Abstract
Over 50% of ST-segment elevation myocardial infarction (STEMI) patients suffer multi-vessel coronary artery disease, which is known to be associated with worse prognosis. Treatment strategies used in clinical practice vary from acute multi-vessel percutaneous coronary intervention (PCI), through staged PCI procedures to a conservative approach with primary PCI of only the infarct-related artery (IRA) and subsequent medical therapy unless recurrent ischaemia occurs. Each approach has advantages and disadvantages. This review paper summarizes the international experience and authors’ opinion on this clinically important question. Multi-vessel disease in STEMI is not a single entity and thus the treatment approach should be individualized. However, the following general rules can be proposed till future large randomized trials prove otherwise:
(i) Single-vessel acute PCI should be the default strategy (to treat only the IRA during the acute phase of STEMI).
(ii) Acute multi-vessel PCI can be justified only in exceptional patients with multiple critical (>90%) and potentially unstable lesions.
(iii) Significant lesions of the non-infarct arteries should be treated either medically or by staged revascularization procedures—both options are currently acceptable.
Keywords: Acute myocardial infarction, Multi-vessel disease, Primary percutaneous coronary intervention, Multi-vessel angioplasty, Staged angioplasty, Medical therapy
Introduction
Primary percutaneous coronary intervention (p-PCI) has become the treatment of choice for patients presenting with ST-segment elevation myocardial infarction (STEMI) when it can be performed expeditiously by an experienced team.1–6 The goal is restoration of flow within 90 min of presentation to a PCI-equipped centre or within 120 min from the time of the first medical contact.7,8 This strategy has been found to be superior to thrombolytic therapy in improving morbidity and mortality. An important piece of information gained at the time of angiography and p-PCI is information not only about the culprit lesion but also about the extent and severity of the underlying coronary artery disease. In patients presenting with STEMI, multi-vessel coronary artery disease is found to be present from 41 to 67% of patients depending upon the baseline characteristics (especially age) of the specific population studied;9–12 however, in one study only 10% of STEMI patients initially treated by p-PCI had a clinical indication for non-culprit PCI during the subsequent follow-up of up to 3 years.13 The presence of multi-vessel disease has been found to be associated with worse prognosis in patients with STEMI.14 Identification of optimal strategies for treating these patients is the subject of considerable interest and controversy.
Treatment strategies vary widely from an aggressive approach which treats all significant lesions in the acute phase of p-PCI to a conservative approach with p-PCI of only the infarct-related artery (IRA) and subsequent medical therapy unless recurrent ischaemia occurs. Between these two extremes are other alternatives; mainly that of staged procedures with the IRA treated acutely and other lesions treated later during the hospital stay or within the first month following discharge. There is no randomized data to definitely answer the issues about the specific scientific merits of any of these approaches. However, there are increasing data from observational series. Each approach has advantages and disadvantages. This review paper summarizes the international experience and authors’ opinion on this clinically important question. The authors have not tried to do a formal ‘meta-analysis’ and rather preferred to describe the current knowledge and to offer their view on the subject.
Aggressive approach: acute multi-vessel percutaneous coronary intervention during ST-segment elevation myocardial infarction
The potential advantages of the aggressive approach are completeness of revascularization, treatment of any secondary potentially unstable lesions, and possible shortening of cumulative hospital stay. The main disadvantages are increased contrast load (and thus increased risk of contrast-induced nephropathy), haemodynamic instability in the acute setting, and potentially disastrous complications of non-infarct artery PCI (Table 1). Several studies reported promising results with multi-vessel PCI strategy. One group15 reported a decrease in recurrent infarctions or ischaemia, but no survival benefit. Rigattieri et al.16 found that p-PCI of the culprit vessel alone with subsequent medical treatment has better early outcomes, whereas multi-vessel-staged PCI tended to better long-term outcomes. Another Italian study17 compared three different revascularization strategies in 214 consecutive patients with STEMI and multi-vessel coronary artery disease undergoing primary angioplasty: culprit vessel angioplasty-only (COR group); staged revascularization (SR group), and simultaneous treatment of non-IRA (CR group). During a mean follow-up of 2.5 years, at least one major adverse cardiac event occurred in 50% patients of the COR group, 20% of the SR group, and 23% of the CR group (P < 0.001).
Table 1.
Acute multi-vessel percutaneous coronary intervention during ST-segment elevation myocardial infarction
| Advantages | Disadvantages |
|---|---|
| Complete revascularization | Increased contrast load/risk of contrast-induced nephropathy |
| Treat ischaemia at a distance | Radiation exposure |
| Treat secondary unstable lesions (plaque instability may not be limited to the culprit lesion) | Complications of treating additional lesions may be potentially fatal |
| Patient preference/comfort | Haemodynamic and general clinical instability treating additional lesions |
| Increased risk of stent thrombosis in patients with clopidogrel resistance/intolerance. | |
| Prothrombotic and inflammatory milieu in the acute phase of STEMI | |
| Coronary spasm may lead to possible overestimation of stenosis severity in non-infarct arteries |
Another single-centre registry18 found among 745 p-PCI patients multi-vessel PCI in STEMI to be feasible and safe. This registry realistically described the relative proportions of single- (46%) vs. multi-vessel (54%) disease and the three most frequently used strategies for multi-vessel disease: p-PCI of the IRA only (39%), staged PCI (24%), and acute multi-vessel (37%) PCI (Figure 1).
Figure 1.
