Abstract
Background
MicroRNAs (miRNAs) are potent regulators of gene expression with proposed roles in brain development and function. We hypothesized that miRNA expression profiles are altered in individuals with severe psychiatric disorders.
Methods
Using real-time quantitative PCR, we compared the expression of 435 miRNAs and 18 snoRNAs in post-mortem brain tissue samples from individuals with schizophrenia, individuals with bipolar disorder, and psychiatrically healthy control subjects (n = 35 each group). Detailed demographic data, sample selection and storage conditions, and drug and substance exposure histories were available for all subjects. Bayesian model averaging was used to simultaneously assess the impact of these covariates as well as the psychiatric phenotype on miRNA expression profiles.
Results
Of the variables considered, sample storage time, brain pH, alcohol at time of death, and post-mortem interval were found to affect the greatest proportion of miRNAs. 19% of miRNAs analyzed exhibited positive evidence of altered expression due to a diagnosis of schizophrenia or bipolar disorder. Both conditions were associated with reduced miRNA expression levels, with a much more pronounced effect observed for bipolar disorder.
Conclusions
This study suggests that modest under-expression of several miRNAs may be involved in the complex pathogenesis of major psychosis.
Keywords: microRNA, major psychosis, schizophrenia, bipolar disorder, Bayesian model averaging, DGCR8
Introduction
Schizophrenia and bipolar disorder are severe psychiatric conditions for which disease pathophysiology is still poorly understood. The association of psychotic features with severe mood disorders, combined with recent genetic insights, suggests that schizophrenia and bipolar disorder are etiologically related (1). Though highly heritable, the complex pathogenesis of major psychosis may involve dozens of genes, as well as epigenetic and environmental factors (reviewed in (2)). The results of genome-wide association studies implicate hundreds of common genetic variants acting in concert to influence disease susceptibility. Such heterogeneity may confound the search for causal genetic mutations and the development of effective, targeted therapeutics.
MicroRNAs (miRNAs) comprise a growing class of endogenous molecules that regulate gene expression post-transcriptionally. By binding to partially complementary regions at the 3′ end of messenger RNAs, these ~22 nucleotide single-stranded molecules induce cleavage or translational repression of targeted transcripts. Several independent studies predict that 20–30% of human genes are regulated by miRNAs (3, 4), but a sensitive pattern-based target prediction algorithm boosts this estimate considerably to 74–92% of genes (5). The ability of miRNAs to influence complex gene networks and pathways suggests that their dysregulation may contribute to the genetic basis of schizophrenia spectrum disorders.
The majority of miRNA genes occur in tandem, operon-like clusters that form polycistronic transcripts. Most miRNAs fall within intergenic regions or within the introns of protein-coding genes (6). Mammalian miRNA biosynthesis begins with RNA polymerase II-dependent transcription, which yields long primary miRNA (pri-miRNA) transcripts (7). Human pri-miRNAs contain one or more hairpins, which are endonucleolytically cleaved by a microprocessor complex consisting of the RNase III enzyme Drosha and the cofactor DGCR8 (8). Drosha cleavage in the nucleus releases a ~70 nucleotide hairpin precursor miRNA (pre-miRNA), which is then exported to the cytoplasm by the Exportin-5-Ran-GTP pathway (9). Cytoplasmic pre-miRNAs are trimmed to their mature length by a second RNase III enzyme called Dicer, and are then unwound and loaded into the RISC effector complex to become biologically active.
Enrichment of predicted miRNA target sites within brain-expressed mRNAs (10), as well as emerging functional evidence, suggests that miRNAs serve important neurobiological roles. Approximately 70% of known miRNAs are expressed in the nervous system, often with a high degree of spatial and temporal specificity (11). A survey of miRNA expression patterns in various organ and tissue types identified several brain-specific and brain-enriched miRNAs (12). Additionally, miRNA profiling of the developing and adult mouse brain revealed a “chronological wave” of expression (13). Thus, the appearance of sequentially expressed groups of miRNAs may coincide with the onset of neurodevelopmental processes, such as neuronal proliferation and migration, neurite growth, and synaptogenesis. There are a growing number of miRNAs with well-characterized neurodevelopmental functions. MiR-124 and miR-9 influence the decision of neural precursors to adopt a neuronal or glial fate. MiR-124 inhibits expression of non-neuronal genes and splicing factors, and transfecting miR-124 duplexes into progenitor cells decreases the number of cells expressing glial markers (GFAP), while increasing the number of neurons (14).
