined CD3- and CD28-specific antibodies40. Thus, these data suggest that the effects of B cell depletion on T cell function are probably due to changes in T cells responding to antigen.
Activation and maintenance of CD4+ T cells
Consistent with the idea that B cells modulate T cell responses to antigen, numerous reports indicate that B cells regulate the initial proliferation of CD4+ T cells after encounter with antigen41–45 and have roles in CD4+ T cell memory responses44, 46–50. For example, the number of IL-4-producing T cells responding to the intestinal nematode Heligmosomoides polygyrus in B cell-deficient (and B cell-depleted) mice was reduced ten-fold compared with that observed in normal mice, even as early as 3 days after infection50. Furthermore, despite the continued presence of antigen, antigen-specific T cell responses remained depressed in B cell-deficient mice for weeks, suggesting that B cells are required for the initiation and expansion of the primary CD4+ T cell response to H. polygyrus. B cells were also required to maintain or reactivate H. polygyrus-induced memory T helper 2 (TH2) cells50. Interestingly, although antibody was essential for successful immunity to H. polygyrus, antibody was completely dispensable for the primary and memory CD4+ T response50. However, primary and secondary Th2 cell responses to H. polygyrus did require antigen-specific antigen-presenting B cells50.
Similar results were reported for CD4+ T cell responses to lymphocytic choriomeningitis virus (LCMV)51. In these experiments, the number of LCMV-specific IFNγ-producing CD4+ T cells was reduced by 60% in B cell-deficient mice on day 8 after primary infection, and was dramatically reduced (by 95%) on day 70 post-infection51. LCMV-specific memory CD4+ T cells were not reduced in mice in which B cells were unable to produce antibody51, which indicates that although B cells were required to maintain CD4+ T cell memory, antibody–antigen complexes were not needed. Interestingly, in B cell-deficient mice LCMV-specific memory CD4+ T cells disappeared relatively early after primary infection (within 1 month), during the contraction phase of the T cell response51, suggesting that B cells play a role in the establishment or early maintenance of LCMV-specific memory CD4+ T cells.
B cells were also required for the reactivation of memory T cells after herpes simplex virus (HSV) reinfection52. In this model, mice were vaccinated with attenuated HSV and then challenged with virulent HSV-2. Protection depended on IFNγ-producing CD4+ T cells that were reactivated by MHC class II-expressing, non-infected antigen presenting cells (APCs)52. When vaccinated mice were depleted of B cells alone, or depleted of either DCs or macrophages, they were still protected after re-infection52. However, TH1 cell-mediated protection against HSV was completely abrogated when both B cells and DCs were depleted before the challenge infection52. Thus, B cells, in combination with DCs, maximize the memory CD4+ T cell recall response to this virus.
Suppression of CD4+ T cells
In addition to activating T cells, B cells may also modulate regulatory T cell development, proliferation or survival. In support of this possibility, one recent study showed that culturing CD19+ human B cells with CD4+CD25+ alloreactive T cells plus IL-2 and CD28-specific antibody induced a 40-fold expansion of regulatory T cells53. The expanded T cells expressed FOXP3 and were highly suppressive in in vitro assays53. Interestingly, the antigen specificity of the regulatory T cells was determined by the stimulating B cells53.
In a second study, CD40L-activated B cells were used to induce regulatory T cells54. Co-culture of CD40L-stimulated human B cells with naïve CD4+CD45RA+CD45RO−CD25− T cells resulted in the expansion of a population of CD4hi T cells that also expressed CD25, FOXP3, CTLA4 and GITR54. These T cells suppressed the proliferation of T cells responding to the original target alloantigens expressed on the B cells54. Importantly, no exogenous stimuli were added to the co-cultures, indicating that the CD40L-activated B cells, which expressed high levels of MHC class II, CD80 and CD86 and secreted substantial amounts of IL-254, provided all of the necessary signals to induce regulatory T cell development.
B cells were also required for regulatory T cell-dependent tolerance induction55. Ovalbumin-specific regulatory T cells can be induced in normal mice by transferring naïve ovalbumin-specific transgenic CD4+ T cells into recipient hosts and then repeatedly administering ovalbumin coupled to cholera toxin B subunit through the sublingual, nasal or oral route56. This tolerance protocol was significantly less effective when the naïve T cells were transferred into B cell-deficient hosts and resulted in fewer Foxp3+ regulatory T cells and poorly suppressed ovalbumin-specific effector T cell responses55. However, transfer of naïve B cells plus naïve ovalbumin-specific CD4+ T cells to the B cell-deficient hosts rescued their ability to generate suppressive ovalbumin-specific Foxp3+ regulatory T cells55. Moreover, B cells from the ovalbumin-tolerized mice expressed higher levels of IL-10 mRNA than did B cells from mice treated with phosphate-buffered saline55 and transfer of the B cells from orally tolerized mice to new hosts significantly suppressed naïve CD4+ T cell responses to vaccination with ovalbumin and adjuvant55. Together, these results suggest that a subset of IL-10-producing B cells, activated in response to a tolerogenic antigen, drove the development of regulatory T cells.
All of these studies, when taken together, show that B cells participate in inducing primary T cell proliferation and generating, maintaining and reactivating TH1 and TH2 memory CD4+ T cells via antigen-specific but antibody-independent mechanisms. The data also show that B cells can modulate regulatory T cells. Therefore, the data predict that B cells have dual and potentially opposing roles in T cell-dependent immune responses. This hypothesis was elegantly tested in recent experiments in which mice were B cell-depleted either before or after the initiation of experimental autoimmune encephalomyelitis (EAE) with myelin oligodendrocyte glycoprotein (MOG) peptide immunization. The severity of clinical disease was significantly higher in mice depleted of B cells seven days prior to immunization with MOG peptide than in non-depleted mice57. Moreover, the number of autoreactive, cytokine-producing T cells in the central nervous system increased significantly in mice depleted of B cells before immunization57. In striking contrast, if B cells were depleted 14 days after immunization with MOG, clinical disease was ameliorated and the numbers of total CD4+ T cells, MOG-specific T cells and IFNγ̃ or IL-17-producing CD4+ T cells in the CNS were reduced57.