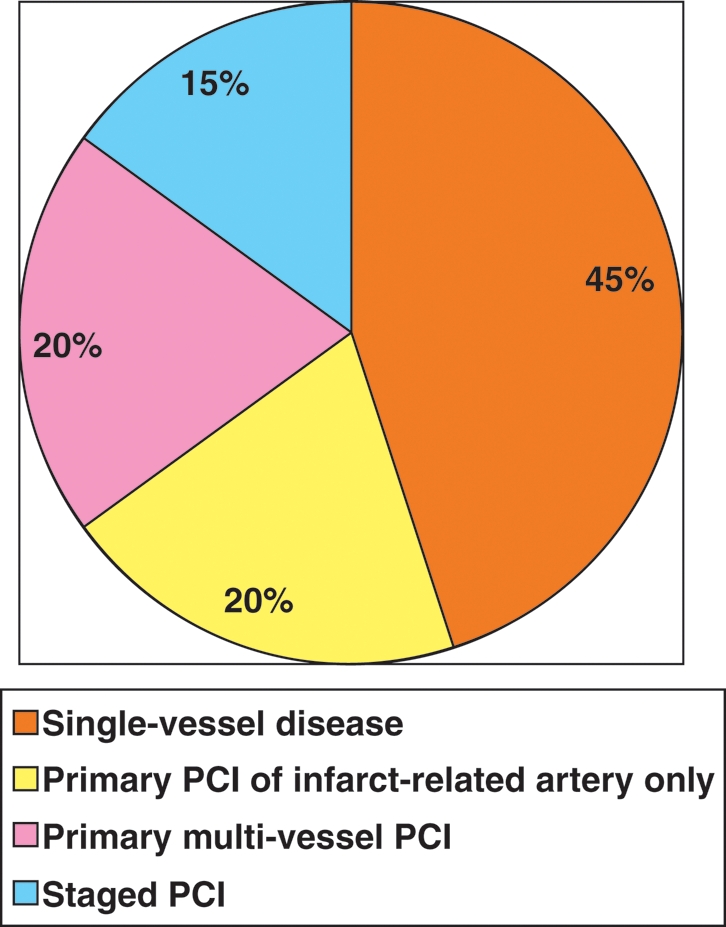
The relative proportion of single- vs. multi-vessel disease and of the three most frequently used PCI (percutaneous coronary intervention) strategies for multi-vessel disease (adopted from Corpus et al.21).
Kong et al.19 analysed patients undergoing PCI for STEMI in 2000–01 (multi-vessel angioplasty, n = 632 and infarct-related vessel angioplasty, n = 1350) from the New York State Angioplasty Registry database. The highest risk patients (previous myocardial infarction, angioplasty, bypass surgery, or cardiogenic shock) were excluded. In-hospital mortality was lower (0.8 vs. 2.3%, P = 0.018) in the multi-vessel angioplasty group. No differences were observed in other ischaemic complications, renal failure, or length of stay. After multivariate analysis, multi-vessel angioplasty remained a significant predictor of lower in-hospital death [odds ratio (OR) = 0.27, 95% confidence interval (CI) = 0.08–0.90, P = 0.03]. However, 0.8% in-hospital mortality for STEMI is not realistic and clearly shows, that this retrospective non-randomized study analysed highly selected, low-risk patients, and thus results cannot be applied to the routine clinical practice.
Chen et al.20 evaluated the outcomes of multi-vessel PCI early after STEMI. Clinical outcomes were compared between patients who underwent multi-vessel PCI (n = 239) and patients who underwent treatment of the IRA alone (n = 1145). The primary endpoint was cumulative survival at 6, 12, and 36 months. Secondary endpoints included a composite of mortality, recurrent infarction, coronary artery bypass graft, or target vessel revascularization at the same time points. The multi-vessel PCI group had a significantly higher prevalence of adverse prognostic indicators. Despite this, observed event rates were similar between the multi-vessel PCI and single-vessel PCI groups. The Kaplan–Meier estimated 1-year survival was 0.91 (95% CI 0.87–0.95) for the multi-vessel PCI group and 0.93 (95% CI 0.92–0.95) for the single-vessel PCI group (P = 0.43). Similarly, 1-year survival free of recurrent infarction and target vessel revascularization rates were similar between the two groups: multi-vessel PCI 0.78 (95% CI 0.73–0.84) and single-vessel PCI 0.78 (95% CI 0.75–0.81).
Conservative approach: acute percutaneous coronary intervention of the infarct-related artery alone (followed by medical therapy unless recurrent ischaemia occurs)
Other studies reported better outcomes with a more conservative strategy of p-PCI only on the IRA in the acute phase (Table 2). The study of Corpus et al.21 described significantly higher risk of re-infarction (13.0 vs. 2.8%, P < .001) and even of repeat revascularization (25 vs. 15%, P = 0,007) among 506 patients when treated by multi-vessel PCI (152 patients) when compared with IRA-only PCI (354 patients).
Table 2.
Infarct-related artery culprit lesions alone then monitor for ischaemia
| Advantages | Disadvantages |
|---|---|
| Treat only culprit lesion | May leave behind significant ischaemia-producing lesions |
| Avoid complications associated with treating other lesions | May not treat other less severe unstable lesions |
| The indication for non-infarct artery PCI can be supported by the objective evidence for myocardial ischaemia in regions supplied by this non-infarct artery | May not prevent recurrent ischaemia |
| The ability to discuss with patients and their families the relative risks and benefits of treating the non-infarct related lesion vs. continued medical therapy or surgical options | Patients have to return to laboratory routinely |
The New York State PCI Registry22 reported that in haemodynamically stable patients with multi-vessel disease, acute phase multi-vessel PCI results in an increased in-hospital mortality (2.4%) when compared with IRA-only PCI (0.9%, P = 0,04). On the contrary, patients undergoing staged multi-vessel PCI within 60 days after the index procedure had a significantly lower 12-month mortality rate than patients undergoing culprit vessel PCI only (1.3 vs. 3.3%, P = 0.04).
