Abstract
Background. Previous studies have suggested that helminth infection exacerbates malaria, but few existing epidemiological studies adequately control for infection heterogeneities and confounding factors. In this study, we investigate spatial and household heterogeneities, predictors, and consequences of Plasmodium species and hookworm coinfection in rural communities in Uganda.
Methods. A cross-sectional study was conducted among 1770 individuals aged 0–88 years in 4 villages. We recorded demographic, socioeconomic, and microgeographic factors during household surveys. We determined malaria parasitemia and hemoglobin concentration and collected stool samples on 2 consecutive days. For data analysis, we used a hierarchical, spatially explicit Bayesian framework.
Results. Prevalence of Plasmodium-hookworm coinfection was 15.5% overall and highest among school-aged children. We found strong evidence of spatial and household clustering of coinfection and an enduring positive association between Plasmodium-species and hookworm infection among preschool-aged children (odds ratio [OR], 2.36; 95% Bayesian credible interval [BCI], 1.26–4.30) and adults (OR, 2.09; 95% BCI, 1.35–3.16) but not among school-aged children. Coinfection was associated with lower hemoglobin level only among school-aged children.
Conclusions. Plasmodium-hookworm coinfection exhibits marked age dependency and significant spatial and household heterogeneity, and among preschool-aged children and adults, occurs more than would be expected by chance. Such heterogeneities provide insight into factors underlying observed patterns and the design of integrated control strategies.
Approximately 21% of the world's population is exposed to stable malaria due to Plasmodium falciparum [1], and an estimated 740 million individuals are infected with hookworms (Necator americanus and Ancylostoma duodenale) [2]. A common complication of P. falciparum malaria is anemia, particularly among children and pregnant women [3–5], whereas hookworm infection results in intestinal blood loss and is probably second only to malaria as an infectious cause of anemia [6]. The widespread occurrence and congruent spatial extents of Plasmodium species and hookworm infections make coinfection common, especially in sub-Saharan Africa: for example, an estimated 45 million school-aged children are at risk of coinfection [7]. Despite this ubiquity of coinfection and its potential consequences for public health, we know little about epidemiology of malaria-hookworm coinfection.
Previous studies have suggested that infection with 1 species may exacerbate infection and disease related to the other [8, 9]. Furthermore, the distinct mechanisms by which Plasmodium species and hookworm infections cause anemia may enhance the risk of anemia and iron deficiency among individuals harboring both species [10–12]. If shown to be correct, these interactions have considerable implications for parasite control in the tropics. Yet there are few detailed epidemiological studies specifically addressing malaria-hookworm coinfection [13, 14], with most existing studies of malaria-helminth coinfection typically focusing on concomitant infection with Schistosoma species and other soil-transmitted helminths [15–18]. These studies have been either retrospective analyses of previously collected data, clinic based, or conducted among specific subgroups, and consequently they are subject to potential bias and confounding due to individual, household, and geographic factors. These features make the contradictory findings of negative, positive, and no associations reported in the literature difficult to interpret [19–21]. Recent studies in Ghana and Uganda do, however, suggest that among pregnant women coinfection with malaria and hookworm occurs more frequently than would be expected by chance after accounting for demographic and socioeconomic characteristics [13, 14].
We report results from a purposively designed, age-stratified study of the epidemiology of Plasmodium-hookworm coinfection in Uganda. The study aims to determine the patterns, risk factors, and hematological consequences of coinfection in a rural community where malaria and hookworm are endemic. We place emphasis on differences by age group and residential location, using a hierarchical, spatially explicit modeling approach.
METHODS
Study Area
This work forms part of a wider investigation of the epidemiology of malaria-helminth coinfection in East Africa. The study was conducted between June and December 2008 in 4 contiguous villages in Mulanda subcounty, Tororo district, eastern Uganda. In the study area, malaria transmission is stable [1], hookworm is prevalent, and other helminth species are relatively rare [22]. Since 2004, periodic mass chemotherapy in schools has been conducted on 2 occasions, although the coverage has been variable, ranging from 30% to 65% [23]; no community-based treatment has been implemented.
Procedures
Details of the study population and recruitment methods are presented elsewhere [24]. We conducted a census and household survey of all households in the subcounty from June to August 2008. We mapped household locations using an eTrex global positioning system (Garmin). Based on these data, we selected 4 neighboring representative villages (Mulanda Stores, Koribudi A, Mulanda Ayago, and Koribudi B) for the biomedical survey because of their size and accessibility, and we subsequently revisited enumerated households. We explained the purpose of the study to all resident adults and parents of children in a language with which they felt most comfortable, and we obtained signed, informed consent for all participants before investigations were undertaken. We administered a standardized questionnaire to each adult and each child’s primary caregiver to record details of medical history and protective behaviors.
From each enrolled individual, we obtained a finger-prick blood sample for hemoglobin measurement using a portable spectrophotometer (HemoCue) and for preparation of thin and thick films for malaria examination. We declared blood slides negative for malaria only after blinded examination of 100 high-powered fields by 2 independent microscopists. The rate of parasite detection did not differ significantly between technicians. We performed a rapid diagnostic test for malaria (OptiMAL; DiaMed) on all participants with fever (axillary temperature, >37.5°C) or with a reported history of fever in the previous 24 h. Any participant with a positive test result but no evidence of danger signs of severe malaria was diagnosed with uncomplicated malaria and treated with coartemether (Coartem; Novartis; 20 mg artemether/120 mg lumefantrine) in accordance with national guidelines. Participants exhibiting danger signs of severe malaria were referred to the local health facility for case management. Those identified with hemoglobin levels of <10.0 g/dL were provided with ferrous sulfate according to national guidelines. Participants were asked to provide 2 daily consecutive stool samples (26.3% of participants provided only 1 sample), which we examined in duplicate for the eggs of intestinal nematodes (Ascaris lumbricoides, Trichuris trichiura, and hookworm species) and Schistosoma mansoni using the Kato-Katz technique. Intensity of infection was expressed as eggs per gram of feces. Infected individuals were treated with albendazole (400 mg) or praziquantel (30 mg/kg) for nematode or S. mansoni infection, respectively.
We used high-resolution (.6 m) QuickBird satellite data (dated 16 October 2003) to georeference, or geographically located, roads and potential mosquito breeding sites, which were verified through ground-truthing, or on-site examination. We compiled geographic data and created maps using ArcGIS (version 9.2; Environmental Systems Research Institute).
The study protocol was approved by the Makerere University Faculty of Medicine Research and Ethics Committee (2008-043), the Uganda National Council of Science and Technology (HS 476), and the London School of Hygiene and Tropical Medicine Ethics Committee (5261).
