Abstract
We showed that F1 hybrid genotypes may provide a broader variety of ethanol drinking phenotypes than the inbred progenitor strains used to create the hybrids (Blednov et al. in Alcohol Clin Exp Res 29:1949–1958–2005). To extend this work, we characterized alcohol consumption as well as intake of other tastants (saccharin, quinine and sodium chloride) in five inbred strains of mice (FVB, SJL, B6, BUB, NZB) and in their reciprocal F1 hybrids with B6 (FVBxB6; B6xFVB; NZBxB6; B6xNZB; BUBxB6; B6xBUB; SJLxB6; B6xSJL). We also compared ethanol intake in these mice for several concentrations before and after two periods of abstinence. F1 hybrid mice derived from the crosses of B6 and FVB and also B6 and SJL drank higher levels of ethanol than their progenitor strains, demonstrating overdominance for two-bottle choice drinking test. The B6 and NZB hybrid showed additivity in two-bottle choice drinking, whereas the hybrid of B6 and BUB demonstrated full or complete dominance. Genealogical origin, as well as non-alcohol taste preferences (sodium chloride), predicted ethanol consumption. Mice derived from the crosses of B6 and FVB showed high sustained alcohol preference and the B6 and NZB hybrids showed reduced alcohol preference after periods of abstinence. These new genetic models offer some advantages over inbred strains because they provide high, sustained, alcohol intake, and should allow mapping of loci important for the genetic architecture of these traits.
Keywords: Alcohol intake, Inbred strains, F1 hybrid, Tastes, Overdominance
Introduction
Recently, we found that C57BL/6JxFVB/NJ F1 hybrid mice self-administered unusually high levels of ethanol during two-bottle preference test (females averaging from 20 to 35 g/kg/day, males 7–25 g/kg/day, depending on concentration) (Blednov et al. 2005). These unexpected results clearly showed that populations of hybrid genotypes may provide a broader range of ethanol drinking than was previously obtained from inbred strains. Indeed, multiple surveys of inbred strains of mice failed to reveal a more extreme preferrer of alcohol solutions than C57BL/6J (B6) mice. In a two bottle choice preference test, where the choice is between a 10% ethanol solution and water, male B6 mice will self-administer ethanol in the range of 10–14 g/kg/day, while female B6 mice will self-administer in the range of 12–18 g/kg/day (Rodgers 1972; Belknap et al. 1993; Wahlsten et al. 2006). This raises the question of whether the FVB strain is unique or can we identify other inbred strains which could be crossed with B6 mice to produce other high drinking hybrids. To approach this question, we considered the genetic origin of these strains and, because the taste plays an important role in regulation of ethanol intake in two-bottle choice model, we also studied the taste characteristics of related inbred strains and hybrid mice.
It should be noted that the genealogies of FVB and B6 inbred strains are quite different (Beck et al. 2000; Festing 1994; Morse 1978). Genealogically, the commercially available SJL/J (SJL) inbred strain is one of the closest relatives of FVB inbred strain (Beck et al. 2000). Remarkably, Petkov et al. (2004) based on analyses of single nucleotide polymorphisms (SNPs) constructed a mouse strain family tree, which in most cases confirmed existing genealogies. In their classification, FVB and SJL inbred strains are in Group 2 whereas B6 inbred strain is in Group 4. This raises the possibility that the common ancestry of the FVB and SJL inbred strains will allow the SJLxB6 F1 hybrid mice to also demonstrate high alcohol intake.
Ethanol consumption in two-bottle choice test depends strongly on sweet taste (Bachmanov et al. 1996; Belknap et al. 1993; Blednov et al. 2008; Blizard and McClearn 2000; Kampov-Polevoy et al. 1995; Kiefer et al. 1990). However, FVB and B6 inbred strains are not very different in consumption of 0.2% of saccharin (Yoneyama et al. 2008), although FVB and B6 differ in preference for some other tastants (Bachmanov et al. 2002). In particular, B6 mice display greater preference for solutions of potassium chloride and ammonium chloride, while FVB mice display greater preference for sodium chloride and sodium lactate (Bachmanov et al. 2002). These researchers also found that BUB/BnJ (BUB) and NZB/B1NJ (NZB) inbred strains, like FVB, demonstrated high preference for different concentrations of sodium chloride. Studies that compared individuals with a paternal history of alcoholism to subjects with no family history of alcoholism noted enhanced unpleasant response to concentrated sodium chloride and citric acid in those with a family history of alcoholism (Scinska et al. 2001; Sandstrom et al. 2003). These data suggest that increased aversive responses to salt taste may predict future development of alcohol dependence. If a high preference for salty taste (or other tastes) was responsible for the ethanol phenotype seen in FVBxB6 hybrids, then similar ethanol as well as taste phenotypes may be present in BUBxB6 and NZBxB6 hybrid mice. Interestingly, in the mouse strain family tree (Petkov et al. 2004) BUB is a member of Group 2 together with FVB and SJL, whereas the NZB strain is a member of Group 3.
Another aspect of models of alcohol consumption is the effect of periods of alcohol deprivation. Recently, Melendez et al. (2006) demonstrated that repeated exposure of B6 mice to alcohol after a period of abstinence may lead to an increase or decrease of alcohol intake depending on the conditions of abstinence and we found that B6 mice with a history of two-bottle choice alcohol consumption reduced alcohol intake after a week of alcohol deprivation (Y. A. Blednov, unpublished). This led us to ask if the unusually high level of alcohol intake observed in FVBxB6 F1 hybrid mice would be stable after abstinence (deprivation).
Overall, there were three goals of this study. The first goal was to investigate the ethanol consumption of five inbred strains (FVB, SJL, B6, BUB, NZB) and in their reciprocal F1 hybrids (FVBxB6; B6xFVB; NZBxB6; B6xNZB; BUBxB6; B6xBUB; SJLxB6; B6xSJL). The second goal was to compare initial ethanol intake with ethanol intake after several periods of abstinence for mice of different genetic backgrounds. The third goal of this study was to investigate non-alcohol (saccharin, quinine and sodium chloride) taste preferences in mice from the genetic backgrounds tested for alcohol consumption.
Materials and methods
Animals
Origin
Studies were conducted in drug-naïve C57BL/6J, FVB/NJ, SJL/J, BUB/BnJ, NZB/B1NJ and reciprocal intercross F1 hybrid mice derived from these five progenitors (B6xFVB F1 and FVBxB6 F1, maternal strain × paternal strain; B6xSJL F1 and SJLxB6 F1, B6xBUB F1 and BUBxB6 F1; B6xNZB F1 and NZBxB6 F1). B6, FVB, SJL, BUB and NZB breeders were purchased from The Jackson Laboratory (Bar Harbor, ME) and mated at age of 8 weeks in the Texas Genetic Animal Core of the INIA (Integrated Neuroscience Initiative on Alcohol) at University of Texas at Austin. Offspring were weaned into isosexual groups of each of the 13 genotypes (B6, FVB, SJL, BUB, NZB, B6xFVB F1, FVBxB6 F1, B6xSJL F1, SJLxB6 F1, B6xBUB F1, BUBxB6 F1, B6xNZB F1, NZBxB6 F1).
Maintenance
Mice (4–5 per cage) were housed in standard polycarbonate shoebox cages with food (Prolab RMH 1800 5LL2 chow) and water provided ad libitum. The colony rooms and testing rooms were maintained in ambient temperature of 21 ± 1°C, humidity (40–60%) and centrally controlled ventilation (12–15 cycles/h with 100% exhaust). Colony rooms were on a 12:12 light/dark light cycle (lights on at 07:00 a.m.). All procedures were approved by the correspondent Institutional Animal Care and Use Committee and adhered to NIH Guidelines. The University of Texas facility is AAALAC accredited. The largest differences between FVBxB6 F1 hybrid mice and B6 inbred strain were previously found for female mice only, therefore only female mice were used in all experiments.
Ethanol intake in two-bottle choice test
Experimentally naïve, adult mice between 60 and 90 days of age were used in all experiments. To avoid seasonal and other time-dependent effects, SJL, FVB, B6 (half of total number of B6), FVBxB6, B6xFVB, SJLxB6 and B6xSJL were tested at the same time and 6 weeks later a second experiment with BUB, NZB, B6 (half of total number of B6), BUBxB6, B6xBUB, NZBxB6 and B6xNZB was started.
Experiments were conducted with conditions of lighting, food, and water like those in the colony rooms, except where stated, and animals were acclimated to testing rooms for 5–7 days before the start of each experiment. Numbers of mice/group are given in figure legends and tables. To avoid a potential parental effect, no more than two mice originating from the same breeder pair were taken for an experiment. To minimize any possible cage effect, no more than two mice from the same cage were taken for an experiment. Body weights were recorded at the beginning of each experiment and at least every 4 days, always on an ethanol concentration change day. Clean cages were provided every 8 days. All animals were acclimated for at least 2 days to fluid bottles with sipper tubes containing water before introduction of an ethanol solution.