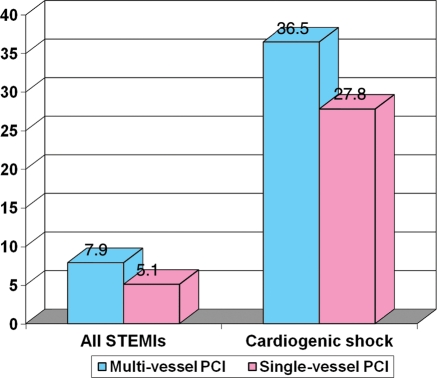
Khattab et al.23 found no mortality benefit from acute multi-vessel PCI. Roe et al.24 described even increased mortality with acute multi-vessel PCI strategy and also an increased risk of major adverse cardiac events with this strategy. The US National Cardiovascular Data Registry (28 936 STEMI patients) found increased mortality among 3087 patients with cardiogenic shock when primary multi-vessel PCI was used25 and also a trend to increased mortality with this strategy among all STEMI patients (Figure 2).
Figure 2.
In-hospital mortality after multi-vessel vs. single-vessel percutaneous coronary intervention in STEMI (ST-elevation myocardial infarction) from the US National Cardiovascular Data Registry (adopted from Chen et al.20).
Dambrink et al.26 randomized 121 patients with multi-vessel disease after p-PCI for STEMI to (i) early fractional flow reserve (FFR)-guided staged PCI (performed 5–20 days after p-PCI, n = 80) vs. (ii) conservative therapy (n = 41). Forty per cent of the non-culprit lesions did not show haemodynamic significance (FFR > 0.75) and subsequent PCI of at least one non-culprit lesion was performed only in 52% of patients in the invasive group. There was no difference in the left ventricular ejection fraction (EF) at 6 months (59 vs. 57%, P = 0.362) or in major adverse cardiovascular events (MACE) (21 vs. 22%, P = 0.929). The authors concluded that an invasive strategy towards non-culprit lesions does not lead to an increase in EF or a reduction in major adverse cardiovascular events (MACE). Furthermore, the functional stenosis severity of non-culprit lesions is frequently overestimated.
The recent meta-analysis of Sethi et al.27 revealed nine non-randomized studies (with a total of 4530 patients treated by acute complete revascularization and 27 323 patients treated by the IRA PCI in the acute phase) and two small randomized studies. Major adverse cardiovascular events (OR = 0.95, 95% CI 0.47–1.90) and long-term mortality (OR = 1.10, 95% CI 0.76–1.59) were similar for both strategies.
Also one small single-centre retrospective study of Mohamad et al.28 concluded that intervention of non-critical lesions did not seem to improve MACEs or all-cause mortality at 1 year of follow-up and might in fact have had a detrimental effect on outcomes.
A recently published secondary analysis of the APEX-acute myocardial infarction trial12 found non-IRA PCI to be performed only in 9.9% of patients with STEMI and multi-vessel disease. Ninety-day death and death/congestive heart failure/shock were higher in this non-IRA group compared with the IRA-only PCI group (12.5 vs. 5.6%, P <0.001 and 17.4 vs. 12.0%, P < 0.020, respectively). After adjusting for patient and procedural characteristics as well as propensity for performing non-IRA PCI, this procedure remained independently associated with an increased hazard of 90-day mortality [adjusted hazard ratio 2.44, 95% CI (1.55–3.83), P <0.001].
Intermediate approach: acute percutaneous coronary intervention of the infarct-related artery followed by staged percutaneous coronary intervention of secondary lesions
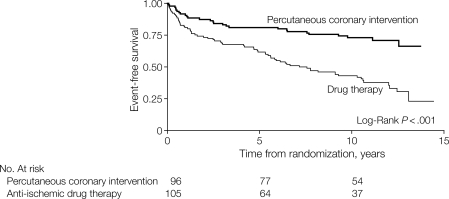
The SWISSI II randomized trial29 found elective (non-acute) PCI to be superior to medical therapy for patients with proven silent myocardial ischaemia after STEMI (Figure 3; Table 3).
Figure 3.
The SWISSI II randomized trial. Kaplan–Meier survivor function for cardiac death, non-fatal myocardial infarction, and symptom-driven revascularization. From Erne et al.29 Permission for publication granted.
Table 3.
Infarct-related artery culprit lesions then staged secondary lesions
| Advantages | Disadvantages |
|---|---|
| Optimize potential for complete revascularization | Economics |
| PCI of a stable stenosis might be intervened more safely at a later phase, after stabilization | May treat asymptomatic lesions |
| Complications of treating secondary lesions early after index event | |
| Timing uncertain |
There are no published large randomized trials. We found only five small randomized studies,17,26,30–32 enrolling between 69 and 219 patients per study. These studies have significant limitations, the main being extremely low mortality (1.9% short-term and 3.8% long-term), which may reflect the high degree of pre-selection with high-risk STEMI patients being excluded. Four of these five randomized trials reported short-term mortality of STEMI patients between 0% (two studies) and 1.4%. Thus, their results may not be applicable to routine clinical practice. One small randomized study33 compared multi-vessel PCI vs. culprit vessel only PCI in a small mixed group of patients including patients without previous acute myocardial infarction.