These mouse model data clearly showed that B cells can both suppress and enhance T cell responses. Likewise, emerging results from patient studies examining the effect of Rituximab treatment to prevent acute rejection of solid organ transplants indicates that B cells have the ability to play opposing roles in T cell-mediated immune responses. Acute cellular rejection of transplanted organs is largely held to be T cell-mediated58. However, it is clear, at least in the case of kidney transplants, that CD20+ B cells can be found in the rejecting kidney59 and that alloantibodies, particularly in pre-sensitized (already immune) individuals contribute to the cellular rejection process60. Treatment of sensitized individuals with Rituximab appears to reduce alloantibody titres and, when combined with other therapies such as corticsteroids and plasmapheresis, can be highly effective in suppressing T cell mediated rejection of the organ following transplant58. By contrast, depletion of T cells from non-sensitized (naïve) individuals appears to greatly enhance acute T cell-mediated rejection61, suggesting the possibility that depletion of a regulatory population of B cells enhances the destructive T cell-mediated alloresponse. Thus, experimental and clinical data predict that there must be multiple functionally distinct populations of regulatory and effector B cells that can modulate T cell responses (Fig 2).
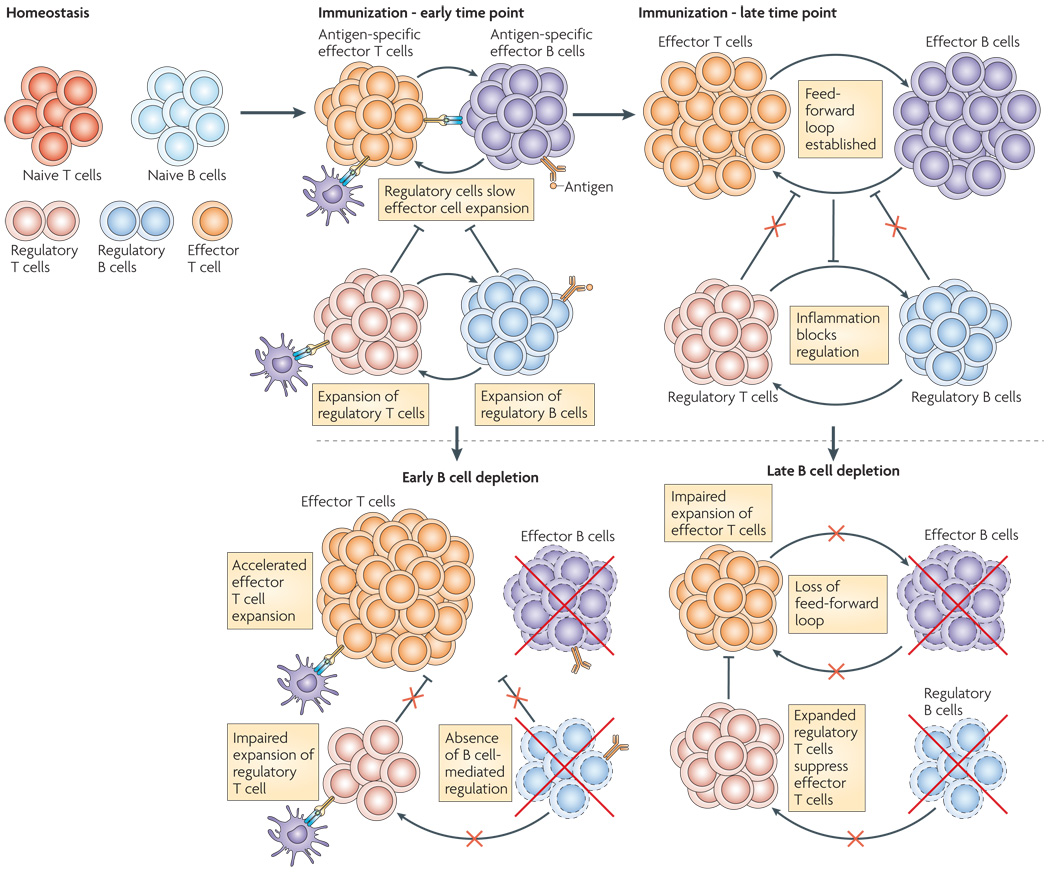
Figure 2. B cells both suppress and enhance T cell mediated immunity.
Depletion of B cells at different times following immunization can have drastically different consequences for T cells (ref 57), suggesting that B cells perform functionally and temporally distinct roles during immune responses. In unmanipulated animals, naïve and regulatory B and T cells are present but few effector cells are found. At early timepoints following immunization, antigen-specific effector T and B cells expand, however regulatory T and B cells are also expanding and can slow the proliferation of the effector T cells. Once the ratio of regulatory to effector cells is biased toward the effector response and the cytokine milieu is dominated by pro-inflammatory cytokines, then the regulatory loop is repressed and the B/T cell antigen-driven feedforward loop is established. When B cells are depleted early, before the immune response is initiated, the regulatory B and T cell loop is not established, leading to an unchecked effector T cell response. By contrast, when effector and regulatory B cells are depleted later in the immune response, then effector T cell expansion and inflammatory cytokine production are curtailed due to interruption of the effector T/B cell feed-forward loop. Regulatory B cells are also eliminated by the depletion therapy, but the remaining regulatory T cells which are no longer exposed to the high level inflammatory cytokines can expand and more efficiently suppress the remaining autoreactive effector T cells.