Data Analysis
We investigated separately 3 infection-status outcomes: presence of malaria infection or presence of hookworm infection (each considered a monoinfection), or concomitant infection with both malaria and hookworm (coinfection). Plasmodium species infection was determined by the thick blood film results. We examined a subsample (20%) of participants’ parasite-positive thin films, which showed that most infections resulted from P. falciparum (94.0%) and the remainder resulted from Plasmodium. malariae (6.0%); we detected no mixed infections. We considered an individual positive for hookworm infection if at least 1 egg was detected in any of the 4 slides examined; we expressed infection intensity as an arithmetic mean. Any child aged between 6 months and 15 years with a height-for-age z score (HAZ) of <−2, calculated from World Health Organization (WHO) reference values [25, 26] using Stata (version 10; Stata), was classified as stunted.
For purposes of spatial analysis and to overcome small denominators, we grouped households into 14 hexagonal .5-km2 subunits, each of which included at least 50 participating residents, and infection prevalence was defined for each hexagon. We investigated associations between Plasmodium species and hookworm infections using Mantel Haenszel χ2 tests, adjusting the analysis for age, sex, and residential location, with stratified and overall odds ratios (ORs), 95% confidence intervals (CI), and associated P values reported.
Univariable analyses of risk factors for Plasmodium species infection, hookworm infection, and coinfection status were undertaken using frequentist logistic regression in Stata (version 10) in order to select candidate variables for spatial multivariable analysis (criteria: Wald test P < .1). Backward-stepwise elimination was used to generate a minimum adequate model; excluded covariates (P > .05) were retested in the minimal model. To investigate age-dependent relationships, we analyzed covariates for the entire population and performed separate analyses for preschool children (aged 0–4 years), school-aged children (5–15 years), and adults (≥16 years).
We included retained covariates in spatial, multivariable, Bayesian, mixed-effect logistic regression models using WinBUGS (version 14.1; Medical Research council Biostatistics Unit). (See the Appendix, which appears only in the online version of the Journal.) These models included a nonspatial household-level random effect and a spatial, area-level random effect: Evaluation of associations between outcomes and the included explanatory variables therefore incorporated a degree of spatial smoothing and adjustment for clustering within households. The area-level effect was modeled using a conditional autoregressive (CAR) prior structure, incorporating a simple adjacency matrix that allowed for correlation between adjacent hexagons and no correlation between nonadjacent hexagons. A 0 value for the random-effect variance parameters would indicate no difference in the baseline infection risk between households/areas after accounting for other included covariates. Back-transforming these random effects from the logit scale to the prevalence scale provides a ratio by which household- and area-level infection prevalence are higher or lower than expected, which can be thought of as a standardized infection ratio (SIR).
We modeled hemoglobin levels using nonspatial Bayesian linear regression, taking into account between-household variation following a backward-stepwise approach as described above. Because of expected hematological differences among preschool children, school-aged children, and adults, analysis was performed separately for each age group.
RESULTS
Of 2358 individuals enumerated in the study census, 2037 individuals chose to participate (86.4%). Of these individuals, 135 had incomplete household questionnaire data, 58 refused to provide a finger-prick blood sample, and 74 refused to provide a stool sample, resulting in a study population of 1770 persons (75.1% of the total population), who were aged between 0 and 88 years and came from 347 households. This final study sample undersampled adult males, as shown by comparison with census data for the study villages, but there was no statistical difference in the size or relative socioeconomic status of participant and nonparticipant households, nor in recruitment rates from the 4 villages (see Pullan et al [24] for further details).
Heterogeneities of Infection
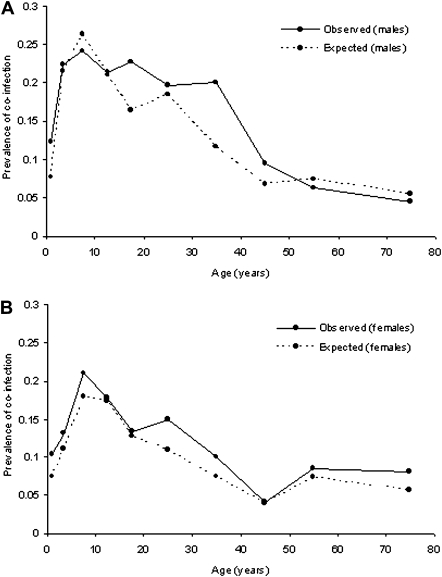
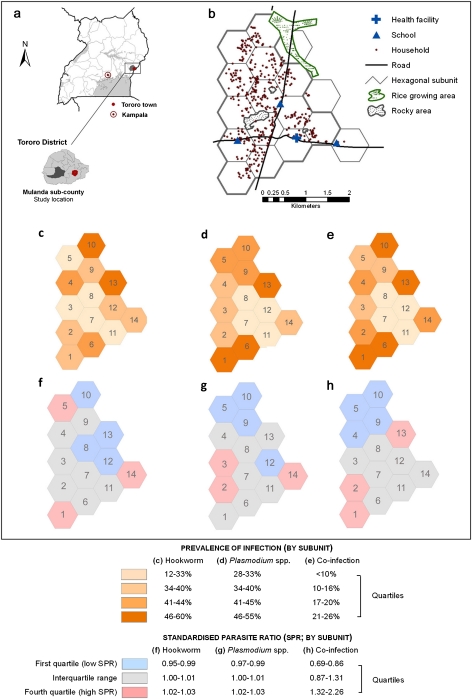
Baseline characteristics of the study population are shown in Table 1. Overall, 38.8% of individuals were infected with Plasmodium species, 39.5% were with hookworm, and 15.5% harbored both parasites (coinfection). Prevalence of coinfection was significantly higher among males than among females (18.2% vs 13.4%; P < .005) and, among both sexes, rose rapidly during early childhood, peaking among children aged 5–9 years and declining during adulthood (Figure 1). Figures 2B–2D present the spatial distribution of infection prevalence, aggregated by hexagonal subunit, and show that both monoinfection and coinfection varied significantly across the study area. Plasmodium species infection was most prevalent in the northeast of the study area (subunit 13; relative risk [RR], 1.76; P < .001), and there was a 2.8-fold difference in prevalence of hookworm between participants living in the areas of highest and lowest prevalence (subunit 6 vs subunit 12; P < .001). Prevalence of coinfection was highest in the northern and southern hexagons, with an overall prevalence of 23%–26%, compared with <10% in the eastern-central hexagons (RR, 5.86; P < .001).
Table 1.