MiRNAs also serve important roles in the fully formed adult nervous system, modulating such diverse functions as synaptic plasticity and circadian rhythm. MiR-134 locally inhibits translation of Limk1 mRNA at postsynaptic sites, which decreases the size of dendritic spines. This effect is reversed upon exposure to BDNF (15). MiR-219 is transcriptionally activated in the suprachasmatic nucleus by the circadian factors CLOCK and BMAL, while miR-132 is induced by CREB in a light-dependent manner. Over-expression of miR-132 in cortical neurons also enhances Ca2+ influx in response to depolarizing agents such as NMDA, while miR-219 has the opposite effect, suggesting that these two miRNAs broadly influence neuronal excitability as well as circadian rhythm (16).
The broad influence of miRNAs on gene expression networks and their known involvement in neurobiological pathways suggests that perturbation of miRNA expression profiles may contribute to the etiology of neuropsychiatric disorders. Altered expression of miRNAs in post-mortem brain samples from individuals with schizophrenia has been reported in several independent studies, but with inconsistent and even contradictory results. Perkins et. al. identified 16 misexpressed miRNAs in individuals with schizophrenia or schizoaffective disorder, 15 of which were under-expressed in the prefrontal cortex (17). Conversely, Beveridge et. al. observed over-expression of 9.5% of all miRNAs expressed in the dorsolateral prefrontal cortex, including four reported to be under-expressed in the Perkins study (18). In the present study, we have performed miRNA expression analysis of 435 miRNAs and 18 small nucleolar RNAs (Sanger miRBase v9.2) using TaqMan® real-time PCR methodology. Expression signatures were obtained from post-mortem brain samples originating from individuals with schizophrenia, individuals with bipolar disorder, and psychiatrically healthy controls. Sample covariates pertaining to demographic variables, sample handling, and substance exposure history were assessed in a statistically rigorous manner. We hope that the use of gold-standard technical methodology and a sophisticated statistical approach could help to rectify the disparities among previous expression studies. We report modest yet significant under-expression of several miRNAs as well as a global trend toward miRNA down-regulation in individuals with psychotic illness.
Methods and Materials
Post-mortem brain tissue samples
A set of samples from individuals with schizophrenia, bipolar disorder, and psychiatrically normal controls were obtained from the Stanley Medical Research Institute (http://www.stanleyresearch.org). This collection contains many more individual subjects than is typical for post-mortem studies of human brain (n=35 each group), and the samples were collected and stored in a standardized fashion with an emphasis on obtaining high-quality RNA for expression studies. For detailed sample information, refer to Supplement 1.
Purification of RNA from tissue samples
Frozen tissue samples originating from Brodmann area 9 (BA9) of the prefrontal cortex were obtained from the Stanley Medical Research Institute, and total RNA extractions were performed using the mirVANA miRNA Isolation Kit (Ambion, Austin, TX, USA). 300–500 mg sections of frozen tissue were added to 3 mL of chilled RNALaterICE (Ambion) and stored at −20°C for a minimum of 16 hours. To prepare samples for RNA purification, RNALaterICE was decanted, and the tissue sample lightly blotted to wick away excess RNALaterICE. Next, 3 mL of chilled mirVana lysis buffer was added to the tissue and the tissue homogenized with a PowerGen 35 handheld homogenizer (Thermo Fisher Scientific, Waltham, MA, USA). The mirVana miRNA isolation kit was used to purify total RNA from a 750 μL aliquot of lysate. Yield and purity were determined using a NanoDrop ND-1000 spectrophotometer.
Real-time quantitative PCR
Real-time qPCR was performed using the TaqMan® (Applied Biosystems, Foster City, CA, USA) single tube human miRNA assay panel (Sanger miRBase v9.2). Multiplexed RT reactions were performed using the TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems). Compatible 8-plexed RT primer pools were selected as subsets of the manufacturer recommended 48-plexed RT pools (Applied Biosystems, Human Multiplex RT Pools 1–8 v1.0). For a 1X reaction, cDNA synthesis was performed in a solution (20 μL) containing 2.0 mM dNTPs (0.50 mM each), 200 U MultiScribe Reverse Transcriptase, 2 μL 10x RT Buffer, 5 U RNase Inhibitor, 0.35 μL nuclease-free water, 12 nM each stem-loop RT primer and 50 ng input RNA. Reactions were incubated in a PTC-200 Thermal Cycler (MJ Research, Waltham, MA, USA) in 96-well format for 30 min at 16°C, 30 min at 42°C, 5 min at 85°C, and then held at 4°C. Real-time PCR reactions were performed using TaqMan® single tube microRNA assays (Applied Biosystems). Reactions were performed in solution (5 μL) containing 1.5 μL nuclease-free water, 2.5 μL TaqMan® Universal PCR Master Mix (No AmpErase UNG), 0.25 μL assay-specific 20x probe/primer mix, and 0.75 μL RT product (1:20 dilution). Reactions were incubated in 384-well format at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. All reactions were carried out in quadruplicate using a 7900HT Real-Time PCR System (Applied Biosystems). Pipetting operations were performed using a JANUS® Automated Workstation (Perkin Elmer) to ensure accuracy and reproducibility of relative expression data. For detailed information regarding selection of internal control genes for normalization, refer to Supplement 1.