Advantages and disadvantages of multi-vessel percutaneous coronary intervention in ST-segment elevation myocardial infarction
The summary of all above-mentioned trials and registries is in Table 4. The reasons supporting multi-vessel PCI in STEMI patients and arguments against this strategy were summarized by Kornowski34:
Table 4.
The published studies comparing multi-vessel percutaneous coronary intervention in AMI vs. single (infarct-related artery)-vessel percutaneous coronary intervention
| First author (reference number) | Number of patients in multi-vessel PCI group | Number of patients in single-vessel PCI group | Main results |
|---|---|---|---|
| Randomized studies | |||
| Ijsselmuiden31 | 108 | 111 | No difference in MACE rates at 1 month (14.4 vs. 9.3%), 1 year (32.4 vs. 26.9%), and 4.6 years (40.4 vs. 34.6%). Study conclusion: the decision of whether to perform culprit vessel or complete revascularization can be made on an individual basis. |
| Politi17 | 130 | 84 | Multi-vessel PCI decreased in-hospital death, repeat revascularisation, and rehospitalization (all P < 0.05). |
| Dambrink26 | 80 | 41 | No difference in ejection fraction or in MACE. The stenosis severity of non-culprit lesions is frequently overestimated. |
| Ochala32 | 92 (this study compared acute multi-vessel PCI vs. staged multi-vessel PCI) | 0 (no such group in this study) | No difference in MACE. This study had too many exclusion criteria (STEMI mortality 0% in both groups!) to be relevant for clinical practice. |
| Di Mario30 | 52 | 17 | Multi-vessel PCI increased the duration of the procedure (69 vs. 53 min, P = 0.032) and the contrast load (341 vs. 242 mL, P = 0.025). Not significantly increased the in-hospital major adverse cardiac events (3.8 vs. 0%, P = 0.164). |
| Non-randomized registries | |||
| Cavender25 | 3134 | 25 802 | Multi-vessel PCI increased in-hospital mortality (7.9 vs. 5.1%, P < 0.01), including patients with cardiogenic shock (36.5 vs. 27.8%; adjusted OR 1.54, 95% confidence interval 1.22–1.95). |
| Hannan22 | 797 | 2724 | After exclusion of cardiogenic shock patients, multi-vessel PCI increased in-hospital mortality (2.4 vs. 0.9%, P = 0.04). Staged multi-vessel PCI within 60 days decreased 1-year mortality (1.3 vs. 3.3%, P = 0.04). |
| Toma12 | 217 | 1984 | Multi-vessel PCI associated with an increased hazard of 90-day mortality [adjusted HR 2.44, 95% CI (1.55–3.83), P < 0.001]. |
| Kong19 | 632 | 1350 | Multi-vessel PCI decreased in-hospital death (OR = 0.27, 95% confidence interval = 0.08–0.90, P = 0.03). |
| Chen20 | 239 | 1145 | No difference in mortality or combined death, myocardial infarction, coronary artery bypass graft, or target vessel revascularization. |
| Corpus21 | 152 | 354 | Multi-vessel PCI increased re-infarction (13.0 vs. 2.8%, P < 0.001), revascularization (25 vs. 15%, P = 0.007), and MACEs (40 vs. 28%, P = 0.006) at 1 year (OR = 1.67, P = 0.01). |
| Varani18 | 243 | 159 | After exclusion of cardiogenic shock patients, multi-vessel PCI decreased 30-day mortality (2.8 vs. 6.3%, P = 0.023). |
| Roe24 | 79 | 79 | Multi-vessel PCI increased non-significantly the risk of death (25 vs. 16.4%, n.s.), re-infarction (8.8 vs. 1.6%, P < 0.07) and significantly the risk of stroke (10.3 vs. 0%, P < 0.01) at 6-month follow-up. |
| Qarawani15 | 95 | 25 | Multi-vessel PCI decreased major cardiac events (recurrent ischaemia, re-infarction, acute heart failure, and in-hospital mortality 16.7 vs. 52%, P = 0.0001). |
| Rigattieri16 | 64 | 46 | Multi-vessel PCI non-significantly increased in-hospital MACE (20.3 vs. 10.8%, P = 0.186); but decreased out of hospital MACE (9.3 vs. 23.9%, P = 0.037). Periprocedural myocardial infarction after the elective procedure occurred in 15.6% of patients. |
| Khattab23 | 28 | 45 | No difference in major adverse cardiac events at 1 year (24 vs. 28%, P = 0.73). |
| Mohamad28 | 34 | 29 | No difference (a trend for better outcomes in IRA PCI only). |
Reasons supporting multi-vessel PCI include:
Plaque instability may not be limited to the culprit lesion,35 but may rather involve larger areas of the coronary tree (systemic endothelial dysfunction and higher platelet activity in acute coronary syndromes).
Complete coronary revascularization is known to be associated with better long-term prognosis.
Patient preference: patients may feel more comfortable when going home ‘after complete repair’. Our experience repeatedly showed, that when we recommend medical therapy to patients with multi-vessel disease after successful p-PCI of the IRA, some patients seek second opinion in other cardiac centres and undergo a second PCI there…..
Kornowski found more reasons against acute multi-vessel PCI:
The acute phase of STEMI is a highly unstable condition (haemodynamic instability, heart failure, arrhythmias, resuscitation, and patient stress among others) and is thus certainly not an ideal situation to perform PCI of a stable stenosis, which might be intervened in probably more safely at a later phase, after stabilization.