Functions of effector and regulatory B cells in T cell-mediated immunity
Antigen presentation and co-stimulation
Many of the studies described above show that B cells modulate effector, memory and regulatory T cell responses by antibody-independent mechanisms. One way in which B cells can modulate T cell responses independently of antibody is by presenting antigen to T cells. Although it is often assumed that antigen-presenting dendritic cells are not only necessary but also sufficient for CD4+ T cell activation, proliferation and differentiation62, it is clear that antigen-presenting B cells can be important for the proliferation and differentiation of T cells1, 42, 44, 45, 50, 63–66 and that B cells are particularly effective APCs when antigen is limiting67–69. Thus, B cells may become increasingly relevant as antigen-presenting cells when antigen load is low. Consistent with this idea, a recent study using B cell depleted mice showed that B cells are essential for CD4+ T cell responses when mice are immunized with limiting amounts of antigen, but are dispensable when larger quantities of antigen are used40.
In addition to antigen presentation by B cells, evidence also suggests that co-stimulatory molecule expressing B cells modulate T cell responses43, 70–72. For example, experiments using mice in which B cells, but not other APCs, are selectively unable to express CD80 and CD86 (B-costimulatory-deficient mice) showed that expression of these costimulatory molecules by B cells was required for the activation and/or proliferation of autoreactive CD4+ T cells in a mouse model of arthritis72. Indeed, CD4+ T cells from the B-costimulatory deficient mice proliferated less following in vitro restimulation with arthritis-inducing cartilage proteoglycan autoantigens than did T cells from the wild-type mice72. Moreover, T cells from B-costimulatory deficient mice with proteoglycan autoantigen-induced arthritis were significantly less effective in inducing severe arthritis when transferred into SCID recipient animals72. Although these experiments did not address whether B cell-dependent co-stimulation is required for memory CD4+ T cells responses to cartilage proteoglycan autoantigens, B cells that lack CD80 and CD86 were far less effective than wild-type B cells in activating CD4+ memory T cells in vitro72, suggesting that B cells likely provide co-stimulatory signals to T cells during both primary and secondary responses to antigen. In follow up experiments, the same group reported that transiently depleting B cells four days after a secondary challenge with cartilage proteoglycan autoantigens reduced the number of IFNγ− and IL-17-producing autoantigen-specific CD4+ T cells73. In addition, T cells from B cell-depleted mice were unable to transfer disease to SCID recipients73. Thus, antigen-presenting, co-stimulatory molecule-expressing B cells are required to activate and expand effector and memory T cell populations.
Cytokine production
In addition to presenting antigen and providing co-stimulation to T cells, B cells secrete cytokines and chemokines either constitutively or in response to antigen, TLR ligands, T cells or combinations of these stimuli74, 75. Although it has been known for decades that B cells can make cytokines76, the concept that the cytokines made by B cells are involved in modulating the activation or function of CD4+ T cells is relatively new74, 75. However, the evidence is growing that cytokine-producing B cells have relevant roles in humoral and cellular immune responses. For example, one group recently showed that Foxp3+ regulatory T cells proliferate in co-cultures containing splenic B cells and anti-CD3 stimulated T cells and that the proliferation of regulatory T cells depends on the production of TGFβ3 by the B cells77. Similarly, TGFβ-expressing B cells, induced in vivo in a model of local inhalation tolerance, were sufficient to promote the conversion of CD25−CD4+ T cells into Foxp3+ regulatory T cells78. Thus, cytokines made by B cells can either directly suppress T cell immune responses or can enhance the development and maintenance of regulatory T cells which subsequently suppress effector T cell proliferation and function.
B cells can functionally sub-divided based on their cytokine profile. Indeed, prior work from our lab revealed that B cells primed by T cells as well as antigen and/or TLR ligands in the presence of TH1-type cytokines secrete IFNγ and IL-12p40 (mouse) or IFNγ and IL-12p70 (human). These B cells, which we have operationally termed B effector-1 cells (Be-1 cells), do not secrete significant amounts of IL-4, IL-13 or IL-2 but can secrete IL-10, TNFα and IL-679, 80. By contrast, B cells primed by T cells and antigen in the presence of TH2-type cytokines (referred to as Be-2 cells by our lab) secrete IL-2, Lymphotoxin, IL-4 and IL-13 but make minimal amounts of IFNγ and IL-1280, 81. Be-2 cells can also secrete IL-10, TNFα and IL-680. Finally, regulatory B cells (also referred to as B10 cells by the Tedder group82) produce IL-10 upon stimulation with combinations of antigen, CD40L and TLR ligands83–86.
Regulatory IL-10 producing B cells suppress CD4+ T cell responses and can prevent the induction of autoimmune disease in several mouse models83–85. IL-10 producing B cells are found in both B1 and B2 lineage cells and recent data suggests that there are multiple phenotypically distinct populations of IL-10 producing B cells that can function in a regulatory capacity83–86. Experiments characterizing one population of regulatory B cells showed that B cells expressing markers of transitional T2 B cells produce IL-10 and can prevent the development of autoimmunity87, 88. Other studies suggested that follicular B cells89 or B cells expressing high levels of CD1d90, 91 can also produce IL-10 and function as regulatory B cells. In the most recent study, a CD5+CD1dhi IL-10 producing regulatory B cell population was assessed in more detail82. Transfer of these IL-10-producing B cells into CD19-deficient mice or B cell-depleted animals blocked the CD4+ T cell-dependent inflammatory response in a contact hypersensitivity model82. Importantly, the suppressive function of CD5+CD1dhi B cells was antigen dependent, as regulatory B cells from mice primed with one antigen could not suppress the activity of T cells primed with a second antigen82. Furthermore, the regulatory function of these B cells was absolutely dependent on their ability to produce IL-1082. Together, these experiments showed that an antigen-specific IL-10-producing B cell subset can down-modulate T cell activation.