Adult female mice were tested in a two-bottle choice experiment as was described earlier (Blednov et al. 2001). Briefly, experiments were carried out in standard 7.5″ × 12.5″ polycarbonate cages in sliding racks. Bottles were placed vertically 3″ from the back wall through two holes in the cage wire-mesh top. The distance between two bottles was about 2″. A feeder was placed on the front wall (opposite from bottles).
The mice were individually housed with access to two 50 ml plastic water bottles with straight sipper tubes containing tap water. Eleven concentrations of ethanol (3, 6, 9, 12, 15, 18, 21, 24, 27, 30 and 35% v/v) in tap water were offered for 4 days each, starting with the lowest concentration and increasing to the highest. Both bottles were weighed daily. As spillage and evaporation controls, average weight of volume depleted from tubes in control cages without mice was subtracted from individual drinking values each day. Tube positions were switched to the opposite side daily. Before placing the next greater concentration onto each cage, all mice were weighed.
After the last day of consumption of the 35% solution, animals had access only to the water bottle for 1 week. After this 1 week of abstinence, the two-bottle choice procedure was repeated with the same mice with 9, 18 and 27% ethanol solutions under conditions described above. The same procedure, including one more week of abstinence from ethanol, was repeated one more time.
Aaper brand (Aaper Alcohol and Chemical, Shelbyville, KY) 200 proof ethanol was used to mix solutions as v/v in tap water.
Preference for non-ethanol tastants in two-bottle choice test
Separate groups of experimentally naïve mice of all genotypes described above were also tested for saccharin, quinine and sodium chloride consumption. Mice were serially offered sodium chloride (75, 150 and 300 mM), quinine hemisulfate (0.03 and 0.06 mM) and saccharin (0.033%) and intakes for 24 h of drinking were calculated. The concentrations were chosen to be sufficient to provide an effect of the tastants without having a ‘floor’ or ‘ceiling’ effect (preference ratio approaching zero or one). Concentrations were based on our pilot experiments (Y. A. Blednov, unpublished data) and on published data (Bachmanov et al. 2002). Each concentration was offered for 4 days, with bottle positions changed every day. Within each tastant, the low concentration was always presented first, followed by the higher concentrations in increasing order. Between tastants, mice had two bottles with water for 2 weeks.
Data analysis
Data are reported as the mean ± SEM value. The dependent measures were weight of ethanol, water and different tastants consumed, ethanol dose (g/kg per day) consumed, preference ratio for ethanol and for the different tastants. When appropriate, trial was included as a repeated measures factor. To evaluate differences between groups, analysis of variance (two-way ANOVA and one-way ANOVA with Post hoc Bonferroni Multiple Comparison) was used. The statistics software programs GraphPad Prizm (Jandel Scientific, Costa Madre, CA) and STATISTICA (StatSoft, Inc., Tulsa, OK) were used throughout.
As noted above, the B6 mice were tested in two different groups but both groups showed very similar ethanol intake they were combined in one group for statistical analyses. Each of the four groups of hybrid mice tested at the same time were analyzed by a Two-Way ANOVA, and for each of these four groups a strong genotype × concentration interaction was found (P<0.0001). Next, two-way ANOVA analyses were performed for pairs of strains from each group; e.g., B6 vs. FVB; B6 vs. FVBxB6, B6 vs. B6xFVB; FVB vs. FVBxB6; FVB vs. B6xFVB; FVBxB6 vs. B6xFVB. In contrast, the omnibus analysis approach which could be used for the analyses of such data set would necessarily include genotypes of mice not tested concurrently, thus risking possible seasonal effects and other genotype × environment confounds arising when the 13 genotypes in this study are tested at different times but analyzed as a single large experiment.
Determination of additive and dominance effects
For each trait, the value of a, the additive effect, or the average effect of an allele substitution (Falconer and Mackay 1996), was calculated as one-half the phenotypic difference between the means of the two homozygous inbred progenitor strains. In this case, the difference yields positive values of a when the B6 genotype showed higher expression values, and negative if the other genotype showed higher expression values. Also, d, the dominance effect (Falconer and Mackay 1996; Kearsey and Pooni 1996), was calculated as the difference between the phenotypic mean of the F1 and the average (midpoint) of the two inbred progenitor strains. In our study, the area under the curve calculated from ethanol intake (g/kg/24 h) vs. concentrations of ethanol solution for each genotype was used as the phenotypic mean.
The next step is to standardize both a (additive) and d (dominance) effects by dividing these two variables by the pooled within genotype standard deviation (SD) units. The pooled within genotype SD is the square root of the mean square within (MSW) from a one-way ANOVA by strain. The sign of d was positive if the F1 mean trait values scored above the mean of the two inbred strains, and was negative if below. The ratio d/a was then determined (Kearsey and Pooni 1996); this value is 0 with no dominance, 1.0 with full or complete dominance, and >1.0 with hybrid overdominance.
Tests of significance for d (dominance) effects on trait values
The presence of dominance (d) was tested vs. the null hypothesis that d = 0 using the equation t = ((|d|) dfd 1/2)/2 as a two-tailed t test (Rosenthal 1994). The test for over-dominance was a test that |d| was significantly greater than |a| using the equation t = ((|d| − |a|) dfd 1/2)/2 as a two-tailed t test. The observed standardized values of a and d were used for these calculations. The values of dfd (degrees of freedom for d) were calculated as dfd = N − 2, and N is the total number of mice (Rosenthal 1994).
Results
Genetic variation in ethanol intake
Data for ethanol intake (amount of ethanol consumed, preference for ethanol and total fluid intake) in a continuous access two-bottle choice test for five inbred strains and eight F1 hybrids are presented in Figs. 1 and 2 (for detailed statistics see Supplemental materials in Table I, Table II, Table III and Table IV). Taken together, these results show that four inbred strains, SJL, BUB, NZB and BUB, consumed less ethanol with lower preference than the B6 inbred strain. Four (FVBxB6; B6xFVB; SJLxB6; B6xSJL) of the eight F1 hybrids showed higher ethanol intake and preference than the B6 parental strain. Ethanol intake and preference in two F1 hybrids (BUBxB6 and B6xBUB) was similar with the B6 inbred strain. Two F1 hybrid lines (NZBxB6 and B6xNZB) showed slightly lower ethanol intake and preference for ethanol than the B6 inbred strain.
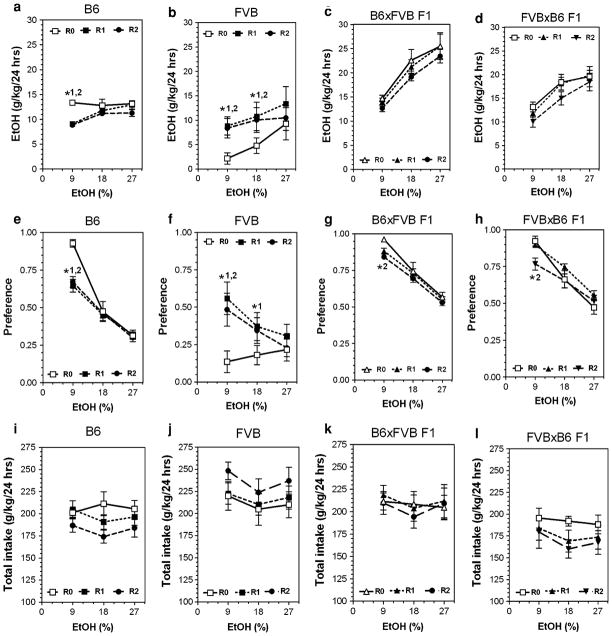
Fig. 1.
Consumption of increasing concentrations of ethanol by B6, FVB, SJL inbred strains and reciprocal F1 hybrids after intercross between B6 and FVB and between B6 and SJL mice in a two-bottle preference test. a Amount of ethanol consumed (g/kg/day) in B6, FVB and their F1 reciprocal hybrids. b Preference for ethanol in B6, FVB and their F1 reciprocal hybrids. c Total fluid intake (g/kg/day) in B6, FVB and their F1 reciprocal hybrids. n = 15 (B6); n = 9 (FVB); n = 12 (both F1 hybrids). d Amount of ethanol consumed (g/kg/day) in B6, SJL and their F1 reciprocal hybrids. e Preference for ethanol in B6, SJL and their F1 reciprocal hybrids. f Total fluid intake (g/kg/day) in B6, SJL and their F1 reciprocal hybrids. n = 15 (B6); n = 7 (FVB); n = 8 (B6xSJL F1 hybrids); n = 8 (SJLxB6 F1 hybrids)
Fig. 2.