Technical validation of real-time qPCR approach
In order to assess the accuracy of our real-time qPCR results, retrospective expression analysis of 10 miRNAs in 7 samples was performed using a second miRNA quantification method. The FlexmiR v2 assay (Luminex, Austin, TX) employs oligonucleotide capture probes bound to magnetic beads impregnated with variable mixtures of two fluorescent dyes. Unmodified template hybridizes to beads in solution, and multiplexed detection occurs using a modified flow cytometer that recognizes 100 unique bead color signatures. Assays were performed using the FlexmiR v2 demo kit according to the manufacturer’s protocol. Absolute expression measures (as quantities plotted on a standard curve for qPCR and median fluorescence intensity for FlexmiR) were compared to generate the correlation coefficient.
Statistical analysis of miRNA expression
Three samples were removed from the statistical analysis due to poor RNA quality or yield following the RNA extraction, and one additional sample was excluded due to organic brain pathology. Statistical analysis was performed using 35, 33, and 33 samples from the schizophrenia, control, and bipolar groups, respectively. Because of concerns that sample covariates may have affected detected expression levels, Bayesian model averaging (BMA) was used to simultaneously assess the impact of these covariates as well as psychiatric diagnosis on miRNA expression profiles. BMA averages over many regression models, using weights equal to the estimated probability of those models, and achieves better predictive performance than use of a single statistical model (19). Because some covariates were missing for some samples, ten distinct imputations of covariates were created (20); missing miRNA expression values (35 total) were not imputed, and were left as missing. The analysis was performed with miRNA expression levels transformed to the log2 scale. Refer to Supplement 1 for further details regarding the implementation of BMA.
Results
The superior sensitivity of TaqMan® assays allows detection of miRNAs that are expressed at less than one copy per cell. Only 9 out of 435 assayed species produced a detection signal that was indistinguishable from background. However, when approaching the limit of detection, the standard deviation in measured expression values across technical replicates dramatically increases due to random sampling error. Therefore, quantitative analysis was restricted to 234 miRNAs and 18 snoRNAs that produced mean Ct values ≤ 34. A second technical approach, the FlexmiR v2 assay, was used for cross-platform evaluation of 10 miRNAs in 7 samples. Even though the FlexmiR method boasts a limit of detection as low as 200 amol, 2 of the 10 miRNAs that produced robust signal with qPCR were indistinguishable from background levels with FlexmiR. Measured expression levels of the 8 remaining miRNAs were highly concordant between the methods, with correlations ranging from 0.761 to 0.980 (Table S1 in Supplement 1). Notably, as the signal-to-background level declined with the FlexmiR assay, so too did the correlation coefficient in most cases. These data suggest that our method of choice achieves accurate and highly sensitive detection of miRNAs in comparison to other commercially available assays.
Bayesian model averaging (BMA) was used to assess the degree to which psychiatric diagnosis as well as sample covariates influence miRNA expression profiles. The regressions implemented through BMA revealed that for most miRNAs, sample covariates accounted for only a small proportion of the variation. The increase in the coefficient of determination R2 (which measures the proportion of variability in the data accounted for by the models) over a model incorporating only plate effect ranged from 0 to 0.3. BMA yields posterior probabilities that a given variable has a nonzero regression coefficient, as well as distributions for each regression coefficient. While this indicates the probability that a given variable influences miRNA expression, it does not show the strength of the effect. A common interpretation of the posterior probabilities in this context is that a posterior probability less than 50% gives no evidence that the variable affects expression, 50% to 75% is weak evidence, 75% to 95% represents positive evidence, 95% to 99% represents strong evidence, and a posterior probability greater than 99% represents very strong evidence (21). Of all sample covariates considered, storage, brain pH, alcohol at TOD, and post-mortem interval influenced the expression of the greatest proportion of miRNAs (25%, 18%, 9%, and 8%, respectively, showing positive evidence or stronger) (Table 1). The impact of the sample covariates on levels of individual miRNAs is presented in Supplement 2.