In addition to suppressing T cell responses, cytokine-producing B cells can also enhance T cell mediated immune responses48, 50, 80, 92–95. For example, in vitro generated effector B cells that produced either TH1- or TH2-type cytokines were shown to promote the activation and differentiation of naïve T cells into effector TH1 and TH2 cells, respectively, through a cytokine-dependent mechanism80. Similarly, IL-12-producing human B cells were shown to promote TH1 cell differentiation in vitro48, 92 and IL-4-producing human B cells were reported to enhance TH2 cell responses95. These in vitro experiments were recently confirmed with in vivo data showing the importance of B cell cytokines in promoting T cell responses. In one set of experiments, TNF production by B cells was shown to be required for the generation of an optimal TH1 cell response to Toxoplasma gondii94. In another set of experiments, the generation of a protective TH2 memory response to H. polygyrus was shown to depend on IL-2-producing B cells50.
In summary, the current evidence suggests that B cells influence CD4+ T cell responses by multiple antibody-independent mechanisms. B cells can enhance T cell responses by presenting antigen and by providing co-stimulation. In addition, phenotypically distinct populations of cytokine-producing B cells can have opposing roles and either enhance or suppress T cell responses (Fig. 3).
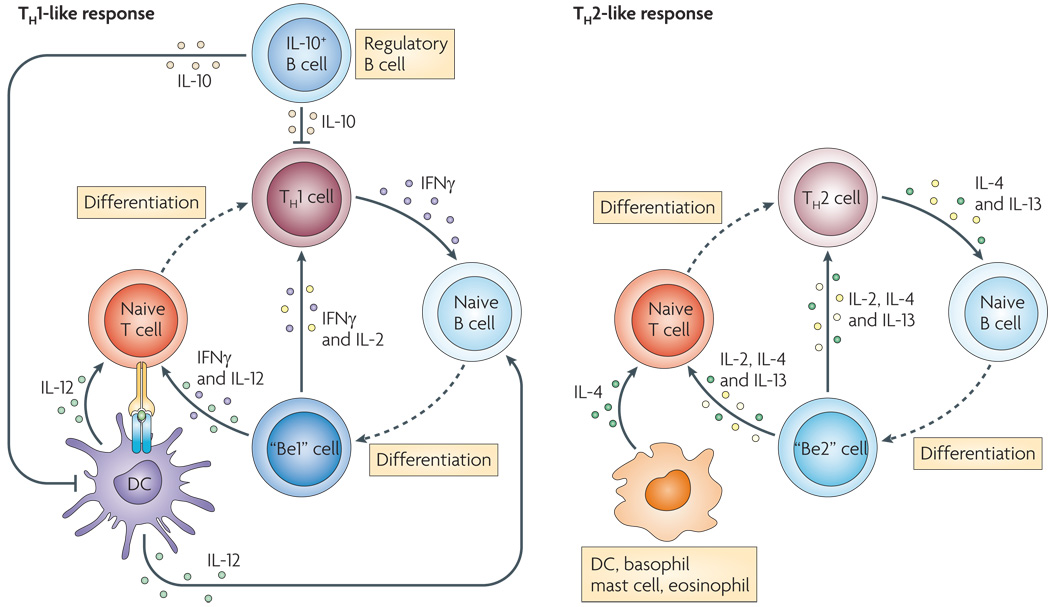
Figure 3. Effector and Regulatory B cells play opposing roles in immune responses.
Effector and regulatory B cells can present antigen to CD4+ T cells and can provide co-stimulation and cytokines. Effector B cells (Be-1 and Be-2) can secrete cytokines such as IFNγ, IL-12, IL-4 and IL-2 that reinforce and stabilize the cytokine profile of effector TH1 and TH2 cells. In addition, the effector B cells can recruit additional naïve T cells into the inflammatory response. In contrast, the regulatory B cells produce IL-10 which suppresses the inflammatory potential of effector T cells, alters the activity of antigen-presenting DCs and promotes regulatory T cell development and expansion. Thus, effector B cells act as accelerators of CD4+ T cell responses while regulatory B cells act as brakes on the response. In autoimmune disease, the ratio between effector B and T cells and regulatory B and T cells likely favors the effector B cells. Drugs that selectively deplete the effector B cells may be highly effective in resetting this balance in favor of the regulatory populations.
Conclusions and perspectives
Antibody is vital for protective immunity to many pathogens and antibody production by B cells is unarguably the most important effector function of these cells and their raison d'être. However, B cells also carry out a variety of other effector and regulatory functions during the course of an immune response. The recent use of B cell depletion therapy is now distinguishing the multiple roles that B cells have during the course of immune responses, independent from antibody production. These experiments reveal that B cells interact in a complex duet with CD4+ T cells to modulate the cellular and humoral immune responses to pathogens and autoantigens. B cells together with DCs, mediate optimal proliferation of CD4+ T cells and can enforce or stabilize the differentiation of T cells into polarized effector cell subsets. B cells also influence the contraction of the CD4+ T cell response and the development of T cell memory. Finally, B cells appear to play an important role in the reactivation of memory CD4+ T cells.