Consumption of increasing concentrations of ethanol by B6, NZB, BUB inbred strains and reciprocal F1 hybrids after intercross between B6 and NZB and between B6 and BUB mice in a two-bottle preference test. Amount of ethanol consumed (g/kg/day) in B6, NZB and their F1 reciprocal hybrids. b Preference for ethanol in B6, NZB and their F1 reciprocal hybrids. c Total fluid intake (g/kg/day) in B6, NZB and their F1 reciprocal hybrids. n = 15 (B6); n = 9 (NZB); n = 10 (both F1 hybrids). d Amount of ethanol consumed (g/kg/day) in B6, BUB and their F1 reciprocal hybrids. e Preference for ethanol in B6, BUB and their F1 reciprocal hybrids. f Total fluid intake (g/kg/day) in B6, BUB and their F1 reciprocal hybrids. n = 15 (B6); n = 10 (BUB); n = 10 (B6xBUB F1 hybrids); n = 9 (BUBxB6 F1 hybrids)
Consumption of ethanol after periods of abstinence
To evaluate the effects of abstinence on ethanol consumption, two trials of alcohol drinking were carried out, each separated by 1 week of no access to ethanol. Ethanol and water intake were measured after first and after second periods of abstinence at 9, 18 and 27% concentrations of ethanol. These numbers were compared with data for experimentally naïve mice (first presentation of ethanol).
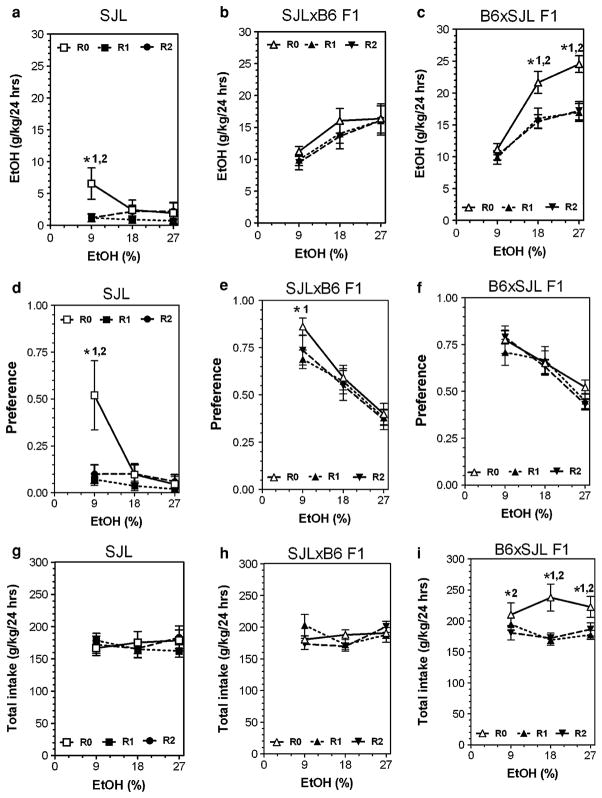
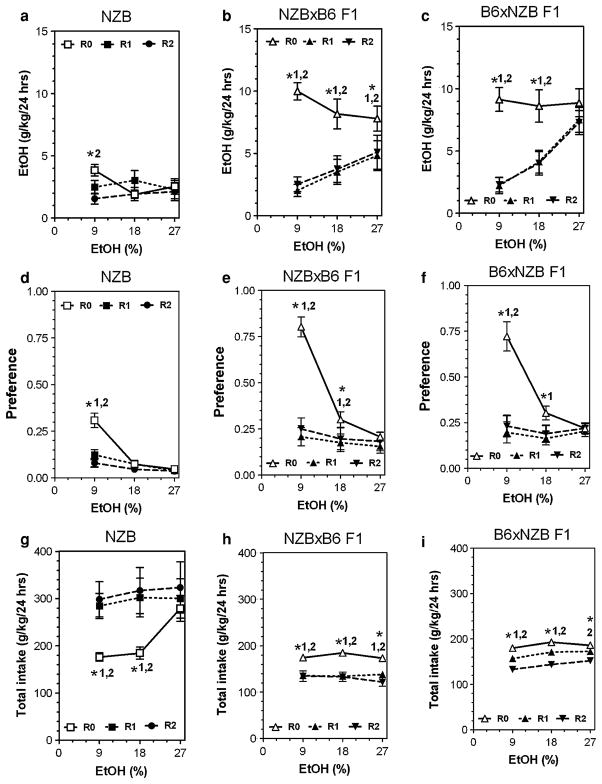
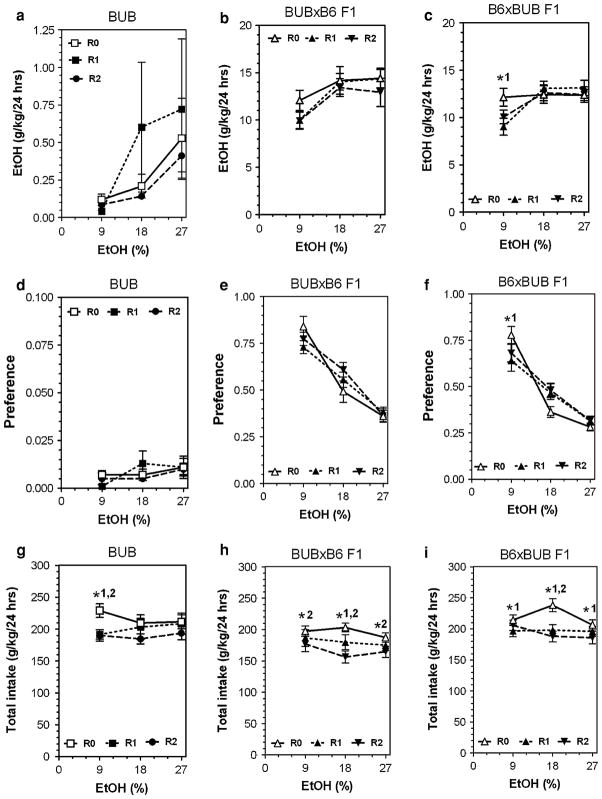
Detailed data for all parameters of ethanol intake after several periods of abstinence are presented in Figs. 3, 4, 5 and 6 (for detailed statistics see Supplemental materials in Table V, Table VI and Table VII). Three strains (B6, SJL and NZB) showed reduction of ethanol intake and preference (mostly at an ethanol concentration of 9%) after periods of abstinence. In contrast, abstinence increased the amount of ethanol consumed as well as preference for ethanol in FVB mice. The BUB mice showed such low consumption of ethanol that it is not meaningful to analyze the effect of abstinence in this strain. Most of the hybrid mice did not show marked changes in alcohol preference or consumption after abstinence. Two hybrids lines (FVBxB6 and B6xFVB) did not show any changes in ethanol intake or preference over several periods of abstinence. Four hybrid lines (SJLxB6; B6xSJL; BUBxB6; B6xBUB) showed little or no effect on preference for ethanol and the amount of ethanol consumed was reduced only for the B6xSJL hybrids. However, this reduction was likely a result of decreased total fluid intake observed in B6xSJL hybrids. In contrast, two hybrids (B6xNZB and NZBxB6) demonstrated strong reduction of ethanol intake and preference after periods of abstinence.
Fig. 3.
Consumption of increasing concentrations of ethanol by B6, FVB inbred strains and their reciprocal F1 hybrids after two consecutive periods of abstinence in a two-bottle preference test. a–d Amount of ethanol consumed (g/kg/day) in B6, FVB, B6xFVB F1 and FVBxB6 F1 hybrids correspondently. e–h Preference for ethanol in B6, FVB, B6xFVB F1 and FVBxB6 F1 hybrids, respectively. i–l Total fluid intake (g/kg/day) in B6, FVB, B6xFVB F1 and FVBxB6 F1 hybrids correspondently. n—See legends to Fig. 1. Ethanol and water intake were measured after first and after second periods of abstinence at 9, 18 and 27% concentrations of ethanol. These numbers were compared with data for experimentally naïve mice (first presentation of ethanol). *1—Statistically significant differences from initial trial (0) and trial 1 within one concentration of ethanol solution (two-way ANOVA with Post hoc Bonferroni Test). *2—Statistically significant differences from initial trial (0) and trial 2 within one concentration of ethanol solution (two-way ANOVA with Post hoc Bonferroni Test). For P values see Supplemental Tables VI, VII. R0—round 1 (ethanol naïve mice); R1—round 2, repeated presentation of ethanol after 1 week of ethanol deprivation; R2—round 3, repeated presentation of ethanol after another week of ethanol deprivation
Fig. 4.
Consumption of increasing concentrations of ethanol by SJL inbred strain and their reciprocal F1 hybrids from intercross with B6 inbred strain after two consecutive periods of abstinence in a two-bottle preference test. a–c Amount of ethanol consumed (g/kg/day) in SJL, SJLxB6 F1 and B6xSJL F1 hybrids correspondently. d–f Preference for ethanol in SJL, SJLxB6 F1 and B6xSJL F1 hybrids correspondently. g–i Total fluid intake (g/kg/day) in SJL, SJLxB6 F1 and B6xSJL F1 hybrids correspondently. n—See legends to Fig. 1. Ethanol and water intake were measured after first and after second periods of abstinence at 9, 18 and 27% concentrations of ethanol. These numbers were compared with data for experimentally naïve mice (first presentation of ethanol). *1—Statistically significant differences from initial trial (0) and trial 1 within one concentration of ethanol solution (two-way ANOVA with Post hoc Bonferroni Test). *2—Statistically significant differences from initial trial (0) and trial 2 within one concentration of ethanol solution (two-way ANOVA with Post hoc Bonferroni Test). For P values see Supplemental Tables VI, VII. R0, R1 and R2—see legends to Fig. 3
Fig. 5.