The acute phase of STEMI is an extremely prothrombotic and inflammatory milieu, which contributes to the potentially higher risk of additional PCI.
Some degree of diffuse coronary spasm (either due to endothelial dysfunction or due to catecholamine use) is frequently present in the acute phase of STEMI, which may lead to possible overestimation of stenosis severity in non-infarct arteries.36
The decision to perform non-infarct artery PCI in the acute phase of STEMI is usually not supported by the objective evidence for myocardial ischaemia in regions supplied by this non-infarct artery. It is thus the classical ‘occulo-stenotic’ indication and not evidence-based indication for PCI.
Multi-vessel PCI increases the contrast overload and further increases (already high) risk of contrast-induced nephropathy.
Any potential PCI complications in the non-infarct artery may lead to catastrophic consequences (double myocardial jeopardy) including periprocedural death.
The limited ability to discuss with patients and their families the relative risks and benefits of treating the non-infarct-related lesion vs. continued medical therapy or surgical options.
From literature review to real life: extreme variation of different clinical and angiographic scenarios!
The main limitation of all the above-mentioned published papers is the simplification. Patients with multi-vessel disease are divided into two groups: those treated by acute or staged multi-vessel PCI and those not treated by this strategy. The every day real-life clinical practice brings much more different clinical scenarios. The decision to proceed with multi-vessel PCI is simple in the rare (∼1% of all p-PCIs) situation when two unstable (thrombus containing) IRA can be clearly demonstrated by the acute phase coronary angiography. This is the only situation, when there is almost no doubt about the PCI strategy (the only doubt is which artery should be dilated first). However, in the vast majority of p-PCIs, a single IRA can be easily recognized and treated.
There are ∼60 possible scenarios based on combinations of angiographic (number of diseased vessels, lesion severity, location and type, chronic total occlusions, thrombolysis in myocardial infarction flow, collaterals, wall motion in the supplied territory, etc.) and clinical (Killip class, immediate post-PCI haemodynamic situation, renal function, etc.) findings in individual patients. Clinical decision-making in this situation is thus really complex and should be always individualized. It is unlikely that any randomized clinical trial in the future can be able to fully address this complexity and thus, experienced, wise clinical judgement will probably remain the most important factor in this difficult situation.
Functional (exercise stress) testing along with the clinical picture (patients’ symptoms) should be an important part of the decision-making algorithm. Patients who are asymptomatic and have negative functional tests and no evidence for silent ischaemia after their first STEMI should currently be treated conservatively.
The role of coronary bypass surgery (CABG) in patients with multi-vessel disease after STEMI was never investigated in a randomized study; however, it is a routine part of the clinical decision making. There is no uniform agreement on the CABG indications after STEMI treated by p-PCI. In most centres, once the culprit artery was treated by PCI, the other stenosed coronary arteries are either revascularized by staged PCI or are treated conservatively. However, in specific situations (e.g. triple-vessel disease with left main stenosis, anatomy not suitable for PCI, diabetes with triple-vessel disease, etc.), CABG should remain an important part of the treatment algorithm. Obviously, the indication for CABG should be supported by residual ischaemia (spontaneous or provoked) after STEMI.
What do the guidelines recommend?
The European Society of Cardiology guidelines for PCI37 state that in multi-vessel disease, p-PCI should be directed only at the infarct-related coronary artery. Decisions about PCI of non-culprit lesions should be done later and guided by objective evidence of residual ischaemia. Only in the setting of cardiogenic shock is there a consensus for attempting multi-vessel PCI in selected patients with multiple critical lesions.
A 2009 report on the appropriateness criteria for coronary revascularization written by the expert panel of the ACCF/SCAI/STS/AATS/AHA/ASNC38 considers revascularization of a non-IRA during index hospitalization inappropriate in STEMI patients with successful treatment of the culprit artery by p-PCI or fibrinolysis when these patients are asymptomatic, without heart failure, have no evidence of recurrent or provocable ischaemia or did not have any unstable ventricular arrhythmias during the index hospitalization and have normal EF. On the other hand, STEMI or non-STEMI myocardial infarction patients after successful PCI of culprit artery with symptoms of recurrent myocardial ischaemia and/or high-risk findings on non-invasive stress testing performed after index hospitalization are suitable candidates for revascularization of additional coronary arteries.
Currently running/future trials
Several studies are currently running trying to contribute to this question. One such study is led by D. Wood (http://clinicaltrialsfeeds.org/clinical-trials/show/NCT01065103), and another by O. Hlinomaz, and L. Groch (the PRAGUE-13 study). None of these is large enough to provide a definite answer. The authors are not currently aware about a larger ongoing study on this topic.
Conclusions
Multi-vessel disease in STEMI is not a single entity and thus the treatment approach should be individualized. However, the following general rules39 can be proposed till future large randomized trials prove otherwise:
Single-vessel acute PCI should be the default strategy (to treat only the IRA during the acute phase of STEMI).
Acute multi-vessel PCI can be justified only in haemodynamically unstable patients with multiple truly critical (>90%) lesions.
Significant lesions of the non-culprit arteries should be treated either medically or by staged revascularization procedures—both options are currently acceptable.
Funding
Funding to pay the Open Access publication charges for this article was provided by The Charles University Prague, research project MSM0021620817.
Conflict of interest: none declared.