Baseline Characteristics of Study Participants by Demographic Group (N = 1770)
| No. (%) stratified by demographic group |
|||
| Personal characteristics | Aged <5 years(n = 378) | Aged 5–15 years(n = 623) | Aged ≥16 years(n = 769) |
| Hookworm infection | |||
| Prevalence, % (n/total) | 93 (24.6) | 204 (33.6) | 397 (51.6) |
| Geometric mean egg count, eggs per gram | 78 | 82 | 198 |
| Malaria infection | |||
| Prevalence, % (n/total) | 169 (44.7) | 381 (61.2) | 139 (17.8) |
| Geometric mean parasite count, n/μL | 2561 | 860 | 295 |
| Coinfection | |||
| Prevalence, % (n/total) | 54 (14.3) | 131 (21.0) | 89 (11.6) |
| Mean hemoglobin level, g/dL | 10.0 | 11.8 | 12.6 |
| Anemia, prevalence, % (n/total) | 258 (68.3) | 242 (38.9) | 305 (39.6) |
| Male sex | 182 (48.2) | 321 (51.5) | 294 (38.2) |
| Anthelmintic in previous 6 months | 135 (36.0) | 420 (67.7) | 137 (17.9) |
| ACT in previous 6 months | 202 (53.4) | 288 (46.2) | 295 (38.4) |
| Wears shoes outside home | 269 (71.2) | 464 (74.5) | 323 (42.0) |
| Slept under a bed net last night | 182 (48.2) | 169 (27.1) | 335 (43.6) |
| Primary caregiver with any education | 281 (74.7) | 433 (70.0) | … |
| Currently attends school | … | 551 (85.8) | … |
| Ever attended school | … | … | 421 (54.7) |
| Occupation | |||
| Farmer | … | … | 316 (41.1) |
| Formal employment | … | … | 60 (7.8) |
| Student | … | … | 54 (7.0) |
| None | … | … | 322 (43.0) |
| Household characteristics (n = 432) | |||
| ≥1 net per 2 residents | 80 (18.2) | ||
| Toilet facilities | |||
| Covered pit/VIP | 91 (20.6) | ||
| Uncovered pit | 350 (79.4) | ||
| None | 22 (5.0) | ||
| Member with formal income | 67 (15.2) | ||
| Educated household heada | 30 (6.8) | ||
NOTE. Data are no. (%) of participants, unless otherwise indicated. Anemia is defined as a hemoglobin concentration of <11.0 g/dL for children aged <5 years; a hemoglobin concentration of <11.5 g/dL for children aged 5–11 years; a hemoglobin concentration of <12.0 g/dL for children aged 12–14 years and women aged ≥15 years; and a hemoglobin concentration of <13.0 for men aged ≥15 years. VIP, ventilated improved pit latrine. ACT, reported coartem (coartemether) medication.
Household head with education above secondary (higher education or vocational training).
Figure 1.
Age-prevalence profiles for coinfection with asymptomatic Plasmodium species parasitemia and hookworm. Observed and expected prevalence of coinfection are shown by age for males (A) and females (B). Estimates for expected prevalence of coinfection within each age group are based on simple probability (ie, the product of proportions infected with each species).
Figure 2.
Spatial distribution of infection status in Mulanda, a rural community in east Uganda. A, Study-site location in eastern Uganda. B, Map of the study households in Mulanda subcounty, showing road networks, main geographic features and important infrastructure. Hexagonal subdivisions are outlined in gray; those with < 25 residents were excluded from further analysis. The shaded charts show the prevalence of Plasmodium-species infection (C), hookworm infection (D), and Plasmodium-hookworm coinfection (E), by hexagonal subunit. Subsequent figures show area (hexagon)–level spatial heterogeneity of infection status, shaded by the quartile of the standardized parasite ratio (SIR; ratio by which the unit's mean prevalence is higher or lower than expected) as derived by Bayesian multivariate spatial conditional autoregressive models: F, hookworm infection; G, Plasmodium species infection; H, hookworm-Plasmodium coinfection.
Associations Between Infections
Mantel-Haenszel stratified ORs indicated that, among males, observed associations between hookworm and Plasmodium species infection were age dependent, with significantly increased odds of Plasmodium species infection in preschool-aged boys (OR, 2.16; P = .02) and men aged 16–24 years (OR, 3.89; P = .002) coinfected with hookworm (Table 2). No age differences in associations were observed among females; after adjusting for age group, hookworm infection was associated with significantly increased odds of Plasmodium species infection (age-adjusted OR, 1.77; P < .001). To explore whether observed associations reflected the spatial distributions of each species, we calculated ORs, adjusted by age group, for each hexagon. Tests for homogeneity of hexagon-specific ORs indicated that associations between species did not differ significantly across the study site (χ2 = .48; P = .3). Overall, after adjusting for hexagon and age group, we found that hookworm infection increased the odds of Plasmodium species infection by 50% (adjusted OR, 1.50; P < .001).
Table 2.
Results of Mantel-Haenszel Adjusted Odds Ratios of the Presence of Hookworm Infection or Plasmodium Species Infection by Sex, Adjusting for Age
| Sex | Age, years | Positive for hookworm eggs, % (n/total subgroup) | Positive for malaria parasitemia, % (n/total subgroup) | OR (95% CI), P | Overall adjusted OR (95% CI), P |
| Male | <5 | 26.9 (49/182) | 49.5 (90/182) | 2.16 (1.09–4.29) | |
| P = .02 | |||||
| 5–9 | 40.4 (67/166) | 65.1 (108/166) | .68 (.35–1.30) | 1.42 (1.03–1.96), P = .03 | |
| P = .2 | |||||
| 10–15 | 34.8 (54/155) | 58.7 (91/155) | 1.17 (.59–2.29) | ||
| P = .7 | |||||
| 16–34 | 52.9 (64/121) | 29.8 (36/121) | 3.89 (1.57–9.67) | ||
| P = .002 | |||||
| >34 | 56.7 (98/173) | 13.3 (23/173) | 1.22 (.50–3.01) | ||
| P = .7 | |||||
| Homogeneity of ORsa: P = .02 | |||||
| Female | <5 | 22.5 (44/196) | 40.3 (79/196) | 1.88 (.94–3.72) | |
| P = .06 | |||||
| 5–9 | 28.7 (45/157) | 62.4 (98/157) | 1.99 (.92–4.30) | 1.77 (1.28–2.44), P = .004 | |
| P = .07 | |||||
| 10–15 | 29.7 (43/145) | 57.9 (84/145) | 1.16 (.56–2.41) | ||
| P = .7 | |||||
| 16–34 | 44.8 (100/223) | 22.9 (51/223) | 2.08 (1.09–3.96) | ||
| P = .02 | |||||
| >34 | 56.3 (135/252) | 10.7 (27/252) | 1.85 (.79–4.31) | ||
| P = .15 | |||||
| Homogeneity of ORsa: P = .8 |
NOTE. CI, confidence interval; OR, odds ratio.
This test compares whether there is a significant difference between age-specific ORs, hence whether the overall adjusted OR is valid.
Predictors of Coinfection
Univariate analysis showed that a number of personal protective factors (bed net use, anthelmintic treatment, and footwear use) and residential environment factors (proximity to paddy fields and village of residence) were significantly associated with both monoinfections and coinfection (P < .01) (Tables 3–5). Tables 4 and 5 present the overall posterior estimates from spatial multivariate logistic regression models for hookworm monoinfection and coinfection (age stratification provided no additional insight for these outcomes); age-stratified estimates are presented for Plasmodium species (Table 3). We found an increased risk of coinfection among children aged 5–9 years, among individuals living in a household with a mud or beaten earth floor, and among individuals who did not wear shoes when outside the home, factors also associated with hookworm infection. Notably, the increased of risk Plasmodium species infection among preschool-aged children and adults infected with hookworm remained after adjusting for individual and household-level covariates as well as residual household and spatial clustering. Among adults, risk of Plasmodium species infection was related to the intensity of hookworm infection (Table 3). However, we found no evidence of an association between malaria and hookworm infection in school-aged children.