Consumption of increasing concentrations of ethanol by NZB inbred strain and their reciprocal F1 hybrids from intercross with B6 inbred strain after two consecutive periods of abstinence in a two-bottle preference test. a–c Amount of ethanol consumed (g/kg/day) in NZB, NZBxB6 F1 and B6xNZB F1 hybrids correspondently. d–f Preference for ethanol in NZB, NZBxB6 F1 and B6xNZB F1 hybrids correspondently. g–i Total fluid intake (g/kg/day) in NZB, NZBxB6 F1 and B6xNZB F1 hybrids correspondently. n—See legends to Fig. 2. Ethanol and water intake were measured after first and after second periods of abstinence at 9, 18 and 27% concentrations of ethanol. These numbers were compared with data for experimentally naïve mice (first presentation of ethanol). *1—Statistically significant differences from initial trial (0) and trial 1 within one concentration of ethanol solution (two-way ANOVA with Post hoc Bonferroni Test). *2—Statistically significant differences from initial trial (0) and trial 2 within one concentration of ethanol solution (two-way ANOVA with Post hoc Bonferroni Test). For P values see Supplemental Tables VI, VII. R0, R1 and R2—see legends to Fig. 3
Fig. 6.
Consumption of increasing concentrations of ethanol by BUB inbred strain and their reciprocal F1 hybrids from intercross with B6 inbred strain after two consecutive periods of abstinence in a two-bottle preference test. a–c Amount of ethanol consumed (g/kg/day) in BUB, BUBxB6 F1 and B6xBUB F1 hybrids, respectively. d–f Preference for ethanol in BUB, BUBxB6 F1 and B6xBUB F1 hybrids correspondently. g–i Total fluid intake (g/kg/day) in BUB, BUBxB6 F1 and B6xBUB F1 hybrids correspondently. n—See legends to Fig. 2. Ethanol and water intake were measured after first and after second periods of abstinence at 9, 18 and 27% concentrations of ethanol. These numbers were compared with data for experimentally naïve mice (first presentation of ethanol). *1—Statistically significant differences from initial trial (0) and trial 1 within one concentration of ethanol solution (two-way ANOVA with Post hoc Bonferroni Test). *2—Statistically significant differences from initial trial (0) and trial 2 within one concentration of ethanol solution (two-way ANOVA with Post hoc Bonferroni Test). For P values see Supplemental Tables VI, VII. R0, R1 and R2—see legends to Fig. 3
Consumption of other tastants
Preference for saccharin
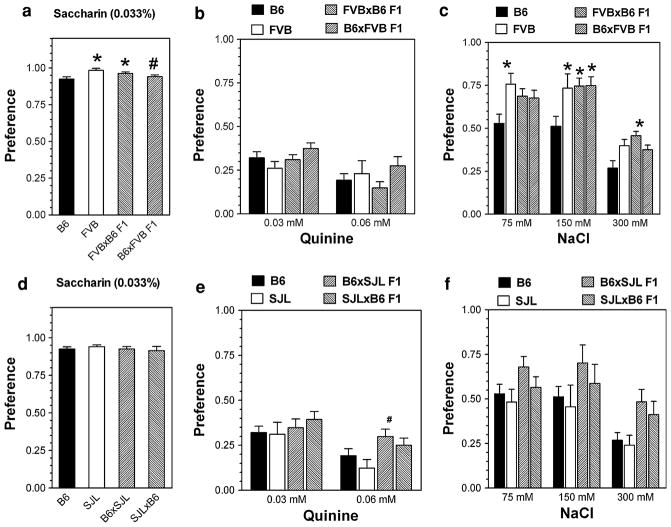
Comparison of preferences for saccharin in B6, FVB, and their reciprocal hybrids demonstrated significant dependence on genotype (F(3,60) = 5.8, P<0.01, one-way ANOVA) (Fig. 7a). Post hoc analyses showed that FVB mice (P<0.01) and FVBxB6 mice (P<0.05) showed a slightly higher preference for saccharin than B6 mice, whereas B6xFVB hybrids showed a slightly lower preference for saccharin as compared with FVB mice (P<0.05).
Fig. 7.
Consumption of saccharin, quinine and sodium chloride by B6, FVB, SJL inbred strains and reciprocal F1 hybrids after intercross between B6 and FVB and between B6 and SJL mice in a two-bottle preference test. a Preference for saccharin in B6, FVB and their reciprocal F1 hybrids. b Preference for quinine in B6, FVB and their reciprocal F1 hybrids. c Preference for sodium chloride in B6, FVB and their reciprocal F1 hybrids. n = 15 (B6); n = 9 (FVB); n = 9 (FVBxB6 F1 hybrids), n = 10 (B6xFVB F1 hybrids). d Preference for saccharin in B6, SJL and their reciprocal F1 hybrids. e Preference for quinine in B6, SJL and their reciprocal F1 hybrids. f Preference for sodium chloride in B6, SJL and their reciprocal F1 hybrids. n = 15 (B6); n = 8 (SJL); n = 8 (both reciprocal F1 hybrids). * Statistically significant differences from B6 inbred strain. # Statistically significant differences from the other progenitor strain. Two-way ANOVA with Post hoc Bonferroni Test has been used. For P values see Supplemental Tables VIII, IX
Preference for a saccharin solution was not dependent on genotype in B6, SJL and their reciprocal hybrids (Fig. 7d). All mouse strains demonstrated a similar preference for saccharin.
Preference for saccharin was shown to be strongly dependent on genotype for B6, NZB and their reciprocal hybrids (F(3,60) = 12.5, P<0.001, one-way ANOVA) (Fig. 8a). Post hoc analyses showed that preference for saccharin was significantly lower for NZB mice than B6 mice (P<0.001). Both reciprocal hybrids showed a higher preference for saccharin as compared with NZB mice (P<0.001), but hybrids did not differ from B6 mice in saccharin preference.
Fig. 8.
Consumption of saccharin, quinine and sodium chloride by B6, NZB, BUB inbred strains and reciprocal F1 hybrids after intercross between B6 and NZB and between B6 and BUB mice in a two-bottle preference test. a Preference for saccharin in B6, NZB and their reciprocal F1 hybrids. b Preference for quinine in B6, NZB and their reciprocal F1 hybrids. c Preference for sodium chloride in B6, NZB and their reciprocal F1 hybrids. n = 15 (B6); n = 7 (NZB); n = 6 (both reciprocal F1 hybrids). d Preference for saccharin in B6, BUB and their reciprocal F1 hybrids. e Preference for quinine in B6, BUB and their reciprocal F1 hybrids. f Preference for sodium chloride in B6, BUB and their reciprocal F1 hybrids. n = 15 (B6); n = 14 (BUB); n = 6 (both reciprocal F1 hybrids). * Statistically significant differences from B6 inbred strain. # Statistically significant differences from another progenitor strain. Two-way ANOVA with Post hoc Bonferroni Test has been used. For P values see Supplemental Tables VIII, IX
Preferences for saccharin was also shown to be significantly dependent on genotype for B6, BUB, and their reciprocal hybrids (F(3,60) = 108, P<0.0001, one-way ANOVA) (Fig. 8d). Post hoc analyses showed that BUB mice showed significantly lower preference for saccharin than B6 mice (P<0.001). Both reciprocal hybrids were not different from B6 mice but showed a significantly higher preference for saccharin as compared with BUB mice (P<0.001).
Preferences for quinine and sodium chloride
Preferences of five inbred strains and eight F1 hybrids for bitter (quinine) and salty (sodium chloride) compounds are presented in Figs. 7 and 8 (for detailed statistics see Supplemental materials in Table VIII and Table IX).
Two strains (FVB and SJL) were not different and two strains (BUB and NZB) showed weaker avoidance of bitter taste (quinine) than B6 mice. Four hybrid lines (NZBxB6; B6xNZB; BUBxB6; B6xBUB) showed an intermediate level of avoidance of quinine solution which was lower than the B6 parental strain but higher than the other progenitor strain.
Two inbred strains (BUB and FVB) showed higher preference for sodium chloride compare with B6 mice especially for low (75 mM) or intermediate (150 mM) concentrations whereas NZB and SJL strains were not different from B6. Five hybrids (BUBxB6; B6xBUB; NZBxB6; B6xNZB; SJLxB6) showed preference for sodium chloride similar to B6. Three other hybrid lines (FVBxB6; B6xFVB; B6xSJL) demonstrated higher preference for sodium chloride compared with B6 mice. FVBxB6 and B6xFVB mice consumed sodium chloride with higher preference than B6 mice with significant differences for 150 and 300 mM of sodium chloride.