References
- 1.Zijlstra F, de Boer MJ, Hoorntje JC, Reiffers S, Reiber JH, Suryapranata H. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med. 1993;328:680–684. doi: 10.1056/NEJM199303113281002. doi:10.1056/NEJM199303113281002. [DOI] [PubMed] [Google Scholar]
- 2.Grines CL, Browne KF, Marco J, Rothbaum D, Stone GW, O'Keefe J, Overlie P, Donohue B, Chelliah N, Timmis GC. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med. 1993;328:673–679. doi: 10.1056/NEJM199303113281001. doi:10.1056/NEJM199303113281001. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons RJ, Holmes DR, Reeder GS, Bailey KR, Hopfenspirger MR, Gersh BJ. Immediate angioplasty compared with the administration of a thrombolytic agent followed by conservative treatment for myocardial infarction. The Mayo Coronary Care Unit and Catheterization Laboratory Groups. N Engl J Med. 1993;328:685–691. doi: 10.1056/NEJM199303113281003. doi:10.1056/NEJM199303113281003. [DOI] [PubMed] [Google Scholar]
- 4.Widimský P, Budesínský T, Vorác D, Groch L, Zelízko M, Aschermann M, Branny M, St'ásek J, Formánek P ‘PRAGUE’ Study Group Investigators. Long distance transport for primary angioplasty vs. immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial—PRAGUE-2. Eur Heart J. 2003;24:94–104. doi: 10.1016/s0195-668x(02)00468-2. doi:10.1016/S0195-668X(02)00468-2. [DOI] [PubMed] [Google Scholar]
- 5.Andersen HR, Nielsen TT, Rasmussen K, Thuesen L, Kelbaek H, Thayssen P, Abildgaard U, Pedersen F, Madsen JK, Grande P, Villadsen AB, Krusell LR, Haghfelt T, Lomholt P, Husted SE, Vigholt E, Kjaergard HK, Mortensen LS DANAMI-2 Investigators. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349:733–742. doi: 10.1056/NEJMoa025142. doi:10.1056/NEJMoa025142. [DOI] [PubMed] [Google Scholar]
- 6.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. doi:10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 7.Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M ESC Committee for Practice Guidelines (CPG) Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Silber S, Aguirre FV, Al-Attar N, Alegria E, Andreotti F, Benzer W, Breithardt O, Danchin N, Di Mario C, Dudek D, Gulba D, Halvorsen S, Kaufmann P, Kornowski R, Lip GY. Management of acute myocardial infarction in patients presenting with persistent ST segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–2945. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- 8.Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC, Jr, Anbe DT, Kushner FG, Ornato JP, Pearle DL, Sloan MA, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:210–247. doi: 10.1016/j.jacc.2007.10.001. doi:10.1016/j.jacc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Cardarelli F, Bellasi A, Fang-Shu Ou, Shaw LJ, Veledar E, Roe MT, Morris DC, Peterson ED, Klein LW, Raggi P. Combined impact of age and estimated glomerular filtration rate on in-hospital mortality after percutaneous coronary intervention for acute myocardial infarction (from the American College of Cardiology National Cardiovascular Data Registry) Am J Cardiol. 2009;103:766–771. doi: 10.1016/j.amjcard.2008.11.033. doi:10.1016/j.amjcard.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 10.Jang Hoon Lee, Hun Sik Park, Shung Chull Chae, Yongkeun Cho, Dong Heon Yang, Myung Ho Jeong, Young Jo Kim, Kee-Sik Kim, Seung Ho Hur, In Whan Seong, Taek Jong Hong, Myeong Chan Cho, Chong Jin Kim, Jae Eun Jun, Wee Hyun Park Korea Acute Myocardial Infarction Registry Investigators. Predictors of six-month major adverse cardiac events in 30-day survivors after acute myocardial infarction (from the Korea Acute Myocardial Infarction Registry) Am J Cardiol. 2009;104:182–189. doi: 10.1016/j.amjcard.2009.03.010. doi:10.1016/j.amjcard.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Rasoul S, Ottervanger JP, de Boer MJ, Dambrink JHE, Hoorntje JCA, Gosselink ATM, Zijlstra F, Suryapranata H, van't Hof AWJ. Predictors of 30-day and 1-year mortality after primary percutaneous coronary intervention for ST-elevation myocardial infarction. Coron Artery Dis. 2009;20:415–421. doi: 10.1097/MCA.0b013e32832e5c4c. doi:10.1097/MCA.0b013e32832e5c4c. [DOI] [PubMed] [Google Scholar]
- 12.Toma M, Buller CE, Westerhout CM, Fu Y, O'Neill WW, Holmes DR, Jr, Hamm CW, Granger CB, Armstrong PW for the APEX-AMI Investigators. Non-culprit coronary artery percutaneous coronary intervention during acute ST-segment elevation myocardial infarction: insights from the APEX-AMI trial. Eur Heart J. 2010;31:1701–1707. doi: 10.1093/eurheartj/ehq129. doi:10.1093/eurheartj/ehq129. [DOI] [PubMed] [Google Scholar]