Table 3.
Logistic Regression Models for Malaria Monoinfection (N = 1770)
| Preschool-aged children (age, <5 years; n = 397) |
School-aged children (age, 5–15 years; n = 643) |
Adults (age, ≥16 years; n = 804) |
||||||||||
| Single variable |
Multivariable |
Single variable |
Multivariable |
Single variable |
Multivariable |
|||||||
| Parameter | OR | (95% CI) | OR | (95% BCI) | OR | (95% CI) | OR | (95% BCI) | OR | (95% CI) | OR | (95% BCI) |
| Male sex | 1.45 | (.96–2.18) | 1.08 | (.78–1.48) | 1.28 | (.88–1.86) | - | |||||
| Age, years | 1 | - | 1 | - | ||||||||
| <2 | ||||||||||||
| 3–4 | 1.78 | (1.18–2.68) | .72 | (.44–1.09) | ||||||||
| 5–9 | 1 | - | ||||||||||
| 10–15 | 1.26 | (.91–1.74) | ||||||||||
| 16–25 | 1 | - | ||||||||||
| 26–49 | 3.05 | (1.79–5.20) | 1 | (.13–.44) | ||||||||
| ≥50 | 1.80 | (1.08–3.01) | .55 | (.31–.83) | ||||||||
| Age in years | 1.42 | (1.20–1.68) | .95 | (.90–1.01) | .95 | (.90–1.00) | .97 | (.96–.98) | ||||
| Personal factors | ||||||||||||
| Used a net last night | .70 | (.46–1.05) | .62 | (.43–.88) | .61 | (.41–.88) | .91 | (.62–1.32) | ||||
| Provided 2 stool samples | .92 | (.59–1.43) | .73 | (.50–1.06) | 1.28 | (.81–2.01) | ||||||
| Bare feet | 1.68 | (1.06–2.57) | 1.08 | (.75–1.56) | 1.26 | (.87–1.83) | ||||||
| BMZ in previous 6 months | 1.11 | (.72–1.69) | .82 | (.58–1.16) | .56 | (.32–.97) | ||||||
| ACT in previous 6 monts | .83 | (.55–1.25) | .82 | (.60–1.14) | .98 | (.67–1.43) | ||||||
| Household factors | ||||||||||||
| ≥1 net per 2 residents | .18 | (.08–.40) | 1.87 | (1.12–3.08) | .81 | (.51–1.31) | .58 | (.33–1.03) | ||||
| Own an ITN/LLIN | .63 | (.41–.97) | .08 | (.00–.35) | 1.15 | (.82–1.60) | 1.26 | (.86–1.84) | ||||
| >500 m from rocky areas | 1.62 | (1.07–2.43) | 1.24 | (.89–1.71) | 1.31 | (.91–1.90) | ||||||
| >750 m from rice paddies | .79 | (.48–1.30) | .82 | (.55–1.24) | .50 | (.34–.76) | .53 | (1.35–3.16) | ||||
| >1000 m from health center | .95 | (.60–1.49) | 1.24 | (.88–1.75) | 2.01 | (1.25–3.22) | 1.58 | (1.52–10.80) | ||||
| SES factors | ||||||||||||
| Relative SES group | ||||||||||||
| 1st (most poor) | 1 | - | 1 | - | 1 | - | ||||||
| 2nd | 1.48 | (.78–2.80) | .52 | (.30–.92) | 1.02 | (.55–1.89) | ||||||
| 3rd | .89 | (.46–1.71) | .74 | (.42–1.29) | 1.66 | (.91–3.02) | ||||||
| 4th | 1.86 | (.96–3.60) | .72 | (.48–1.40) | 1.25 | (.68–2.27) | ||||||
| 5th (least poor) | .86 | (.43–1.68) | .80 | (.47–1.35) | .87 | (.46–1.62) | ||||||
| Educated PCG | 1.31 | (.82–2.10) | 1.03 | (.72–1.46) | 1.14 | (.75–1.72) | ||||||
| Educated head | 3.12 | (1.08–9.06) | 2.77 | (1.26–6.11) | .48 | (.19–1.24) | ||||||
| Formal income | 1.15 | (.75–1.77) | 1.19 | (.85–1.67) | .83 | (.56–1.25) | ||||||
| Coinfection | ||||||||||||
| Hookworm | ||||||||||||
| Egg-positive | 1.95 | (1.33–2.86) | 2.36 | (1.26–4.30) | 1.14 | (.81–1.60) | 1.13 | (.78–1.62) | 1.95 | (1.33–2.86) | - | |
| Low intensity | 1.96 | (1.20–3.19) | 1.16 | (.82–1.65) | 2.00 | (1.34–2.98) | 2.09 | (1.35–3.16) | ||||
| High intensity | 3.70 | (.70–19.4) | .80 | (.24–2.65) | 1.72 | (.89–3.33) | 4.57 | (1.52–10.80) | ||||
| Variance parameters | ||||||||||||
| Nonspatial household | .90 | (.005–3.43) | .12 | (.002–76) | .14 | (.002–.99) | ||||||
| Spatial area variation | .18 | (.002–1.42) | .01 | (.001–-.08) | .01 | (.001–.10) | ||||||
NOTE. Comparison of results between single-variable regression and spatially explicit Bayesian multivariable logistic regression models for malaria infection status, stratified by age group. The baseline comparison group had no infection or hookworm only. Odds ratios shown in bold had P < .1 in single-variable regression and were subsequently tried in multivariable regression models. Personal factors include the following: provided 2 stool samples vs 1 sample; and bare feet vs shoes/sandals outside home. Socioeconomic (SES) factors are defined as follows: educated primary caregiver (PCG), primary caregiver formally educated to any level; educated head, household head educated to further/higher level; formal income, household member with formal income. Hookworm infection is defined as follows: low intensity, <1000 eggs per gram; high intensity ≥1000 eggs per gram. ACT, reported Coartem (coartemether) medication; BCI, Bayesian credible interval; BMZ, reported benmidazole (anthelmintic) medication; CI, confidence interval; OR, odds ratio; ITN/LLIN, insecticide-treated net/long-lasting insecticidal net.
Table 4.