Table 1. Posterior probabilities of covariate inclusion.
Values in cells indicate the percentage of analyzed miRNAs for which the posterior probability of a non-zero covariate effect falls into the bin in the column heading.
| Covariates | [0,75] | (75,95] | (95,99] | (99,100] |
| Diagnosis | 81 | 7 | 2 | 10 |
| Age | 95 | 2 | 0 | 2 |
| Sex | 99 | 1 | 0 | 0 |
| Brain PH | 82 | 9 | 4 | 5 |
| Brain Hemisphere | 99 | 0 | 1 | 0 |
| Lifetime Alcohol Use | 100 | 0 | 0 | 0 |
| Lifetime Drug Use | 99 | 1 | 0 | 0 |
| TOD Alcohol Use | 91 | 5 | 1 | 3 |
| TOD Drug Use | 100 | 0 | 0 | 0 |
| Smoking at TOD | 100 | 0 | 0 | 0 |
| Mood Stabilizer at death | 99 | 0 | 1 | 0 |
| Antidepressants at death | 97 | 2 | 1 | 0 |
| Anticholinergic at death | 100 | 0 | 0 | 0 |
| Storage | 75 | 10 | 2 | 13 |
| Refrigerator Interval | 100 | 0 | 0 | 0 |
| Post-mortem Interval | 92 | 5 | 1 | 2 |
| Brain Weight | 100 | 0 | 0 | 0 |
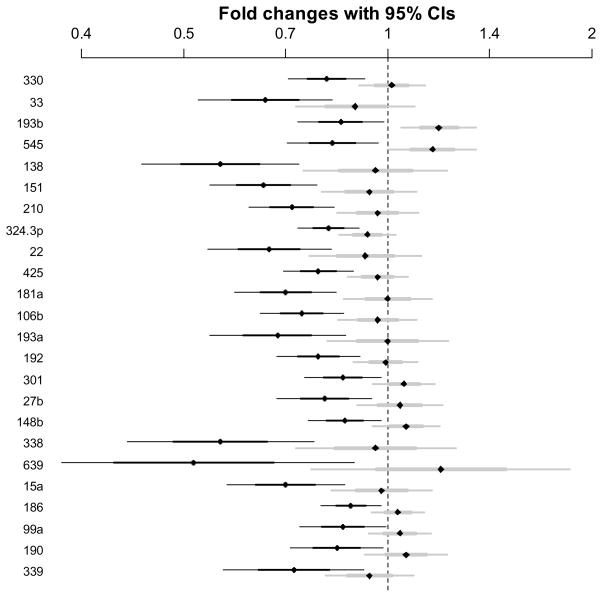
According to the above evidence thresholds, 19% of miRNAs analyzed exhibited positive evidence of altered expression based on diagnostic classification, with 12% showing strong or very strong evidence. miRNAs with posterior probabilities of a nonzero diagnostic effect greater than 95% are listed in Table 2. None of the identified miRNAs are among the most highly expressed miRNAs in the adult prefrontal cortex, but are instead expressed at intermediate or modest levels. None of the listed miRNAs arise from genomically proximal hairpins, suggesting that transcriptional co-regulation is unlikely. Only two of the misexpressed miRNAs, miR-193a and miR-193b, are closely related family members with nearly identical sequences.
Table 2. Misexpressed miRNAs.
miRNAs having greater than 95% posterior probability of non-zero effect of psychiatric diagnosis are listed, along with chromosomal positions and host genes. The rightmost column indicates miRNAs found by Stark et. al. to be under-expressed in a 22q11 hemizygous knockout mouse.