- 13.Lemesle G, de Labriolle A, Bonello L, Torguson R, Kaneshige K, Xue Z, Suddath WO, Satler LF, Kent KM, Lindsay J, Pichard AD, Waksman R. Incidence, predictors, and outcome of new, subsequent lesions treated with percutaneous coronary intervention in patients presenting with myocardial infarction. Am J Cardiol. 2009;103:1189–1195. doi: 10.1016/j.amjcard.2009.01.029. doi:10.1016/j.amjcard.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Jaski BE, Cohen JD, Trausch J, Marsh DG, Bail GR, Overlie PA, Skowronski EW, Smith SC., Jr Outcome of urgent percutaneous transluminal coronary angioplasty in acute myocardial infarction: comparison of single-vessel versus multivessel coronary artery disease. Am Heart J. 1992;124:1427–1433. doi: 10.1016/0002-8703(92)90053-x. doi:10.1016/0002-8703(92)90053-X. [DOI] [PubMed] [Google Scholar]
- 15.Qarawani D, Nahir M, Abboud M, Hazanov Y, Hasin Y. Culprit only versus complete coronary revascularization during primary PCI. Int J Cardiol. 2008;123:288–292. doi: 10.1016/j.ijcard.2006.12.013. doi:10.1016/j.ijcard.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Rigattieri S, Biondi-Zoccai G, Silvestri P, Di Russo C, Musto C, Ferraiuolo G, Loschiavo P. Management of multivessel coronary disease after ST elevation myocardial infarction treated by primary angioplasty. J Interv Cardiol. 2008;21:1–7. doi: 10.1111/j.1540-8183.2007.00317.x. doi:10.1111/j.1540-8183.2007.00317.x. [DOI] [PubMed] [Google Scholar]
- 17.Politi L, Sgura F, Rossi R, Monopoli D, Guerri E, Leuzzi C, Bursi F, Sangiorgi GM, Modena MG. A randomised trial of target-vessel versus multi-vessel revascularisation in ST-elevation myocardial infarction: major adverse cardiac events during long-term follow-up. Heart. 2010;96:662–667. doi: 10.1136/hrt.2009.177162. doi:10.1136/hrt.2009.177162. [DOI] [PubMed] [Google Scholar]
- 18.Varani E, Balducelli M, Aquilina M, Vecchi G, Hussien MN, Frassineti V, Maresta A. Single or multivessel percutaneous coronary intervention in ST-elevation myocardial infarction patients. Catheter Cardiovasc Interv. 2008;72:927–933. doi: 10.1002/ccd.21722. doi:10.1002/ccd.21722. [DOI] [PubMed] [Google Scholar]
- 19.Kong JA, Chou ET, Minutello RM, Wong SC, Hong MK. Safety of single versus multi-vessel angioplasty for patients with acute myocardial infarction and multi-vessel coronary artery disease: report from the New York State Angioplasty Registry. Coron Artery Dis. 2006;17:71–75. doi: 10.1097/00019501-200602000-00012. doi:10.1097/00019501-200602000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Chen LY, Lennon RJ, Grantham JA, Berger PB, Mathew V, Singh M, Holmes DR, Jr, Rihal CS. In-hospital and long-term outcomes of multivessel percutaneous coronary revascularization after acute myocardial infarction. Am J Cardiol. 2005;95:349–354. doi: 10.1016/j.amjcard.2004.09.032. doi:10.1016/j.amjcard.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 21.Corpus RA, House JA, Marso SP, Grantham JA, Huber KC, Jr, Laster SB, Johnson WL, Daniels WC, Barth CW, Giorgi LV, Rutherford BD. Multivessel percutaneous coronary intervention in patients with multivessel disease and acute myocardial infarction. Am Heart J. 2004;148:493–500. doi: 10.1016/j.ahj.2004.03.051. doi:10.1016/j.ahj.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 22.Hannan EL, Samadashvili Z, Walford G, Holmes DR, Jr, Jacobs AK, Stamato NJ, Venditti FJ, Sharma S, King SB., 3rd Culprit vessel percutaneous coronary intervention versus multivessel and staged percutaneous coronary intervention for ST-segment elevation myocardial infarction patients with multivessel disease. JACC Cardiovasc Interv. 2010;3:22–31. doi: 10.1016/j.jcin.2009.10.017. doi:10.1016/j.jcin.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Khattab AA, Abdel-Wahab M, Röther C, Liska B, Toelg R, Kassner G, Geist V, Richardt G. Multi-vessel stenting during primary percutaneous coronary intervention for acute myocardial infarction. A single-center experience. Clin Res Cardiol. 2008;97:32–38. doi: 10.1007/s00392-007-0570-4. doi:10.1007/s00392-007-0570-4. [DOI] [PubMed] [Google Scholar]
- 24.Roe MT, Cura FA, Joski PS, Garcia E, Guetta V, Kereiakes DJ, Zijlstra F, Brodie BR, Grines CL, Ellis SG. Initial experience with multivessel percutaneous coronary intervention during mechanical reperfusion for acute myocardial infarction. Am J Cardiol. 2001;88:170–173. doi: 10.1016/s0002-9149(01)01615-0. doi:10.1016/S0002-9149(01)01615-0. [DOI] [PubMed] [Google Scholar]
- 25.Cavender MA, Milford-Beland S, Roe MT, Peterson ED, Weintraub WS, Rao SV. Prevalence, predictors, and in-hospital outcomes of non-infarct artery intervention during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction (from the National Cardiovascular Data Registry) Am J Cardiol. 2009;104:507–513. doi: 10.1016/j.amjcard.2009.04.016. doi:10.1016/j.amjcard.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Dambrink JH, Debrauwere JP, van 't Hof AW, Ottervanger JP, Gosselink AT, Hoorntje JC, de Boer MJ, Suryapranata H. Non-culprit lesions detected during primary PCI: treat invasively or follow the guidelines? EuroIntervention. 2010;5:968–975. doi:10.4244/EIJV5I8A162. [PubMed] [Google Scholar]