In addition to promoting effector and memory CD4+ T cell responses, B cells influence the development, expansion and survival of regulatory T cell populations. The evidence suggests that activated effector B cells enhance effector T cell responses in autoimmune settings, while simultaneously blocking the survival or expansion of regulatory T cell subsets. Although B cell depletion experiments do not discriminate whether the activated effector B cells directly inhibit regulatory T cells or whether the B cells contribute to the inhospitable inflammatory milieu that prevents the survival or expansion of regulatory T cells, other experiments suggest that under some conditions, naïve and appropriately activated B cells can provide signals that actually facilitate tolerance and the development and expansion of regulatory T cells. Thus, B cell depletion may promote the development of a more tolerogenic environment in autoimmune disease through multiple mechanisms. First, the depletion of autoreactive effector B cells can block the B cell–T cell feed-forward loop, preventing the further proliferation and reactivation of pathogenic T cells and thereby reducing organ-specific and systemic inflammation. Second, the decrease in inflammatory cytokine production will favour the development and survival of regulatory T and B cells that can further dampen the autoimmune response. Finally, the transitional and naïve B cells that initially repopulate the depleted host seem to express a distinct transcriptional profile and may be particularly effective in inducing or expanding regulatory T cell populations. Thus, B cell depletion therapy, when most effective, likely resets the balance between effector and regulatory populations of both B and T cells.
Although we are making significant strides in our understanding of the multi-faceted roles of B cells in humoral and cellular immune responses, there is much that we still do not understand. The data convincing show that B cells modulate T cell responses by presenting antigen, by providing co-stimulation and by secreting cytokines. Furthermore, it is becoming clear that the different effector functions of B cells are tightly controlled and that these tasks are carried out by functionally and phenotypically distinct effector and regulatory B cell subsets. What is less clear, is whether these functionally defined Be-1, Be-2 or B10 cells truly represent distinct lineages or subsets. Furthermore, we do not know whether the antibody-independent effector functions are hard-wired into certain B cells or whether B cells have the plasticity to change their effector functions depending on their local environment. In addition, we do not know whether B cells that develop an effector or regulatory phenotype represent an end fate decision for the B cells or whether they can enter the memory or plasma cell pools while maintaining some or all of their unique effector functions. Finally, we have not yet identified all of the in vivo signals that are required to trigger the development and activation of B cells with regulatory or effector functions. Although there are a large number of important questions remaining to be addressed, it is clear that the scope of B cell biology has expanded beyond its initial boundaries of humoral immunity to encompass the wider field of CD4+ T cell-mediated immunity.
Acknowledgements
The authors gratefully acknowledge discussion and comments from Dr. W. Wojciechowski, R. Misra and B. Leon. This work was supported by NIH grants R01AI068056 and R01AI061511 and the University of Rochester.
Glossary Terms
- Follicular B cells
A re-circulating, mature B-cell subset that populates the follicles of the spleen and lymph nodes.
- Transitional T2 B cells
Transitional B cells are short-lived immature B cells, typically found in the spleen, that either die or are selected into the peripheral mature B cell repertoire. Transitional B cells can be subdivided into three subsets (T1, T2 and T3 cells) based on differential phenotypic and functional characteristics.
References
- 1.Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocytes can be competent antigen-presenting cell for CD4+ T cells to protein antigens in vivo. J. Immunol. 1995;155:3734–3741. [PubMed] [Google Scholar]
- 2.Langhorne J, Cross C, Seixas E, Li C, von der Weid T. A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc. Natl. Acad. Sci. 1997;95:1730–1734. doi: 10.1073/pnas.95.4.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mastroeni P, Simmons C, Fowler R, Hormaeche CE, Dougan G. Igh-6−/− (B cell deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica Serovar Typhimurium and show impaired Th1 T-cell responses to Salmonella Antigens. Infect. Immun. 2000;68:46–53. doi: 10.1128/iai.68.1.46-53.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmann CC, Ramakrishna C, Kornacki M, Stohlman SA. Impaired T cell immunity in B cell-deficient mice following viral central nervous system infection. J Immunol. 2001;167:1575–1583. doi: 10.4049/jimmunol.167.3.1575. [DOI] [PubMed] [Google Scholar]
- 5.Homann D, et al. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: Failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from µMT/µMT mice. J. Virol. 1998;72:9208–9216. doi: 10.1128/jvi.72.11.9208-9216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen H, et al. A specific role for B cells in the generation of CD8 T cell memory by recombinant Listeria monocytogenes. J Immunol. 2003;170:1443–1451. doi: 10.4049/jimmunol.170.3.1443. [DOI] [PubMed] [Google Scholar]
- 7.Epstein MM, Di Rosa F, Jankovic D, Sher A, Matzinger P. Successful T cell priming in B cell-deficient mice. J. Exp. Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips JA, et al. CD4+ T cell activation and tolerance induction in B cell knockout mice. J. Exp. Med. 1996;183:1339–1344. doi: 10.1084/jem.183.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Rosa F, Matzinger P. Long-lasting CD8 T cell memory in the absence of CD4 T cells or B cells. J Exp Med. 1996;183:2153–2163. doi: 10.1084/jem.183.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asano MS, Ahmed R. CD8 T cell memory in B cell-deficient mice. J Exp Med. 1996;183:2165–2174. doi: 10.1084/jem.183.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golovkina TV, Shlomchik M, Hannum L, Chervonsky A. Organogenic role of B lymphocytes in mucosal immunity. Science. 1999;286:1965–1968. doi: 10.1126/science.286.5446.1965. [DOI] [PubMed] [Google Scholar]