| miRNA | Posterior probability diagnosis | Chromosomal Position | Host Gene | Under-expressed in 22q11 Del mouse PFC* |
|---|---|---|---|---|
| miR-330 | 100 | 19q13.32 | EML2 | |
| miR-33 | 100 | 22q13.2 | SREBF2 | Below detection threshold |
| miR-193b | 100 | 16p13.12 | intergenic | No probe for sequence |
| miR-545 | 100 | Xq13.2 | intergenic | No probe for sequence |
| miR-138 | 100 | 3p21.33/16q13 | Intergenic/intergenic | |
| miR-151 | 100 | 8q24.3 | PTK2 | Yes** |
| miR-210 | 100 | 11p15.5 | intergenic | |
| miR-324-3p | 100 | 17p13.1 | ACADVL | Yes |
| miR-22 | 100 | 17p13.3 | C17orf91 | Yes |
| miR-425 | 100 | 3p21.31 | DALRD3 | Yes |
| miR-181a | 100 | 1q31.3/9q33.3 | Intergenic/NR6A1 | |
| miR-106b | 100 | 7q22.1 | MCM7 | Yes |
| miR-193a | 99.95 | 17q11.2 | intergenic | |
| miR-192 | 99.92 | 11q13.1 | intergenic | Yes |
| miR-301 | 99.91 | 17q22 | FAM33A | |
| miR-27b | 99.82 | 9q22.32 | C9orf3 | |
| miR-148b | 99.8 | 12q13.13 | COPZ1 | |
| miR-338 | 99.58 | 17q25.3 | AATK | Yes |
| miR-639 | 99.57 | 19p12.13 | GPSN2 | No probe for sequence |
| miR-15a | 99.5 | 13q14.3 | DLEU2 | |
| miR-186 | 98.8 | 1p31.1 | ZRANB2 | Yes |
| miR-99a | 98.52 | 21q21.1 | C21orf34 | |
| miR-190 | 97.25 | 15q22.2 | TLN2 | Below detection threshold |
| miR-339 | 96.09 | 7p22.3 | C7orf50 |
From Stark et. al., 2008
The mouse homolog mmu-miR-151 differs at a single base pair position from the corresponding human sequence
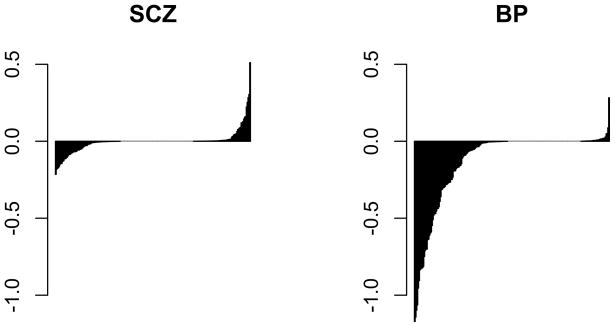
A diagnostic classification of either schizophrenia or bipolar disorder, but especially bipolar disorder, seemingly corresponds to reduced miRNA expression levels. All miRNAs that are under-expressed in the schizophrenia group relative to controls are also under-expressed in the bipolar group, and to a greater degree (Figure 1). Notably, eight of the identified miRNAs were also found by Stark et. al. to be under-expressed in the prefrontal cortex of a 22q11 hemizygous knockout mouse with haploinsufficiency of the DGCR8 miRNA processing gene (22) (Table 2). The observed overlap in differentially expressed miRNAs between these expression studies is unlikely to occur by chance (p=0.0373 using Fisher’s exact test), and suggests that aberrant miRNA processing may underlie observed expression changes. The overall trend toward under-expression of miRNAs in bipolar disorder is especially evident when examining standardized effect sizes of all analyzed miRNAs (Figure 2). Regression coefficients depicted in this figure are standardized so that expression levels have a standard deviation equal to 1. For example, a standardized coefficient of 0.5 corresponds to a change in miRNA expression of 0.5 standard deviations. Taken together, the data suggest that the presence of major psychosis is associated with globally reduced miRNA expression levels in the prefrontal cortex of affected adults.
Figure 1. Magnitude of expression changes.
Fold changes with 95% confidence intervals for miRNAs with posterior probability of non-zero effect of diagnosis exceeding 95% (black bars represent the bipolar group, gray bars represent the schizophrenia group).
Figure 2. Separately sorted effect sizes for diagnostic classes.
Expression levels were standardized such that transitioning from normal to the indicated affected category shifts miRNA expression by units of standard deviation on the Y-axis. Bolder negative effects for the bipolar group are evident.
Discussion
MicroRNA expression profiling of the adult prefrontal cortex has revealed altered expression of several miRNAs in individuals affected with schizophrenia or bipolar disorder as compared to psychiatrically normal controls. Sensitivity and accuracy are essential when evaluating subtle differences in expression between closely related sample groups, and the TaqMan® assay has demonstrated superior sensitivity and linear dynamic range compared to Northern blotting and microarrays (23). Furthermore, expression values were normalized to multiple internal control genes with empirically validated expression stability in this tissue. While the sensitivity of the TaqMan® assay allows for detection of miRNAs expressed at less than one copy per cell, miRNAs present at such low levels are not accurately quantified and so were excluded from our statistical analyses. It is unclear if these miRNAs might be present at a higher concentration within a specific subset of cells within the tissue blocks analyzed and of regulatory significance within those cells.