Logistic Regression Models for Hookworm Monoinfection(N = 1770)
| Single variable |
Multivariable |
||||
| Parameter | n | OR | (95% BCI) | OR | (95% BCI) |
| Male sex | 797 | 1.18 | (.97–1.43) | 1.28 | (1.02–1.62) |
| Age in years | 1.02 | (1.02–1.04) | |||
| Personal factors | |||||
| Used a net last night | 686 | .75 | (.61–.91) | ||
| Provided 2 stool samples | 1312 | 2.13 | (1.69–2.69) | 2.14 | (1.56–2.88) |
| Bare feet | 1056 | 1.35 | (1.11, 1.64) | 1.29 | (.99–1.67) |
| BMZ in previous 6 months | 692 | .43 | (.35–.53) | .51 | (.39–.66) |
| ACT in previous 6 months | 785 | .87 | (.72–1.06) | ||
| Household factors | |||||
| Latrine (vs none) | 81 | 1 | - | ||
| Uncovered pit | 1331 | .51 | (.32–.80) | ||
| Covered pit/VIP | 358 | .38 | (.23–.63) | ||
| Mud/beaten earth floor | 1586 | 4.48 | (2.92–6.86) | 4.30 | (2.37–8.00) |
| >500 m from rocky areas | 1396 | 1.79 | (1.47–2.17) | 1.63 | (1.07–2.42) |
| >750 m from rice paddies | 791 | .98 | (.78–1.24) | ||
| >1000 m from health center | 1255 | 1.88 | (1.51–2.35) | ||
| SES factors | |||||
| Relative SES group | |||||
| 1st (most poor) | 309 | 1 | - | ||
| 2nd | 354 | 1.09 | (.80–1.48) | ||
| 3rd | 327 | .83 | (.60–1.13) | ||
| 4th | 385 | .77 | (.57–1.04) | ||
| 5th (least poor) | 395 | .40 | (.29–.55) | ||
| Educated PCG | 1248 | .57 | (.46–.70) | .42 | (.22–.78) |
| Educated head | 109 | .44 | (.28–.69) | .69 | (.51–.94) |
| Formal income | 293 | .43 | (.32–.58) | .49 | (.32–.74) |
| Coinfection | |||||
| Malaria | |||||
| Slide-positive | 687 | 1.04 | (.85–1.26) | 1.46 | (1.12–1.88) |
| Low density | 579 | 1.08 | (.88–1.32) | ||
| High density | 108 | .84 | (.56–1.28) | ||
| Variance parameters | |||||
| Nonspatial household | .72 | (.41–1.12) | |||
| Spatial area variation | .06 | (.004–.37) | |||
NOTE. Comparison of results between single variable nonspatial regression and spatially explicit Bayesian multivariable logistic regression models for hookworm infection status. The baseline comparison group had no infection or malaria only. Personal factors included the following: provided 2 stool samples vs 1 sample; bare feet vs shoes/sandals outside home; ACT, reported Coartem (coartemether) medication. Household factors included mud/beaten earth floor vs concrete. Socioeconomic (SES) factors were defined as follows: educated primary caregiver (PCG), primary caregiver formally educated to any level; educated head, household head educated to further/higher level; formal income, household member with formal income. Density of parasitemia was defined as follows: low density, <5000 n/μL; high intensity, ≥5000 n/μL. BCI, Bayesian credible interval; BMZ, reported benmidazole (anthelmintic) medication; OR, odds ratio; VIP; ventilated improved pit latrine.
Table 5.
Logistic Regression Models for Coinfection (N = 1770)
| Single variable |
Multivariable |
||||
| Parameter | n | OR | (95% BCI) | OR | (95% BCI) |
| Male sex | 797 | 1.44 | (1.11–1.87) | 1.43 | (1.05–1.92) |
| Age, years | |||||
| <2 | 202 | 1 | - | 1 | - |
| 3–4 | 176 | 1.66 | (.93–2.98) | 1.81 | (.92–3.52) |
| 5–9 | 323 | 2.72 | (1.37–3.77) | 3.07 | (1.73–5.48) |
| 10–15 | 300 | 1.91 | (1.13–3.20) | 2.78 | (1.55–5.10) |
| 16–25 | 201 | 1.58 | (.90–2.80) | 2.12 | (1.11–3.96) |
| 26–49 | 349 | 1.01 | (.58–1.74) | 1.04 | (.56–1.92) |
| ≥50 | 219 | .57 | (.29–1.13) | .53 | (.25–1.13) |
| Personal factors | |||||
| Used a net last night | 686 | .68 | (.52–.89) | ||
| Provided 2 stool samples | 1312 | 1.60 | (1.16–2.21) | 1.76 | (1.21–2.59) |
| Bare feet | 1056 | 1.72 | (1.31–2.28) | 1.44 | (1.02–2.05) |
| BMZ in previous 6 months | 692 | .87 | (.67–1.14) | .54 | (.37–.76) |
| ACT in previous 6 months | 785 | .97 | (.75–1.25) | ||
| Household factors | |||||
| ≥1 net per 2 residents | 259 | .43 | (.27–.69) | ||
| LLIN/ITN in household | 627 | 1.30 | (1.00–1.69) | ||
| Latrine (vs none) | 81 | 1 | - | ||
| Uncovered pit | 1331 | .64 | (.37–1.10) | ||
| Covered pit/VIP | 358 | .58 | (.32–1.06) | ||
| Mud/beaten earth floor | 1586 | 5.14 | (2.39–11.07) | 5.09 | (2.09–13.57) |
| >500 m from rocky areas | 1396 | 1.81 | (1.39–2.34) | 1.63 | (1.07–2.42) |
| >750 m from rice paddies | 791 | .75 | (.55–1.01) | ||
| >1000 m from health center | 1255 | 2.14 | (1.54–2.98) | ||
| SES factors | |||||
| Relative SES group | |||||
| 1st (most poor) | 309 | 1 | - | ||
| 2nd | 354 | 1.12 | (.76–1.67) | ||
| 3rd | 327 | .85 | (.56–1.30) | ||
| 4th | 385 | .98 | (.66–1.45) | ||
| 5th (least poor) | 395 | .49 | (.31–.76) | ||
| Educated PCG | 1,248 | .74 | (.56–.97) | ||
| Educated head | 109 | .47 | (.26–.95) | ||
| Formal income | 293 | .50 | (.33–.76) | ||
| Variance parameters | |||||
| Nonspatial household | .88 | (.44–1.45) | |||
| Spatial area variation | .15 | (.002–1.17) | |||
NOTE. Comparison of results between single-variable nonspatial regression and spatially explicit Bayesian multivariable logistic regression models for hookworm-malaria coinfection. The baseline comparison group had no infection, hookworm only, or malaria only. Personal factors included the following: provided 2 stool samples vs 1 sample; bare feet vs shoes/sandals outside home. Household factors included mud/beaten earth floor vs concrete. Socioeconomic (SES) factors were defined as follows: educated primary caregiver (PCG), primary caregiver formally educated to any level; educated head, household head educated to further/higher level; formal income, household member with formal income. Density of parasitemia was defined as follows: low density, <5000 n/μL; high intensity ≥5000 n/μL. ACT, reported Coartem (artemether-lumefantrine) medication; BCI, Bayesian credible interval; BMZ, reported benmidazole (anthelmintic) medication; LLIN/ITN, long-lasting insecticidal net/insecticide-treated net; OR, odds ratio; VIP; ventilated improved pit latrine.