Correlation of ethanol intake and preference with preference for other tastants
Results of complete correlational analyses are presented in Supplemental Table X. For ethanol concentrations from 9 to 35% ethanol intake and preference was positively correlated with preference for saccharin (r = 0.58–0.68). Ethanol drinking was not correlated with quinine preference except that preference for 12% ethanol was negatively correlated with preference for 0.06 mM quinine. No correlations were found between ethanol drinking and preference for 75 mM NaCl. However, preference for 150 mM NaCl was positively correlated with drinking (amount of ethanol consumed and preference for ethanol) of the more concentrated (21–35%) ethanol solutions (r = 0.56–0.62). For 300 mM NaCl, preference was positively correlated with ethanol intake and preference with the most concentrated (30 and 35%) ethanol solutions (r = 0.58–0.60).
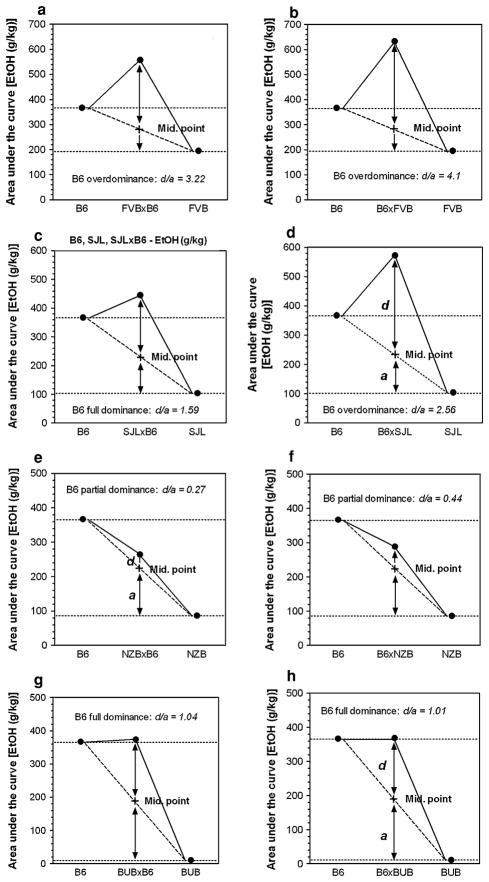
Determination of dominance
The results of calculation for dominant effects of B6 alleles on ethanol intake in different hybrids are presented in Fig. 9. Because the signs of d/a were positive for all the hybrids, we can conclude that the B6 allele showed dominance (for statistics see Supplemental Table XI). Based on our calculations, FVBxB6 and B6xFVB hybrids showed clear overdominance of the B6 allele (Fig. 9a, b). Overdominance was demonstrated for B6xSJL but not for SJLxB6 hybrids (Fig. 9c, d). Partial dominance of the B6 allele was shown for both B6xNZB and NZBxB6 mice (Fig. 9e, f), whereas in BUBxB6 and B6xBUB mice the B6 allele demonstrated full or complete dominance (Fig. 9g, h). Similar results were obtained from calculations for dominant effects of B6 alleles on preference for ethanol, except no overdominance was found for B6xSJL hybrids (for statistics see Supplemental Table XII). Results in section “Genetic variation in ethanol intake” show that high levels of alcohol consumption by FVBxB6, B6xFVB, SJLxB6 and B6xSJL hybrids are seen mainly with the higher concentrations of ethanol (above 12%). In section “Correlation of ethanol intake and preference with preference for other tastants”, we showed that correlations between preference for ethanol and preference for sodium chloride were also restricted to the higher concentrations of ethanol. Therefore, we asked if the dominance and overdominance for the preference for ethanol depends on concentration. Results for 9, 18 and 27% ethanol are shown in Table 1. The sign of d/a was positive for all the hybrids and for all concentrations of ethanol showing that the B6 allele showed dominance. No significant overdominance was found for any of the hybrids at 9% ethanol. For 18% ethanol, only two hybrids (B6xFVB and B6xSJL) showed marginal overdominance, For 27% ethanol, three hybrids (FVBxB6, B6xFVB and B6xSJL) showed overdominance, It should be noted, that d/a values substantially increased for all three hybrids from ethanol 9% to ethanol 27% showing the gradual increase of overdominance as a function of increasing concentration.
Fig. 9.
Examples of partial dominance, full dominance and overdominance of the B6 allele relative to the NZB, BUB, SJL and FVB. a Phenotypic means for B6, FVB and FVBxB6 F1 hybrids. b Phenotypic means for B6, FVB and B6xFVB F1 hybrids. c Phenotypic means for B6, SJL and SJLxB6 F1 hybrids. d Phenotypic means for B6, SJL and B6xSJL F1 hybrids. e Phenotypic means for B6, NZB and NZBxB6 F1 hybrids. f Phenotypic means for B6, NZB and B6xNZB F1 hybrids. g Phenotypic means for B6, BUB and BUBxB6 F1 hybrids. h Phenotypic means for B6, BUB and B6xBUB F1 hybrids. The area under the curve for ethanol intake (g/kg/24 h) vs. concentrations of ethanol solution for each genotype was used as the phenotypic mean. These areas were calculated from data shown in Figs. 1 and 2
Table 1.
Calculations of dominance and overdominance for preference for 9, 18 and 27% ethanol solutions
| Strains | Preference (ethanol 9%) |
Preference (ethanol 18%) |
Preference (ethanol 27%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| d/a | Dom. P value | Ovdom. P value | d/a | Dom. P value | Ovdom. P value | d/a | Dom. P value | Ovdom. P value | |
| FVBxB6 | 0.95 | < 0.0001 | 2.07 | < 0.001 | 3.67 | < 0.01 | < 0.05 | ||
| B6xFVB | 1.09 | < 0.0001 | 2.85 | < 0.0001 | < 0.01 | 6.26 | < 0.0001 | < 0.0001 | |
| SJLxB6 | 0.68 | 1.63 | < 0.001 | 1.64 | < 0.0001 | ||||
| B6xSJL | 0.26 | 1.95 | < 0.0001 | < 0.05 | 2.56 | < 0.0001 | < 0.0001 | ||
| NZBxB6 | 0.60 | < 0.001 | 0.14 | 0.21 | |||||
| B6xNZB | 0.34 | 0.15 | 0.29 | ||||||
| BUBxB6 | 0.64 | < 0.0001 | 0.85 | < 0.05 | 1.07 | < 0.0001 | |||
| B6xBUB | 0.68 | < 0.0001 | 0.53 | 0.79 | < 0.0001 | ||||
Dom. dominance, Ovdom. overdominance
Discussion
These results confirm and extend previous studies (Blednov et al. 2005) showing that hybrid mice from the cross of B6 and FVB strains drink substantially more ethanol than either progenitor strain when given a choice of ethanol solution or water. We found that hybrid mice from a cross of SJL (genealogically and genetically close to FVB strain, Beck et al. 2000; Festing 1994; Morse 1978; Petkov et al. 2004) with B6 also showed higher ethanol consumption than either progenitor strain. It should be noted that increased consumption was observed mainly for high concentrations of ethanol (above 9%). This suggests that the common genetics of FVB and SJL inbred mouse strains may be important in determining the increased ethanol consumption for both hybrids over the already high level of ethanol drinking in B6 mice. It is of interest to note that in this study and many others, mice ‘titrate’ their intake by reducing preference for more concentrated alcohol solutions. This sets a ‘ceiling’ for alcohol intake and suggests that continuous two bottle choice drinking may model social drinking rather than binge or abuse patterns of intake for most strains. However, the hybrids of B6 with either FVB or SJL show altered ‘titration’ such that presentation of more concentrated alcohol solutions results in higher alcohol intake. Titration of alcohol intake to different levels was also seen in longitudinal studies of human alcoholics (Young 1994). Thus, these hybrids provide a new approach to understand the genetics and neurobiology of regulation of alcohol intake by titration.
Hybrid lines were traditionally evaluated in terms of heterosis or hybrid “vigour”, which describes the deviation of the hybrid line from the two parental or progenitor strains. This phenomenon was extensively studied in plants (Shull 1948), and in animals, where “behavioral heterosis” was documented (Bruell 1964a, b, 1965). The genetic basis of heterosis remains murky but ‘dominance’ and ‘overdominance’ are usually invoked as mechanisms. In addition, epistatic interactions between non-allelic genes at two or more loci may also contribute to the phenotypic expression of a trait in hybrids (see Hochholdinger and Hoecker 2007, for review). However, it is important to note that overdominance reported here refers to the aggregate effect of one to many loci, and cannot be ascribed to any one locus based on the data presented. Nonetheless, because overdominance at known single loci or QTL is relatively rare (Valdar et al. 2006), this suggests that the observed overdominance is due to relatively few loci. The present findings also demonstrate that alleles do not always affect alcohol drinking behavior in a simple additive or dominant fashion in all crosses. Indeed, hybrids from the intercross of B6 and NZB inbred strains demonstrated either additivity or partial dominance, whereas hybrids from the intercross of B6 and BUB inbred strains showed full or complete dominance, i.e., d = a.