- 12.Fu Y-X, Huang G, Wang Y, Chaplin DD. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin a-dependent fashion. J. Exp. Med. 1998;187:1009–1018. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endres R, et al. Mature follicular dendritic cell networks depend on expression of lymphotoxin b receptor by radioresistant stromal cells and of lymphotoxin b and tumor necrosis factor by B cells. J. Exp. Med. 1999;189:159–167. doi: 10.1084/jem.189.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngo VN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. J Exp Med. 2001;194:1649–1660. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabashima K, et al. Intrinsic lymphotoxin-beta receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity. 2005;22:439–450. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Joao C, Ogle BM, Gay-Rabinstein C, Platt JL, Cascalho M. B cell-dependent TCR diversification. J Immunol. 2004;172:4709–4716. doi: 10.4049/jimmunol.172.8.4709. [DOI] [PubMed] [Google Scholar]
- 17.Hauser SL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 18.Pescovitz MD, et al. Rituximab, B-Lymphocyte Depletion, and Preservation of Beta-Cell Function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen SB, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 20.Dorner T, et al. Current status on B-cell depletion therapy in autoimmune diseases other than rheumatoid arthritis. Autoimmun Rev. 2009;9:82–89. doi: 10.1016/j.autrev.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Levesque MC. Translational Mini-Review Series on B Cell-Directed Therapies: Recent advances in B cell-directed biological therapies for autoimmune disorders. Clin Exp Immunol. 2009;157:198–208. doi: 10.1111/j.1365-2249.2009.03979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levesque MC, St Clair EW. B cell-directed therapies for autoimmune disease and correlates of disease response and relapse. J Allergy Clin Immunol. 2008;121:13–21. doi: 10.1016/j.jaci.2007.11.030. quiz 22-13. [DOI] [PubMed] [Google Scholar]
- 23.Liossis SN, Sfikakis PP. Rituximab-induced B cell depletion in autoimmune diseases: potential effects on T cells. Clin Immunol. 2008;127:280–285. doi: 10.1016/j.clim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Gong Q, et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 25.Taylor RP, Lindorfer MA. Immunotherapeutic mechanisms of anti-CD20 monoclonal antibodies. Curr Opin Immunol. 2008;20:444–449. doi: 10.1016/j.coi.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proc Natl Acad Sci U S A. 2008;105:4802–4807. doi: 10.1073/pnas.0800555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahuja A, et al. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 28. Eming R, et al. Rituximab exerts a dual effect in pemphigus vulgaris. J Invest Dermatol. 2008;128:2850–2858. doi: 10.1038/jid.2008.172.. This manuscript demonstrates that the number and responsiveness of peripheral blood autoreactive CD4+ T cells, but not tetanus toxoid specific T cells, declines significantly following B cell depletion therapy
- 29. Stasi R, et al. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood. 2007;110:2924–2930. doi: 10.1182/blood-2007-02-068999.. This manuscript shows that the altered T cell responses observed in ITP patients are reversed following B cell depletion and that successful therapy correlates strongly with the reversal of T cell pathology
- 30.Stasi R, et al. Idiopathic thrombocytopenic purpura: current concepts in pathophysiology and management. Thromb Haemost. 2008;99:4–13. doi: 10.1160/TH07-08-0513. [DOI] [PubMed] [Google Scholar]
- 31.Sfikakis PP, et al. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 2005;52:501–513. doi: 10.1002/art.20858. [DOI] [PubMed] [Google Scholar]
- 32.Sfikakis PP, et al. Increased expression of the FoxP3 functional marker of regulatory T cells following B cell depletion with rituximab in patients with lupus nephritis. Clin Immunol. 2007;123:66–73. doi: 10.1016/j.clim.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Vigna-Perez M, et al. Clinical and immunological effects of Rituximab in patients with lupus nephritis refractory to conventional therapy: a pilot study. Arthritis Res Ther. 2006;8:R83. doi: 10.1186/ar1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallerskog T, et al. Treatment with rituximab affects both the cellular and the humoral arm of the immune system in patients with SLE. Clin Immunol. 2007;122:62–74. doi: 10.1016/j.clim.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Landau DA, Saadoun D, Calabrese LH, Cacoub P. The pathophysiology of HCV induced B-cell clonal disorders. Autoimmun Rev. 2007;6:581–587. doi: 10.1016/j.autrev.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 36. Saadoun D, et al. Restoration of peripheral immune homeostasis after rituximab in mixed cryoglobulinemia vasculitis. Blood. 2008;111:5334–5341. doi: 10.1182/blood-2007-11-122713.. This manuscript shows that B cell depletion in MCV patients can ameliorate the production of TH1 cytokines typically observed in these patients and promotes the expansion of a FOXP3+CD25+ T cell population. The efficacy of treatment correlates with the changes in T cell populations and not with changes in autoantibody titres.
- 37. Hu CY, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405.. This manuscript demonstrates that Rituximab treatment of autoimmune prone mice expressing a human CD20 transgene prevents the development of autoimmune disease and can alleviate disease in animals with clinical manifestations of disease. The authors also show that B cell depletion promoted the induction of protective regulatory T and B cell populations.