Household and Spatial Clustering of Coinfection
Comparison of random effects from the Bayesian mixed models shows that most variation in the population occurred between households. Specifically, of the total household-level and area (hexagon)–level variation for hookworm and for P. falciparum infection, 6%–8% occurred at the area level and 92%–94% occurred at the household level (Tables 3 and 4). Within-area clustering was considerably more important for coinfection, accounting for 15% of the total variability at household and area levels (Table 5). The interquartile ranges for household-level SIR values (the ratio by which mean household risk was higher or lower than expected) were .70–1.51 for Plasmodium species infection, .44–1.97 for hookworm infection, and .53–1.69 for coinfection, indicating considerable residual household clustering. Figures 2E–2G presents the residual spatial variation of infection prevalence arising from the CAR models; area-level SIR estimates for coinfection exhibited residual high-prevalence clustering in the southern (subunits 1 and 2) and eastern (subunit 13) regions of the study area.
Consequences of Coinfection on Hemoglobin Levels
In total, hemoglobin-level data were available for 1698 participants (96.0%). Mean hemoglobin level (standard deviation) among preschool children was 10.0 (.09) g/dL; among school-aged children, 11.8 (.05) g/dL; and among adults, 12.6 (.07) g/dL. Among preschool-aged children, high-density malaria parasitemia (>5000 parasites/μL) was associated with lower hemoglobin levels after adjusting for age and stunting (−1.02 g/dL; 95% BCI, −1.50 to −.55 g/dL) compared with uninfected children, whereas among adults, hookworm intensity of ≥1000 eggs per gram resulted in a .42 g/dL (95% BCI, −.71 to −.12 g/dL) reduction in hemoglobin levels after adjusting for sex and socioeconomic status. School-aged children singly infected with either hookworm or malaria parasites did not have lower hemoglobin levels, although coinfection was associated with .34 g/dL (95% BCI, −.68 to −.01 g/dL) lower hemoglobin levels after adjusting for age and stunting.
DISCUSSION
This study is, to our knowledge, the first population-level, purposive epidemiological investigation of Plasmodium-hookworm coinfection. In the rural community studied, coinfection was most common among school-aged children and exhibited marked spatial heterogeneity and household clustering, with a number of predictors identified. Taking account of these epidemiological features, we show that Plasmodium species infection was positively associated with hookworm among preschool-aged children and adults. Previous studies have reported either positive [13, 14, 27–30] or negative [28, 31, 32] association, or no association [17, 33, 34], between malaria phenotypes and helminth infections (including both soil-transmitted helminth and Schistosoma species), but few of these studies appropriately adjusted for socioeconomic or household risk factors, age, or spatial location [19–21, 35]. Our data show how relatively minor spatial heterogeneities in the distribution of individual species can cause pronounced spatial aggregation of coinfection, a phenomenon previously demonstrated at larger spatial scales [36, 37]. Furthermore, we show that clustering of coinfection within households represents a major source of population-level variability, even after adjustment for peridomestic and microenvironmental factors. Failure to account for such heterogeneities may lead to statistical problems and spurious associations [20, 21].
We found significant positive association between Plasmodium species and hookworm, controlling for possible sources of variability and confounding, which indicates an enduring association between species. Whereas this study design does not allow for exploration of mechanisms underlying this association, previous studies have suggested that the immunoregulatory effects of helminths, which promote their survival within humans, may impair the immune response required to protect against or eliminate malaria parasites [8, 38]. Alternatively, extrinsic behavioral or environmental factors that result in increased exposure to both parasites may play a role [19]. Studies of polyhelminth infection have shown that factors associated with the domestic environment play an important role in associations between species [39, 40]. The lack of Plasmodium-hookworm association among school-aged children in this study may, for example, reflect more homogeneous behaviors or exposures in this demographic group. Whether the observed association among parasite infections translates into an increased risk of clinical malaria is also unclear. Intervention studies are therefore warranted to examine the impact of helminth infection and deworming on incidence of clinical malaria.
Plasmodium-hookworm coinfection was found to be associated with slightly lower hemoglobin levels among school-aged children. The mechanisms by which Plasmodium species infection causes anemia are multifactorial, including increased destruction of red blood cells through rupturing, phagocytosis, and hypersplenism and decreased red blood cell production through inflammation and dyserythropiesis [41]. Hookworms contribute to anemia by direct intestinal blood loss and anemia of inflammation [42]. Based on these distinct mechanisms, an additive effect of coinfection on hemoglobin level is plausible; however, longitudinal studies may provide further insight. Thus, although these findings suggest that coinfection may not occur more than would be expected by chance in school-aged children, coinfection is still associated with morbidity and is of substantial public health relevance. School health programs already deliver anthelmintic treatment and school feeding and provide an under-utilised framework for delivering malaria interventions targeted at school-aged children [43]. Possible options include school-based, intermittent, preventive treatment and delivery of mosquito nets, interventions that have already been shown to reduce malaria-related anemia in school-aged children [44–46]. Further studies are warranted to evaluate the impact of combined malaria control and deworming interventions on child health outcomes.
Our results represent a single time point, yet the detectability of Plasmodium species and hookworm infection fluctuates [47, 48]. In addition, light microscopy can miss a substantial proportion of Plasmodium species infections when compared with more sensitive polymerase chain reaction–based techniques [49], whereas the Kato-Katz method can miss light hookworm infections [50]. However, the relevance of these light and submicroscopic infections, for both transmission and morbidity, is uncertain. Finally, our analysis grouped households into ∼.5 km2 hexagon subunits to overcome the problem of small denominators. This assumption may have masked spatial variation at very small scales, although we could find no evidence that association between species varied by subunit.
This study also has several strengths. Interpretation of the co-occurrence of parasitic infections can be fraught with problems, not least the risk of false conclusions through failure to control for factors that aggregate parasites in subsets of the population [20, 21]. This is a large, purposive, population-level study enhanced by the collection of detailed socioeconomic, geographic, and other contextual data and powered to detect potential associations between helminths and malaria infection within population subgroups. Explicitly modeling the spatial distribution of each infection provided a means to adjust for the spatial dependency of each parasite species during analysis.
In conclusion, we have demonstrated in a large, population-based study that coinfection with Plasmodium species and hookworm exhibits important population heterogeneities, but that an enduring positive association between species infections remains. This observation remained after spatially explicit multilevel logistic regression to control for potential confounding factors. These results warrant further investigation but also have implications for a targeted approach to parasite control in developing countries. In particular, they show that certain subpopulations are more at risk for coinfection and its consequences, and that these subpopulations should be targeted for combined malaria and helminth control. The previous decade has witnessed an unprecedented commitment in tackling malaria and in implementing deworming programs, but such efforts require a tailored integration to ensure that they can concomitantly tackle the problem of malaria-helminth coinfection.
Funding
This work was supported by the Wellcome Trust (Career Development Fellowship 081673 to S.B.); and the UK Medical Research Council (studentship to R.L.P.).