Data obtained in this study clearly show that the range of ethanol consumption in a standard two bottle preference test is not restricted to that seen in standard inbred strains but is substantially broader when hybrids are included. Previous studies of ethanol consumption in BXD recombinant inbred strains (Tarantino et al. 1998; Phillips et al. 1998; Gill et al. 1996) found that the distribution of ethanol consumption is skewed towards low consumption and falls within the range of ethanol consumption of the two parental strains. Similarly, the F1 hybrid cross of 129P3/JxC57BL/6ByJ (Bachmanov et al. 1996) showed lower ethanol preference than C57BL/6ByJ. Other F1 crosses reported to date include C57BL/Crgl by DBA/NCrgl, A/Crgl/2, C3H/Crgl/2, and BALB/cCrgl (McClearn and Rodgers 1961) and DBA/2JxA/J, DBA/2JxC3HeB/FeJ, C57BL/6JxDBA/2J, C57BL/6JxC3HeB/FeJ, and C57BL/6JxA/J (Fuller 1964). In these studies, preference for ethanol instead of consumption was reported, but the hybrids in all of these crosses showed lower preference than B6. This conclusion, in conjunction with the present results, is that alcohol preference drinking does not show overdominance as a rule, but rather is restricted to specific progenitor strain crosses, specifically B6 crossed with FVB or SJL in the present study. These new hybrid models should prove useful for exploring the underlying genetic basis of overdominance and its’ contribution to individual differences in alcohol drinking in mice.
Our data also show a maternal effect which increases ethanol consumption. Indeed, both pairs of reciprocal hybrid mice with B6 mothers (B6xFVB and B6xSJL) consumed significantly more ethanol than hybrids with B6 fathers (FVBxB6 and SJLxB6) (Table 2). It should be noted that for hybrids obtained from B6 and SJL inbred strains, the effect of overdominance was significant only for the B6xSJL mice. This suggests the possible importance of cytoplasmic heredity, the particular role of some genes located on the X chromosome or epigenetic effects of maternal environment. For example, hybrids obtained from B6 and DBA/2J inbred strains reared by B6 dams consumed more ethanol during forced exposure than did hybrids reared by DBA dams (Gabriel and Cunningham 2008).
Table 2.
Summary of consumption data for all inbred strains and hybrids
| Strains | EtOH |
Sacch. | Quin. | NaCl | EtOH | |||
|---|---|---|---|---|---|---|---|---|
| Max. intake (g/kg) | EtOH (%) | Max. pref. | EtOH (%) | Pref. (0.03%) | Pref. (0.06 mM) | Pref. (150 mM) | Changes across trials | |
| B6 | 14.3 | 12 | 0.93 | 9 | 0.92 | 0.19 | 0.51 | ↓ |
| FVB | 11.8 | 30 | 0.24 | 30 | 0.98 | 0.23 | 0.73 | ↑ |
| SJL | 7.6 | 12 | 0.52 | 9 | 0.94 | 0.12 | 0.46 | ↓ |
| NZB | 4.6 | 6 | 0.53 | 3 | 0.69 | 0.54 | 0.46 | ↓ |
| BUB | 0.9 | 30 | 0.11 | 3 | 0.60 | 0.49 | 0.71 | – |
| B6xFVB | 25.5 | 27 | 0.96 | 9 | 0.94 | 0.28 | 0.75 | 0 |
| FVBxB6 | 24.5 | 21 | 0.94 | 12 | 0.96 | 0.15 | 0.75 | 0 |
| B6xSJL | 24.5 | 27 | 0.8 | 12 | 0.92 | 0.30 | 0.70 | ↓ |
| SJLxB6 | 18.4 | 30 | 0.86 | 9 | 0.91 | 0.25 | 0.59 | 0 |
| B6xNZB | 12.2 | 35 | 0.72 | 9 | 0.88 | 0.33 | 0.57 | ↓ |
| NZBxB6 | 10.5 | 12 | 0.8 | 9 | 0.89 | 0.30 | 0.53 | ↓ |
| B6xBUB | 13.5 | 15 | 0.78 | 9 | 0.91 | 0.40 | 0.67 | ↓ |
| BUBxB6 | 13.6 | 15 | 0.76 | 9 | 0.92 | 0.37 | 0.66 | 0 |
For ethanol (EtOH), the maximal intake in g/kg/day is given with the alcohol concentration (% v/v) that gave the maximal intake and the maximal preference (ratio of alcohol consumption to total fluid consumption) is given with the alcohol concentration that gave the maximal preference. The preference (pref.) ratio for consumption of saccharin (0.03%), quinine (0.06 mM) and NaCl (150 mM) is also given for each strain or hybrid. In addition, the direction of change (increase, decrease or no change) in alcohol consumption across the three trials is given
It is well documented that taste perception is a critical factor in determining ethanol consumption in the two-bottle choice test. A positive relationship between ethanol and sweet intake had been known for more than 40 years (Rodgers et al. 1963; Rodgers and McClearn 1964). These findings have been confirmed in many studies in inbred strains of mice (Bachmanov et al. 1996; Belknap et al. 1993; Yoneyama et al. 2008), congenic mouse strains (Blizard and McClearn 2000), outbred rats (Gosnell and Krahn 1992), genetically selected alcohol preferring rats (Kampov-Polevoy et al. 1995; Sinclair et al. 1992; Stewart et al. 1994) and monkeys (Higley and Bennett 1999). Furthermore, rats selected for high or low saccharin consumption consumed more or less ethanol, respectively (Dess et al. 1998). Recently, we directly showed that the deletion of any one of three different genes expressed in taste buds and involved in detection of sweet taste leads to a substantial reduction of alcohol intake without any changes in the pharmacological actions of ethanol (Blednov et al. 2008). Despite the limited number of genotypes used in our study (five inbred strains and eight F1 hybrids), we were able to detect the well established positive correlation between preference for saccharin and preference for ethanol. However, despite this correlation, sensitivity to sweet taste cannot explain the increased ethanol consumption observed in hybrids from B6 and FVB strains or B6 and SJL strains because both pairs of parents and reciprocal hybrids show similar, high, preference for saccharin solutions. Moreover, overdominance was seen only for ethanol and not for saccharin preference drinking.
Differences between FVB and B6 strains in preference for some other tastants were noted previously (Bachmanov et al. 2002). Thus, B6 mice display greater preference for solutions of potassium chloride and ammonium chloride, while FVB mice display greater preference for solutions of sodium chloride and sodium lactate. Preference for sodium chloride in the SJL inbred strain was similar to FVB and significantly higher than in B6 (Tordoff et al. 2007). Little information about a possible connection between sensitivity to salt and ethanol consumption is available. Two human studies reported that individuals with a paternal history of alcoholism showed significantly enhanced unpleasant response to concentrated sodium chloride and citric acid compared to subjects with no family history of alcoholism (Scinska et al. 2001; Sandstrom et al. 2003). Hellekant et al. (1997) showed that high concentrations of ethanol specifically stimulated individual taste fibers with selective response to sodium chloride in rhesus monkey. Consistent with this possibility, we found a correlation between consumption of alcohol and sodium chloride, particularly for the higher concentrations of alcohol and the higher concentrations of sodium chloride. This relationship is illustrated by the B6xSJL mice which showed higher preference for sodium chloride solutions than B6 mice, whereas no differences were found between SJLxB6 mice and B6 mice. Furthermore, B6xSJL, but not SJLxB6, hybrids consumed more ethanol than B6 (Table 2). Consistent with earlier published results (Bachmanov et al. 2002; Tordoff et al. 2007), BUB mice, like FVB and SJL mice, showed higher preference for sodium chloride than B6 mice. However, BUBxB6 and B6xBUB mice did not differ from B6 mice in ethanol preference and consumption. Although similar in preference for sodium chloride, the BUB strain is genealogically different from the FVB and SJL strains (Beck et al. 2000; Petkov et al. 2004). Therefore, probably both genealogical origin and sensitivity to the salty taste are factors which regulate to some degree ethanol consumption in these hybrids.
It is generally thought that the avoidance of more concentrated ethanol solutions can be related to bitterness. For example, the alcohol consumption in rats was positively correlated with intake of quinine, suggesting that sensitivity to bitter taste influences alcohol acceptance (Kampov-Polevoy et al. 1990; Goodwin et al. 2000). Using conditioned taste aversion, Blizard (2007) showed that B6 mice generalized taste aversions from sucrose and quinine solutions to 10% ethanol and, reciprocally, aversions to 10% ethanol generalized to each of these solutions presented separately. Thus, considering these two gustatory qualities, 10% ethanol should taste both sweet and bitter to B6 mice. However, under conditions of free choice drinking, quinine intake (Phillips et al. 1991) and ethanol consumption (Fernandez et al. 1999) were not correlated for the BXD recombinant inbred mouse strains (WebQTL, The Gene Network; http://www.genenetwork.org/). In agreement with results from this analysis, we did not find any clear correlations between quinine and ethanol consumption for our inbred strains and hybrid mice. Specifically, the high ethanol consuming hybrids (FVB and B6 crosses; SJL and B6 crosses) did not differ from the B6 progenitor strain in avoidance of quinine solutions. Also, hybrids (BUB and B6 crosses) showed significantly lower avoidance of bitter solutions of quinine than B6 mice but were not different from B6 mice in ethanol preference and consumption.