- 38.Marino E, et al. CD4(+)CD25(+) T-cells control autoimmunity in the absence of B-cells. Diabetes. 2009;58:1568–1577. doi: 10.2337/db08-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiorina P, et al. Targeting CD22 reprograms B-cells and reverses autoimmune diabetes. Diabetes. 2008;57:3013–3024. doi: 10.2337/db08-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouaziz JD, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci U S A. 2007;104:20878–20883. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christensen JP, Kauffmann SO, Thomsen AR. Deficient CD4+ T cell priming and regression of CD8+ T cell functionality in virus-infected mice lacking a normal B cell compartment. J Immunol. 2003;171:4733–4741. doi: 10.4049/jimmunol.171.9.4733. [DOI] [PubMed] [Google Scholar]
- 42.Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 43.Linton PJ, et al. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197:875–883. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lund FE, et al. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J Immunol. 2006;176:6147–6154. doi: 10.4049/jimmunol.176.10.6147. [DOI] [PubMed] [Google Scholar]
- 45.Liu Q, et al. The Role of B Cells in the Development of CD4 Effector T Cells during a Polarized Th2 Immune Response. J Immunol. 2007;179:3821–3830. doi: 10.4049/jimmunol.179.6.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elkins KL, Bosio CM, Rhinehart-Jones TR. Importance of B cells, but not specific antibodies, in primary and secondary protective immunity to the intracellular bacterium Francisella tularensis Live Vaccine Strain. Infect. Immun. 1999;67:6002–6007. doi: 10.1128/iai.67.11.6002-6007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linton P-J, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J. Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 48.Schultze JL, et al. Human non-germinal center B cell interleukin (IL)-12 production is primarily regulated by T cell signals CD40 ligand, interferon γ, and IL-10: Role of B cells in the maintenance of T cell responses. J. Exp. Med. 1999;189:1–11. doi: 10.1084/jem.189.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephens R, Langhorne J. Priming of CD4+ T cells and development of CD4+ T cell memory; lessons for malaria. Parasite Immunol. 2006;28:25–30. doi: 10.1111/j.1365-3024.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- 50. Wojciechowski W, et al. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30:421–433. doi: 10.1016/j.immuni.2009.01.006.. This manuscript demonstrates that antigen-presenting, cytokine-producing B cells are required to generate effective primary and memory CD4+ TH2 responses to an infectious nematode
- 51. Whitmire JK, et al. Requirement of B cells for generating CD4+ T cell memory. J Immunol. 2009;182:1868–1876. doi: 10.4049/jimmunol.0802501.. This manuscript shows the CD4 memory responses to the systemic virus LCMV is dependent on the presence of B cells but not antibody
- 52. Iijima N, et al. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J Exp Med. 2008;205:3041–3052. doi: 10.1084/jem.20082039.. This manuscript reports that TH1 memory recall responses to Herpes Virus are dependent on the presence of B cells and DCs at the time of challenge infection
- 53.Chen LC, Delgado JC, Jensen PE, Chen X. Direct expansion of human allospecific FoxP3+CD4+ regulatory T cells with allogeneic B cells for therapeutic application. J Immunol. 2009;183:4094–4102. doi: 10.4049/jimmunol.0901081. [DOI] [PubMed] [Google Scholar]
- 54.Tu W, et al. Efficient generation of human alloantigen-specific CD4+ regulatory T cells from naive precursors by CD40-activated B cells. Blood. 2008;112:2554–2562. doi: 10.1182/blood-2008-04-152041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun JB, Flach CF, Czerkinsky C, Holmgren J. B lymphocytes promote expansion of regulatory T cells in oral tolerance: powerful induction by antigen coupled to cholera toxin B subunit. J Immunol. 2008;181:8278–8287. doi: 10.4049/jimmunol.181.12.8278. [DOI] [PubMed] [Google Scholar]
- 56.Sun JB, Raghavan S, Sjoling A, Lundin S, Holmgren J. Oral tolerance induction with antigen conjugated to cholera toxin B subunit generates both Foxp3+CD25+ and Foxp3-CD25-CD4+ regulatory T cells. J Immunol. 2006;177:7634–7644. doi: 10.4049/jimmunol.177.11.7634. [DOI] [PubMed] [Google Scholar]
- 57. Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030.. This manuscript shows that the therapeutic effectiveness of B cell depletion in autoimmune disease is dependent on when the B cells are depleted during the course of disease and suggests that B cells can both inhibit and exacerbate autoimmune disease progression
- 58.Becker YT, Samaniego-Picota M, Sollinger HW. The emerging role of rituximab in organ transplantation. Transpl Int. 2006;19:621–628. doi: 10.1111/j.1432-2277.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 59.Sarwal M, et al. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med. 2003;349:125–138. doi: 10.1056/NEJMoa035588. [DOI] [PubMed] [Google Scholar]
- 60.Zarkhin V, Li L, Sarwal M. "To B or not to B?" B-cells and graft rejection. Transplantation. 2008;85:1705–1714. doi: 10.1097/TP.0b013e318177793e. [DOI] [PubMed] [Google Scholar]
- 61.Clatworthy MR, et al. B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med. 2009;360:2683–2685. doi: 10.1056/NEJMc0808481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 63.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol. 1998;161:1163–1168. [PubMed] [Google Scholar]
- 65.Serreze DV, et al. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161:3912–3918. [PubMed] [Google Scholar]
- 66.O'Neill SK, et al. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J Immunol. 2005;174:3781–3788. doi: 10.4049/jimmunol.174.6.3781. [DOI] [PubMed] [Google Scholar]