Acknowledgments
The authors are very grateful to inhabitants of Mulanda who kindly participated in the study and thank all the members of the study team in Uganda for their tireless effort and excellent work. We also thank Hugh Sturrock and Archie Clements for statistical advice and constructive comments.
Appendix
Bayesian spatially explicit logistic regression models included a nonspatial household-level random effect (with an exchangeable correlation structure) and a spatial area-level random effect. Models were of the following form:
Yij ∼ binomial(μij, k)
where Yij is a binary indicator of the presence of monoinfection or coinfection for individual i in household j = 1…N in hexagon k = 1…N; α is the intercept and Σ βn is a vector of n regression parameters corresponding to the set of n covariates (Xij). The component ui is the unstructured household level heterogeneity. The area-level effect (vk) was modeled using a conditional autoregressive (CAR) prior structure, in which an adjacency matrix was specified with a weight of 1 given to adjacent hexagons and a weight of 0 given to nonadjacent hexagons, thus incorporating a degree of spatial smoothing dependent upon covariance between neighboring hexagons. This error term yields a ratio by which area-level infection prevalence is higher or lower than expected, which can be thought of as a standardized infection ratio [SIR; exp(vk)].
Noninformative priors were used for the coefficients (normal prior with a mean 0 and precision 1 × 10−6), and the precision of ui and vk was given a noninformative γ distribution. Posterior distributions for the unknown parameters were formed using a Monte Carlo Markov chain and Gibbs sampling. For each model, 35,000 iterations were run—with the first 10,000 discarded (“burn in”)—and the remaining iterations were subsampled at every fifth observation, giving a final sample size of 5000, for which the model parameters were estimated. Model convergence was evaluated on the basis of inspection of sample traces, and inference was based on the better-fitting model using the deviance information criteria as a goodness-of-fit measure. Posterior distributions of model parameters were summarized using descriptive statistics (posterior mean and 95% Bayesian credible intervals).
References
- 1.Hay SI, Guerra CA, Gething PW, et al. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;24:e1000048. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: Updating the global picture. Trends Parasitol. 2003;19:547–51. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Desai MR, Mei JV, Kariuki SK, et al. Randomized, controlled trial of daily iron supplementation intermittent sulfadoxine-pyrimethamine for the treatment of mild childhood anemia in western Kenya. J Infect Dis. 2003;187:658–66. doi: 10.1086/367986. [DOI] [PubMed] [Google Scholar]
- 4.Nyakeriga AM, Troye-Blomberg M, Dorfman JR, et al. Iron deficiency and malaria among children living on the coast of Kenya. J Infect Dis. 2004;190:439–47. doi: 10.1086/422331. [DOI] [PubMed] [Google Scholar]
- 5.Brabin BJ, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. J Nutr. 2001;131:604S–615S. doi: 10.1093/jn/131.2.604S. [DOI] [PubMed] [Google Scholar]
- 6.Stoltzfus RJ, Dreyfuss ML, Chwaya HM, Albonico M. Hookworm control as a strategy to prevent iron deficiency. Nutr Rev. 1997;55:223–32. doi: 10.1111/j.1753-4887.1997.tb01609.x. [DOI] [PubMed] [Google Scholar]
- 7.Brooker S, Clements AC, Hotez PJ, et al. The co-distribution of Plasmodium falciparum and hookworm among African school children. Malar J. 2006;5:99. doi: 10.1186/1475-2875-5-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Druilhe P, Tall A, Sokhna C. Worms can worsen malaria: Towards a new means to roll back malaria? Trends Parasitol. 2005;21:359–62. doi: 10.1016/j.pt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Nacher M. Worms malaria: Noisy nuisances and silent benefits. Parasite Immunol. 2002;24:391–3. doi: 10.1046/j.1365-3024.2002.00470.x. [DOI] [PubMed] [Google Scholar]
- 10.Brooker S, Akhwale WS, Pullan R, et al. Epidemiology of Plasmodium-helminth co-infection in Africa: Potential impact on anemia, prospects for combining control. Am J Trop Med Hyg. 2007;77:88–98. [PMC free article] [PubMed] [Google Scholar]
- 11.Demissie F, Kebede A, Shimels T, Beyene P. Assessment of public health implication of malaria-geohelminth co-infection with an emphasis on hookworm-malaria anaemia among suspected malaria patients in Asendabo, southwest Ethiopia. Ethiop Med J. 2009;47:153–58. [PubMed] [Google Scholar]
- 12.Egwunyenga AO, Ajayi JA, Nmorsi OP, Duhlinska-Popova DD. Plasmodium/intestinal helminth co-infections among pregnant Nigerian women. em Inst Oswaldo Cruz. 2001;96:1055–9. doi: 10.1590/s0074-02762001000800005. [DOI] [PubMed] [Google Scholar]
- 13.Hillier S, Booth M, Muhangi L, et al. Plasmodium falciparum and helminth coinfection in a semi urban population of pregnant women in Uganda. J Infect Dis. 2008;198:920–7. doi: 10.1086/591183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yatich NJ, Yi J, Agbenyega T, et al. Malaria and intestinal helminth co-infection among pregnant women in Ghana: Prevalence and risk factors. Am J Trop Med Hyg. 2009;80:896–901. [PubMed] [Google Scholar]
- 15.Nacher M. Interactions between worm infections and malaria. Clin Rev Allergy Immunol. 2004;26:85–92. doi: 10.1007/s12016-004-0003-3. [DOI] [PubMed] [Google Scholar]
- 16.Sokhna C, Le Hesran JY, Mbaye PA, et al. Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal. Malar J. 2004;3:43. doi: 10.1186/1475-2875-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briand V, Watier L, Hesran JY, Garcia A, Cot M. Coinfection with Plasmodium falciparum and Schistosoma haematobium: Protective effect of schistosomiasis on malaria in Senegalese children? Am J Trop Med Hyg. 2005;72:702–7. [PubMed] [Google Scholar]
- 18.Lyke KE, Dicko A, Dabo A, et al. Association of Schistosoma haematobium infection with protection against acute Plasmodium falciparum malaria in Malian children. Am J Trop Med Hyg. 2005;73:1124–30. [PMC free article] [PubMed] [Google Scholar]
- 19.Mwangi TW, Bethony JM, Brooker S. Malaria and helminth interactions in humans: an epidemiological viewpoint. Ann Trop Med Parasitol. 2006;100:551–70. doi: 10.1179/136485906X118468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Booth M. The role of residential location in apparent helminth and malaria associations. Trends Parasitol. 2006;22:359–62. doi: 10.1016/j.pt.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Behnke JM. Structure in parasite component communities in wild rodents: Predictability, stability, associations interactions…or pure randomness? Parasitology. 2008;135:751–66. doi: 10.1017/S0031182008000334. [DOI] [PubMed] [Google Scholar]