One would expect to find a large number of polymorphisms between two pairs of inbred strains—FVB vs. B6 and SJL vs. B6, as their genealogies are quite different (Beck et al. 2000; Petkov et al. 2004). We searched several public databases for genetic polymorphisms between these strains. Indeed, the Mouse Genome Database (searched March 21, 2009) found 158 polymorphisms identified by polymerase chain reaction between B6 and FVB inbred strains (http://www.informatics.jax.org/searches/polymorphism_form.shtml). The search for genetic polymorphisms between B6 and SJL strains found 189 identified polymorphisms. Some of these polymorphic minisatellites are located within quantitative trait loci (QTL) for ethanol preference on chromosomes 1, 2 and 9 (for B6 and FVB comparison) and on chromosomes 1, 2 (for B6 and SJL comparison) (Tarantino et al. 1998; Melo et al. 1996). However, it should be noted that the QTL for ethanol preference mentioned above were obtained for crosses between B6 and DBA inbred strains and we do not know if crosses between B6 and FVB inbred strains will have similar QTL. Consistent with their common genealogy, only three polymorphisms were found between FVB and SJL inbred strains. The Center for Inherited Disease Research Mouse Microsatellite Studies website was searched March 18 (2009) (http://www.cidr.jhmi.edu/mouse/mouse_strp.html). One hundred and ninety-one polymorphic markers between B6 and FVB were identified with a mean distance of 8.0 cM between markers, 186 polymorphic markers between B6 and SJL were identified with a mean distance of 8.2 cM between markers, and 116 polymorphic markers between FVB and SJL were identified with a mean distance of 12.5 cM between markers.
It is of potential interest to evaluate the emerging SNP databases for differences between the B6 and FVB strains. For chromosome 2, which is strongly implicated in genetic differences in alcohol consumption, the Mouse Phenome Database Mouse SNP site (http://aretha.jax.org/pub-cgi/phenome/mpdcgi?rtn=snps/door) shows 20008 SNPs between FVB and B6 strains. However, it is important to note that the QTL on chromosome 2 (as well as other QTLs) for alcohol consumption are from B6 and DBA recombinant inbred mice consuming 10% ethanol (Tarantino et al. 1998; Melo et al. 1996) and our data suggest different genetic determinants for intake of low (6–10%) and high (30%) concentrations of ethanol. To explore the genetic differences important for the high intake of 30% ethanol in the B6xFVB hybrids with SNP data will require mapping of QTLs in these mice using a range of alcohol consumption.
It should be noted that ethanol consumption in the two-bottle choice test is not always stable over time. In our study, repeated presentation of ethanol after two 1-week periods of abstinence (ethanol deprivation) dramatically reduced consumption, especially of previously highly preferred concentrations of ethanol. However, the genetic dependence of this behavior in ethanol-experienced mice is very different from genetic influences on consumption in ethanol-naïve mice. Thus, genetically similar FVB and SJL inbred strains show opposite changes in ethanol preference and intake after repeated presentation of ethanol. Also, reduction of ethanol preference and intake after ethanol deprivation was found in two other genetically unrelated strains: B6 and NZB. The very low ethanol intake and preference for ethanol observed in BUB mice makes it impossible to evaluate changes in alcohol consumption in this strain in contrast to the other inbred strains. For the hybrids, six of the eight showed stable ethanol preference and intake after repeated ethanol deprivation (Table 2). The slight reduction of ethanol intake (but not preference) found in both B6 and SJL reciprocal hybrids after ethanol deprivation can be explained by reduced total fluid intake in these mice. Only the B6xNZB and NZBxB6 reciprocal hybrids showed strong reduction of ethanol preference and intake after ethanol deprivation. This could represent the additive effects of ethanol deprivation observed in both progenitor strains, B6 and NZB although SJL showed a reduction in ethanol consumption after periods of ethanol deprivation, but the hybrids did not show this reduction.
Presentation of high ethanol concentrations and repeated ethanol presentation/deprivation pairings are key challenges known to produce experience dependent changes in ethanol consumption in mice (Melendez et al. 2006; Y. A. Blednov, unpublished data). Without challenges such as these, some strains will stably drink ethanol for long periods of time; this behavior is thought to model controlled drinking (Melendez et al. 2006; Y. A. Blednov, unpublished data). After forced deprivations, subsequent increased ethanol consumption is referred to as a positive alcohol deprivation effect (ADE) and is thought to model uncontrolled drinking, whereas decreased ethanol consumption has been referred to as a negative ADE and could represent a change in the threshold for the aversive properties of ethanol (Sinclair and Senter 1968; Sinclair and Sheaff 1973; DiBattista 1991; Melendez et al. 2006). The contribution of taste learning should also be considered, as it is critical for survival to develop associations between taste and safe/unsafe outcomes. Gutiérrez et al. (2003) showed that the taste memory trace is simultaneously processed by two mechanisms in the insular cortex, and that their interaction determines the degree of preference or aversion learned to a novel taste. The possible importance of aversive memory in regulating alcohol consumption is supported by data in our companion paper showing differences in development of conditioned taste aversion to ethanol between B6xFVB and B6xNZB mice (A. R. Ozburn et al., companion paper). Future studies will further characterize behaviors of these hybrids to define differences in innate and ethanol-related responses which can cause these differences in ethanol preference.
In conclusion, mice derived from the hybrid crosses of B6 and FVB and B6 and SJL drank higher levels of ethanol than their progenitor strains in the two bottle choice test. The B6 and FVB hybrid is noteworthy for two reasons. First, it demonstrates the occurrence of overdominance in two-bottle choice drinking in mice (i.e., whereby alleles interact to cause the hybrids to score outside the range of the inbred progenitors, where the interaction could occur at alleles within a locus (dominance) or between loci (epistasis), or (more likely) a combination across all loci which influence alcohol preference drinking). Second, it identifies a mouse genotype that shows sustained alcohol preference and consumption in response to the challenges of repeated high ethanol concentrations and periods of abstinence. The hybrid of B6 and NZB demonstrates genetic additivity in two-bottle choice drinking in mice, but shows markedly reduced alcohol preference in response to the challenges of repeated high ethanol concentrations and periods of abstinence. The differences in these phenotypes are explored in the accompanying manuscript (A. R. Ozburn et al., companion paper). It is interesting to note that the inbred mouse strains reduced their ethanol consumption after repeated presentation of ethanol whereas most of the hybrids showed stable drinking. Although inbred mouse strains are a pillar of alcohol genetics research, humans are heterozygous at many loci and we speculate that hybrid mice will provide a wider range of alcohol responses and perhaps a better model of some human responses to alcohol than inbred strains.
Supplementary Material
Acknowledgments
This study or research was supported by grants from the National Institute of Alcohol Abuse and Alcoholism (AA U01 13520 and AA U01 AA016655—INIA West Projects), NIH A06399 and AA01760, and SRCS Award from the Department of Veterans Affairs. The authors would like to thank Virginia Bleck for excellent technical assistance.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10519-009-9298-4) contains supplementary material, which is available to authorized users.