- 27.Sethi A, Bahekar A, Bhuriya R, Singh S, Ahmed A, Khosla S. Complete versus culprit only revascularization in acute ST elevation myocardial infarction - a meta-analysis. Catheter Cardiovasc Interv. 2010 doi: 10.1002/ccd.22647. Published online ahead of print 1 June 2010. [DOI] [PubMed] [Google Scholar]
- 28.Mohamad T, Bernal JM, Kondur A, Hari P, Nelson K, Niraj A, Badheka A, Hassna S, Kiernan T, Elder MD, Gardi D, Schreiber T. Coronary Revascularization Strategy for ST Elevation Myocardial Infarction with Multivessel Disease: Experience and Results at 1-Year Follow-Up. Am J Ther. 2010 doi: 10.1097/MJT.0b013e3181b809ee. Published online ahead of print 4 January 2010. [DOI] [PubMed] [Google Scholar]
- 29.Erne P, Schoenenberger AW, Burckhardt D, Zuber M, Kiowski W, Buser PT, Dubach P, Resink TJ, Pfisterer M. Effects of percutaneous coronary interventions in silent ischemia after myocardial infarction: the SWISSI II randomized controlled trial. JAMA. 2007;297:1985–1991. doi: 10.1001/jama.297.18.1985. doi:10.1001/jama.297.18.1985. [DOI] [PubMed] [Google Scholar]
- 30.Di Mario C, Mara S, Flavio A, mad S, Antonio M, Anna P, Emanuela P, Stefano DS, Angelo R, Stefania C, Anna F, Carmelo C, Antonio C, Monzini N, Bonardi MA. Single vs multivessel treatment during primary angioplasty: results of the multicentre randomised HEpacoat for cuLPrit or multivessel stenting for Acute Myocardial Infarction (HELPAMI) Study. Int J Cardiovasc Intervent. 2004;6:128–133. doi: 10.1080/14628840310030441. [DOI] [PubMed] [Google Scholar]
- 31.Ijsselmuiden AJ, Ezechiels J, Westendorp IC, Tijssen JG, Kiemeneij F, Slagboom T, van der Wieken R, Tangelder G, Serruys PW, Laarman G. Complete versus culprit vessel percutaneous coronary intervention in multivessel disease: a randomized comparison. Am Heart J. 2004;148:467–474. doi: 10.1016/j.ahj.2004.03.026. doi:10.1016/j.ahj.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Ochala A, Smolka GA, Wojakowski W, Dudek D, Dziewierz A, Krolikowski Z, Gasior Z, Tendera M. The function of the left ventricle after complete multivessel one-stage percutaneous coronary intervention in patients with acute myocardial infarction. J Invasive Cardiol. 2004;16:699–702. [PubMed] [Google Scholar]
- 33.Kussmaul WG, 3rd, Krol J, Laskey WK, Herrmann HC, Hirshfeld JW., Jr One-year follow-up results of ‘culprit’ versus multivessel coronary angioplasty trial. Am J Cardiol. 1993;71:1431–1433. doi: 10.1016/0002-9149(93)90605-c. doi:10.1016/0002-9149(93)90605-C. [DOI] [PubMed] [Google Scholar]
- 34.Kornowski R. Completeness of revascularization in patients with ST-Elevation acute myocardial infarction. Catheter Cardiovasc Interv. 2008;72:934–936. doi: 10.1002/ccd.21875. doi:10.1002/ccd.21875. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein JA, Demetriou D, Grines CL, Pica M, Shoukfeh M, O'Neill WW. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343:915–922. doi: 10.1056/NEJM200009283431303. doi:10.1056/NEJM200009283431303. [DOI] [PubMed] [Google Scholar]
- 36.Hanratty CG, Koyama Y, Rasmussen HH, Nelson GI, Hansen PS, Ward MR. Exaggeration of non culprit stenosis severity during acute myocardial infarction: implications for immediate multivessel revascularization. J Am Coll Cardiol. 2002;40:911–916. doi: 10.1016/s0735-1097(02)02049-1. doi:10.1016/S0735-1097(02)02049-1. [DOI] [PubMed] [Google Scholar]
- 37.Silber S, Albertsson P, Avilés FF, Camici PG, Colombo A, Hamm C, Jørgensen E, Marco J, Nordrehaug JE, Ruzyllo W, Urban P, Stone GW, Wijns W Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Guidelines for percutaneous coronary interventions. The Task Force for percutaneous coronary interventions of the European Society of Cardiology. Eur Heart J. 2005;26:804–847. doi: 10.1093/eurheartj/ehi138. doi:10.1093/eurheartj/ehi138. [DOI] [PubMed] [Google Scholar]
- 38.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA American College of Cardiology Foundation Appropriateness Criteria Task Force; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; American Association for Thoracic Surgery; American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography; Heart Failure Society of America; Society of Cardiovascular Computed Tomography. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2009;53:530–553. doi: 10.1016/j.jacc.2008.10.005. doi:10.1016/j.jacc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas PE, Widimsky P, Alfieri O, Dunning J, Elia S, Kappetein P, Lockowandt U, Sarris G, Vouhe P, Kearney P, von Segesser L, Agewall S, Aladashvili A, Alexopoulos D, Antunes MJ, Atalar E, Brutel de la Riviere A, Doganov A, Eha J, Fajadet J, Ferreira R, Garot J, Halcox J, Hasin Y, Janssens S, Kervinen K, Laufer G, Legrand V, Nashef SA, Neumann FJ, Niemela K, Nihoyannopoulos P, Noc M, Piek JJ, Pirk J, Rozenman Y, Sabate M, Starc R, Thielmann M, Wheatley DJ, Windecker S, Zembala M. Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2010 doi: 10.1093/eurheartj/ehu278. Published online ahead of print 29 August 2010. [DOI] [PubMed] [Google Scholar]