- 22.Kabatereine NB, Tukahebwa EM, Kazibwe F, et al. Soil-transmitted helminthiasis in Uganda: Epidemiology and cost of control. Trop Med Int Health. 2005;10:1187–89. doi: 10.1111/j.1365-3156.2005.01509.x. [DOI] [PubMed] [Google Scholar]
- 23.Kabatereine NB, Brooker S, Koukounari A, et al. Impact of a national helminth control programme on infection and morbidity in Ugandan school children. Bull World Health Organ. 2007;85:91–9. doi: 10.2471/BLT.06.030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pullan RL, Bukirwa H, Staedke SG, Snow RW, Brooker S. Plasmodium infection and its risk factors in eastern Uganda. Malar J. 2010;9:2. doi: 10.1186/1475-2875-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–7. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. WHO child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height body mass index-for age: Methods and development. Geneva, Switzerland: World Health Organisation; 2006. [Google Scholar]
- 27.Degarege A, Animut A, Legesse M, Erko B. Malaria severity status in patients with soil-transmitted helminth infections. Acta Trop. 2009;112:8–11. doi: 10.1016/j.actatropica.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Nacher M, Singhasivanon P, Yimsamran S, et al. Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand. J Parasitol. 2002;88:55–8. doi: 10.1645/0022-3395(2002)088[0055:IHIAAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Tshikuka JG, Scott ME, Gray-Donald K, Kalumba ON. Multiple infection with Plasmodium and helminths in communities of low and relatively high socio-economic status. Ann Trop Med Parasitol. 1996;90:277–93. doi: 10.1080/00034983.1996.11813053. [DOI] [PubMed] [Google Scholar]
- 30.Midzi N, Sangweme D, Zinyowera S, et al. The burden of polyparasitism among schoolchildren in rural farming areas in Zimbabwe. Trans R Soc Trop Med. 2008;102:1039–45. doi: 10.1016/j.trstmh.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 31.Nacher M, Singhasivanon P, Treeprasertsuk S, et al. Intestinal helminths and malnutrition are independently associated with protection from cerebral malaria in Thailand. Ann Trop Med Parasitol. 2002;96:5–13. doi: 10.1179/000349802125000448. [DOI] [PubMed] [Google Scholar]
- 32.Nacher M, Singhasivanon P, Silachamroon U, et al. Helminth infections are associated with protection from malaria-related acute renal failure and jaundice in Thailand. Am J Trop Med Hyg. 2001;65:834–6. doi: 10.4269/ajtmh.2001.65.834. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro AE, Tukahebwa EM, Kasten J, et al. Epidemiology of helminth infections and their relationship to clinical malaria in southwest Uganda. Trans R Soc Trop Med Hyg. 2005;99:18–24. doi: 10.1016/j.trstmh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Achidi EA, Apinjoh TO, Mbunwe E, et al. Febrile status, malarial parasitaemia gastro-intestinal helminthiases in schoolchildren resident at different altitudes, in south-western Cameroon. Ann Trop Med Parasitol. 2008;102:103–18. doi: 10.1179/136485908X252287. [DOI] [PubMed] [Google Scholar]
- 35.Howard SC, Donnell CA, Chan MS. Methods for estimation of associations between multiple species parasite infections. Parasitology. 2001;122:233–51. doi: 10.1017/s0031182001007272. [DOI] [PubMed] [Google Scholar]
- 36.Brooker S, Clements AC. Spatial heterogeneity of parasite co-infection: Determinants and geostatistical prediction at regional scales. Int J Parasitol. 2009;39:591–7. doi: 10.1016/j.ijpara.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonardo LR, Rivera PT, Crisostomo BA, et al. A study of the environmental determinants of malaria and schistosomiasis in the Philippines using Remote Sensing and Geographic Information Systems. Parassitologia. 2005;47:105–14. [PubMed] [Google Scholar]
- 38.Hartgers FC, Yazdanbakhsh M. Co-infection of helminths malaria: Modulation of the immune responses to malaria. Parasite Immunol. 2006;28:497–506. doi: 10.1111/j.1365-3024.2006.00901.x. [DOI] [PubMed] [Google Scholar]
- 39.Pullan RL, Bethony JM, Geiger SM, Correa-Oliveira R, Brooker S, Quinnell RJ. Human helminth co-infection: No evidence of common genetic control of hookworm and Schistosoma mansoni infection intensity in a Brazilian community. Int J Parasitol. 2010;40:299–306. doi: 10.1016/j.ijpara.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellis MK, Raso G, Li YS, Rong Z, Chen HG, McManus DP. Familial aggregation of human susceptibility to co- and multiple helminth infections in a population from the Poyang Lake region, China. Int J Parasitol. 2007;37:1153–61. doi: 10.1016/j.ijpara.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menendez C, Fleming AF, Alonso PL. Malaria-related Anaemia. Parasitol Today. 2000;16:469–76. doi: 10.1016/s0169-4758(00)01774-9. [DOI] [PubMed] [Google Scholar]
- 42.Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. New Engl J Med. 2004;351:799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- 43.Brooker S, Clarke S, Snow RW, Bundy DA. Malaria in African schoolchildren: Options for control. Trans R Soc Trop Med. 2008;102:304–5. doi: 10.1016/j.trstmh.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barger B, Maiga H, Traore OB, et al. Intermittent preventive treatment using artemisinin-based combination therapy reduces malaria morbidity among school-aged children in Mali. Trop Med Int Health. 2009;14:784–91. doi: 10.1111/j.1365-3156.2009.02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leenstra T, Phillips-Howard PA, Kariuki SK, et al. Permethrin-treated bed nets in the prevention of malaria anemia in adolescent schoolgirls in western Kenya. Am J Trop Med Hyg. 2003;68:86–93. [PubMed] [Google Scholar]
- 46.Clarke SE, Jukes M, Njagi JK, et al. Effect of intermittent preventive treatment of malaria on health and education in schoolchildren: A cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:127–38. doi: 10.1016/S0140-6736(08)61034-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Färnert A. Plasmodium falciparum population dynamics: Only snapshots in time? Trends Parasitol. 2008;24:340–4. doi: 10.1016/j.pt.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Anderson RM, Schad GA. Hookworm burdens faecal egg counts: An analysis of the biological basis of variation. Trans R Soc Trop Med Hyg. 1985;79:812–25. doi: 10.1016/0035-9203(85)90128-2. [DOI] [PubMed] [Google Scholar]
- 49.Okell LC, Ghani AC, Lyons E, Drakeley CJ. Submicroscopic infection in Plasmodium falciparum–endemic populations: A systematic review and meta-analysis. J Infect Dis. 2009;15:1509–17. doi: 10.1086/644781. [DOI] [PubMed] [Google Scholar]
- 50.Booth M, Vounatsou P, N’Goran EK, Tanner M, Utzinger J. The influence of sampling effort and the performance of the Kato-Katz technique in diagnosing Schistosoma mansoni and hookworm co-infections in rural Côte d'Ivoire. Parasitology. 2003;127:525–31. doi: 10.1017/s0031182003004128. [DOI] [PubMed] [Google Scholar]