Contributor Information
Y. A. Blednov, Email: yablednov@mail.utexas.edu, Waggoner Center for Alcohol and Addiction Research, University of Texas, 2500 Speedway MBB 1.124, Austin, TX 78712, USA. Waggoner Center for Alcohol and Addiction Research, 1 University Station A4800, Austin, TX 78712-0159, USA
A. R. Ozburn, Waggoner Center for Alcohol and Addiction Research, University of Texas, 2500 Speedway MBB 1.124, Austin, TX 78712, USA
D. Walker, Waggoner Center for Alcohol and Addiction Research, University of Texas, 2500 Speedway MBB 1.124, Austin, TX 78712, USA
S. Ahmed, Waggoner Center for Alcohol and Addiction Research, University of Texas, 2500 Speedway MBB 1.124, Austin, TX 78712, USA
J. K. Belknap, Portland Alcohol Research Center, Department of Veterans Affairs Medical Center and Department of Behavioral Neuroscience, Oregon Health & Science University, Portland, OR 97239, USA
R. A. Harris, Waggoner Center for Alcohol and Addiction Research, University of Texas, 2500 Speedway MBB 1.124, Austin, TX 78712, USA
References
- Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res. 1996;20:201–206. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Beauchamp GK, Tordoff MG. Voluntary consumption of NaCl, KCl, CaCl2, and NH4Cl solutions by 28 mouse strains. Behav Genet. 2002;32:445–457. doi: 10.1023/a:1020832327983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MF, Fisher EM. Genealogies of mouse inbred strains. Nat Genet. 2000;24:23–25. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Chang SR, Harris RA. Potassium channels as targets for ethanol: studies of G-protein-coupled inwardly rectifying potassium channel 2 (GIRK2) null mutant mice. J Pharmacol Exp Ther. 2001;298:521–530. [PubMed] [Google Scholar]
- Blednov YA, Metten P, Finn DA, Rhodes JS, Bergeson SE, Harris RA, Crabbe JC. Hybrid C57BL/6J × FVB/NJ mice drink more alcohol than do C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1949–1958. doi: 10.1097/01.alc.0000187605.91468.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 2008;7:1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA. Sweet and bitter taste of ethanol in C57BL/6J and DBA2/J mouse strains. Behav Genet. 2007;37:146–159. doi: 10.1007/s10519-006-9121-4. [DOI] [PubMed] [Google Scholar]
- Blizard DA, McClearn GE. Association between ethanol and sucrose intake in the laboratory mouse: exploration via congenic strains and conditioned taste aversion. Alcohol Clin Exp Res. 2000;24:253–258. [PubMed] [Google Scholar]
- Bruell JH. Heterotic inheritance of wheelrunning in mice. J Comp Physiol Psychol. 1964a;58:159–163. doi: 10.1037/h0043142. [DOI] [PubMed] [Google Scholar]
- Bruell JH. Inheritance of behavioral and physiological characters of mice and the problem of heterosis. Am Zool. 1964b;4:125–138. doi: 10.1093/icb/4.2.125. [DOI] [PubMed] [Google Scholar]
- Bruell JH. Mode of inheritance of response time in mice. J Comp Physiol Psychol. 1965;60:147–148. doi: 10.1037/h0022304. [DOI] [PubMed] [Google Scholar]
- Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol. 1998;16:275–278. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- DiBattista D. Examination of the negative alcohol-deprivation effect in the golden hamster (Mesocricetus auratus) Alcohol. 1991;8:337–343. doi: 10.1016/0741-8329(91)90528-5. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. 4. Longman; Essex: 1996. [Google Scholar]
- Fernandez JR, Vogler GP, Tarantino LM, Vignetti S, Plomin R, McClearn GE. Sex-exclusive quantitative trait loci influences in alcohol-related phenotypes. Am J Med Genet. 1999;88:647–652. doi: 10.1002/(sici)1096-8628(19991215)88:6<647::aid-ajmg13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Festing MFW. Inbred strains of mice. Mouse Genome. 1994;92:420–426. [Google Scholar]
- Fuller JL. Measurement of alcohol preference in genetic experiments. J Comp Physiol Psychol. 1964;57:85–88. doi: 10.1037/h0043100. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Cunningham CL. Effects of maternal strain on ethanol responses in reciprocal F1 C57BL/6J and DBA/2J hybrid mice. Genes Brain Behav. 2008;7:276–287. doi: 10.1111/j.1601-183X.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- Gill K, Liu Y, Deitrich RA. Voluntary alcohol consumption in BXD recombinant inbred mice: relationship to alcohol metabolism. Alcohol Clin Exp Res. 1996;20:185–190. doi: 10.1111/j.1530-0277.1996.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Goodwin FL, Bergeron N, Amit Z. Differences in the consumption of ethanol and flavored solutions in three strains of rats. Pharmacol Biochem Behav. 2000;65:357–362. doi: 10.1016/s0091-3057(99)00222-1. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Krahn DD. The relationship between saccharin and alcohol intake in rats. Alcohol. 1992;9:201–206. doi: 10.1016/0741-8329(92)90054-e. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R, Rodriguez-Ortiz CJ, De La Cruz V, Núñez-Jaramillo L, Bermudez-Rattoni F. Cholinergic dependence of taste memory formation: evidence of two distinct processes. Neurobiol Learn Mem. 2003;80:323–331. doi: 10.1016/s1074-7427(03)00066-2. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Danilova V, Roberts T, Ninomiya Y. The taste of ethanol in a primate model: I. Chorda tympani nerve response in Macaca mulatta. Alcohol. 1997;14:473–484. doi: 10.1016/s0741-8329(96)00215-7. [DOI] [PubMed] [Google Scholar]
- Higley JD, Bennett AJ. Central nervous system serotonin and personality as variables contributing to excessive alcohol consumption in non-human primates. Alcohol Alcohol. 1999;34:402–418. doi: 10.1093/alcalc/34.3.402. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Hoecker N. Towards the molecular basis of heterosis. Trends Plant Sci. 2007;12:427–432. doi: 10.1016/j.tplants.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Kasheffskaya OP, Sinclair JD. Initial acceptance of ethanol: gustatory factors and patterns of alcohol drinking. Alcohol. 1990;7:83–85. doi: 10.1016/0741-8329(90)90065-k. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Overstreet DH, Rezvani AH, Janowsky DS. Suppression of ethanol intake in alcohol-preferring rats by prior voluntary saccharin consumption. Pharmacol Biochem Behav. 1995;52:59–64. doi: 10.1016/0091-3057(94)00430-q. [DOI] [PubMed] [Google Scholar]
- Kearsey MJ, Pooni HS. The genetical analysis of quantitative traits. Chapman and Hall; London: 1996. [Google Scholar]
- Kiefer SW, Bice PJ, Orr MR, Dopp JM. Similarity of taste reactivity responses to alcohol and sucrose mixtures in rats. Alcohol. 1990;7:115–120. doi: 10.1016/0741-8329(90)90071-j. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW. Development of an alcohol deprivation and escalation effect in C57BL/6J mice. Alcohol Clin Exp Res. 2006;30:2017–2025. doi: 10.1111/j.1530-0277.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Melo JA, Shendure J, Pociask K, Silver LM. Identification of sex-specific quantitative trait loci controlling alcohol preference in C57BL/6 mice. Nat Genet. 1996;13:147–153. doi: 10.1038/ng0696-147. [DOI] [PubMed] [Google Scholar]
- Morse HC., III . Origins of inbred mice. Academic Press; New York: 1978. [Google Scholar]
- Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, Crew V, Johnson KA, Robinson P, Scott VE, Wiles MV. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004;14:1806–1811. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Belknap JK, Crabbe JC. Use of recombinant inbred strains to assess vulnerability to drug abuse at the genetic level. J Addict Dis. 1991;10:73–87. doi: 10.1300/J069v10n01_06. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Belknap JK, Buck KJ, Cunningham CL. Genes on mouse chromosomes 2 and 9 determine variation in ethanol consumption. Mamm Genome. 1998;9:936–941. doi: 10.1007/s003359900903. [DOI] [PubMed] [Google Scholar]
- Rodgers DA. Factors underlying differences in alcohol preference in inbred strains of mice. In: Kissin B, Begleiter H, editors. The biology of alcoholism. Plenum; New York: 1972. pp. 107–130. [Google Scholar]
- Rodgers DA, McClearn GE, Bennett EL, Hebert M. Alcohol preference as a function of its caloric utility in mice. J Comp Physiol Psychol. 1963;56:666–672. doi: 10.1037/h0040350. [DOI] [PubMed] [Google Scholar]
- Rodgers DA, McClearn GE. Sucrose versus ethanol appetite in inbred strains of mice. Q J Stud Alcohol. 1964;25:26–35. [PubMed] [Google Scholar]
- Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges LV, editors. The handbook of research synthesis. Russell Sage Foundation; New York: 1994. pp. 231–244. [Google Scholar]
- Sandstrom KA, Rajan TM, Feinn R, Kranzler HR. Salty and sour taste characteristics and risk of alcoholism. Alcohol Clin Exp Res. 2003;27:955–961. doi: 10.1097/01.ALC.0000071931.33565.54. [DOI] [PubMed] [Google Scholar]
- Scinska A, Bogucka-Bonikowska E, Koros E, Polanowska B, Habrat A, Kukwa A, Kostowski W, Bienkowski P. Taste responses in sons of male alcoholics. Alcohol Alcohol. 2001;36:79–84. doi: 10.1093/alcalc/36.1.79. [DOI] [PubMed] [Google Scholar]
- Shull GH. What is heterosis? Genetics. 1948;33:439–446. doi: 10.1093/genetics/33.5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Sinclair JD, Sheaff B. A negative alcohol-deprivation effect in hamsters. Q J Stud Alcohol. 1973;34:71–77. [PubMed] [Google Scholar]
- Sinclair JD, Kampov-Polevoy A, Stewart E, Li TK. Taste preferences in rat lines selected for high and low ethanol consumption. Alcohol. 1992;9:155–160. doi: 10.1016/0741-8329(92)90027-8. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Russell RN, Lumeng L, Li TK, Murphy JM. Consumption of sweet, salty, sour, and bitter solutions by selectively bred alcohol-preferring and alcohol-nonpreferring lines of rats. Alcohol Clin Exp Res. 1994;18:375–381. doi: 10.1111/j.1530-0277.1994.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Tarantino LM, McClearn GE, Rodriguez LA, Plomin R. Confirmation of quantitative trait loci for alcohol preference in mice. Alcohol Clin Exp Res. 1998;22:1099–1105. [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA, Reed DR. Forty mouse strain survey of water and sodium intake. Physiol Behav. 2007;91:620–631. doi: 10.1016/j.physbeh.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdar W, Solberg LC, Gauguier D, Burnett S, Klenerman P, Cookson WO, Taylor MS, Nicholas J, Rawlins P, Mott R, Flint J. Genome-wide genetic association of complex traits in heterogeneous stock mice. Nat Genet. 2006;38:879–887. doi: 10.1038/ng1840. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci USA. 2006;103:16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JL. Influence of self-titration on the relationships between ethanol dose and chronic tissue toxicities: theoretical considerations. Alcohol. 1994;11:219–223. doi: 10.1016/0741-8329(94)90034-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.