- 67.Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class-II restricted T lymphocytes. Annu. Rev. Immunol. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- 68.Malynn BA, Romeo DT, Wortis HH. Antigen-specific B cells efficiently present low doses of antigen for induction of T cell proliferation. J Immunol. 1985;135:980–988. [PubMed] [Google Scholar]
- 69.Townsend SE, Goodnow CC. Abortive proliferation of rare T cells induced by direct or indirect antigen presentation by rare B cells in vivo. J Exp Med. 1998;187:1611–1621. doi: 10.1084/jem.187.10.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noorchashm H, et al. Impaired CD4 T cell activation due to reliance upon B cell-mediated costimulation in nonobese diabetic (NOD) mice. J. Immunol. 2000;165:4685–4696. doi: 10.4049/jimmunol.165.8.4685. [DOI] [PubMed] [Google Scholar]
- 71.Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- 72.O'Neill SK, et al. Expression of CD80/86 on B cells is essential for autoreactive T cell activation and the development of arthritis. J Immunol. 2007;179:5109–5116. doi: 10.4049/jimmunol.179.8.5109. [DOI] [PubMed] [Google Scholar]
- 73. Hamel K, et al. Suppression of proteoglycan-induced arthritis by anti-CD20 B Cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J Immunol. 2008;180:4994–5003. doi: 10.4049/jimmunol.180.7.4994.. This manuscript directly examines antigen-specific CD4+ T cell responses in autoimmune mice that have been B cell depleted and demonstrates that the maintenance or expansion of autoreactive, cytokine-producing T cells is highly dependent on the presence of B cells
- 74.Lund FE. Cytokine-producing B lymphocytes - key regulators of immunity. Curr. Opin. Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr. Dir. Autoimmunity. 2005;8:25–54. doi: 10.1159/000082086. [DOI] [PubMed] [Google Scholar]
- 76.Pistoia V. Production of cytokines by human B cells in health and disease. Immunol. Today. 1997;18:343–350. doi: 10.1016/s0167-5699(97)01080-3. [DOI] [PubMed] [Google Scholar]
- 77.Shah S, Qiao L. Resting B cells expand a CD4+CD25+Foxp3+ Treg population via TGF-beta3. Eur J Immunol. 2008;38:2488–2498. doi: 10.1002/eji.200838201. [DOI] [PubMed] [Google Scholar]
- 78.Gros MJ, Naquet P, Guinamard RR. Cell intrinsic TGF-beta 1 regulation of B cells. J Immunol. 2008;180:8153–8158. doi: 10.4049/jimmunol.180.12.8153. [DOI] [PubMed] [Google Scholar]
- 79.Harris DP, Goodrich S, Gerth AJ, Peng SL, Lund FE. Regulation of IFN-gamma production by B effector 1 cells: essential roles for T-bet and the IFN-gamma receptor. J Immunol. 2005;174:6781–6790. doi: 10.4049/jimmunol.174.11.6781. [DOI] [PubMed] [Google Scholar]
- 80. Harris DP, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nature Immunol. 2000;1:475–482. doi: 10.1038/82717.. This manuscript describes the initial characterization of functionally defined cytokine producing B cell effector subsets
- 81.Harris DP, Goodrich S, Mohrs K, Mohrs M, Lund FE. Cutting edge: the development of IL-4-producing B cells (B effector 2 cells) is controlled by IL-4, IL-4 receptor alpha, and Th2 cells. J Immunol. 2005;175:7103–7107. doi: 10.4049/jimmunol.175.11.7103. [DOI] [PubMed] [Google Scholar]
- 82. Yanaba K, et al. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017.. This manuscript provides a comprehensive phenotypic and functional characterization of IL-10 producing regulatory B cells in mice
- 83.Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat Rev Immunol. 2008;8:391–397. doi: 10.1038/nri2315. [DOI] [PubMed] [Google Scholar]
- 84.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 85.Mauri C, Ehrenstein MR. The 'short' history of regulatory B cells. Trends Immunol. 2008;29:34–40. doi: 10.1016/j.it.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 86.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blair PA, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182:3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Evans JG, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 89.Wei B, et al. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc Natl Acad Sci U S A. 2005;102:2010–2015. doi: 10.1073/pnas.0409449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 91.Duan B, Croker BP, Morel L. Lupus resistance is associated with marginal zone abnormalities in an NZM murine model. Lab Invest. 2007;87:14–28. doi: 10.1038/labinvest.3700497. [DOI] [PubMed] [Google Scholar]
- 92.Wagner M, et al. IL-12p70-dependent Th1 induction by human B cells requires combined activation with CD40 Ligand and CpG DNA. J. Immunol. 2004;172:954–963. doi: 10.4049/jimmunol.172.2.954. [DOI] [PubMed] [Google Scholar]
- 93.Sugimoto K, et al. Inducible IL-12-producing B cells regulate Th2-mediated intestinal inflammation. Gastroenterology. 2007;133:124–136. doi: 10.1053/j.gastro.2007.03.112. [DOI] [PubMed] [Google Scholar]
- 94.Menard LC, et al. B cells amplify IFN-gamma production by T cells via a TNF-alpha-mediated mechanism. J Immunol. 2007;179:4857–4866. doi: 10.4049/jimmunol.179.7.4857. [DOI] [PubMed] [Google Scholar]
- 95.Johansson-Lindbom B, Ingvarsson S, Borrebaeck CA. Germinal centers regulate human Th2 development. J Immunol. 2003;171:1657–1666. doi: 10.4049/jimmunol.171.4.1657. [DOI] [PubMed] [Google Scholar]
- 96.Tamimoto Y, et al. A dose-escalation study of rituximab for treatment of systemic lupus erythematosus and Evans' syndrome: immunological analysis of B cells, T cells and cytokines. Rheumatology (Oxford) 2008;47:821–827. doi: 10.1093/rheumatology/ken071. [DOI] [PubMed] [Google Scholar]
- 97.Xiu Y, et al. B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions. J Immunol. 2008;180:2863–2875. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- 98.Yanaba K, et al. B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. J Immunol. 2007;179:1369–1380. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- 99.Yu S, Dunn R, Kehry MR, Braley-Mullen H. B cell depletion inhibits spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2008;180:7706–7713. doi: 10.4049/jimmunol.180.11.7706. [DOI] [PubMed] [Google Scholar]