Post-mortem expression studies are frequently confounded by variables pertaining to tissue source and quality. Sethi et. al. recently observed limited stability and short half-lives of certain brain-enriched miRNAs, and also reported over-expression of several such miRNAs only in short post-mortem interval Alzheimer’s disease-affected tissue samples (24). This underscores the importance of controlling for sample selection variables either experimentally (25) or statistically. Our study used a collection of anatomically homogeneous samples, collected and stored with an emphasis on obtaining high quality RNA for expression studies. The sample group was comprised of specimens originating from 105 subjects divided equally among three diagnostic groups, many more than is typical for expression studies of the post-mortem human brain. Besides basic demographic variables (age, race, and sex), lifetime exposure histories to alcohol, nicotine, psychoactive medications, and illicit drugs were also available. The effect of all sample covariates was assessed in a statistically rigorous manner, such that the influence of underlying neuropathology on miRNA expression levels could be evaluated.
Bayesian model averaging takes into account the inherent uncertainty in modeling the effect size of sample covariates in case-control studies (19). The output of BMA is the posterior probability that a given covariate influences the dependent variable, in this case miRNA expression. BMA revealed that basic demographic variables such as age and sex were unlikely to influence expression levels of most miRNAs. Pre-mortem acidosis and post-mortem interval have been shown to affect mRNA expression levels in past studies (26). Variables related to sample selection and handling, particularly storage time, pH, and post-mortem interval, appeared to influence expression levels of many miRNAs, with storage time having a particularly strong influence.
Besides sample selection and handling variables, we also assessed the potentially confounding effects of exposure to psychoactive drugs and medications. Given the severity of schizophrenia and bipolar disorder, it is tremendously difficult to find large collections of brain tissue from untreated subjects for gene expression studies. Importantly, treatment with antipsychotics was excluded from BMA because all subjects with schizophrenia and more than two-thirds of those with bipolar disorder received such treatment. In this sample set, antipsychotic treatment history acts as a proxy for the presence of psychosis, the phenotype of interest. Thus, inclusion of this variable would obscure the detection of disease-related alterations in miRNA expression. The psychoactive substances considered affected only a small proportion of miRNAs, with the exception of alcohol at time of death. To clarify, this variable describes an individual’s pattern of alcohol use prior to death, not their actual state upon autopsy. Alcohol at TOD influenced the expression of 9% of miRNAs analyzed, even though lifetime alcohol use had no measurable effect, implying a short term, acute effect of alcohol on miRNA expression levels in the brain.
This study contributes to a growing body of research examing the role of miRNAs in psychiatic disorders. Our expression analysis indicated that psychiatric diagnosis strongly influences brain expression levels of 24 miRNAs. Notably, most of the differentially expressed miRNAs were under-expressed in both the schizophrenia and bipolar sample groups relative to controls. Although expression differences are subtle, consistent trends toward under-expression of several miRNAs in both of the affected groups not only legitimizes these characteristic expression signatures, but also further supports the idea of genetic overlap between schizophrenia and bipolar disorder. This observation of under-expression of the majority of misexpressed miRNAs is in agreement with the work of others (17). Perkins et. al. measured the expression of 264 miRNAs in the prefrontal cortex from 15 individuals with schizophrenia or schizoaffective disorder versus 21 psychiatrically healthy controls. They observed altered expression of 16 miRNAs, 15 of which were under-expressed in schizophrenia. These miRNAs also displayed lower mature miRNA to pri-miRNA expression ratios, suggesting a possible defect in miRNA biogenesis.
Contrary to dramatic expression differences of individual miRNAs observed in human cancers (27), subtle perturbations in expression levels suggest that combinatorial effects of several misexpressed miRNAs may contribute to the pathogenesis of major psychosis. This finding holds critical implications for future studies of miRNAs in psychiatric illness. First, miRNAs appear to be very tightly regulated in the human adult brain, with little inter-individual variability. Consequently, it is important to implement the most sensitive and precise technical methodologies in order to detect subtle expression differences between sample groups. Second, rather than selecting particular miRNA genes as candidate susceptibility loci, genetic studies should focus on factors that regulate transcription or processing of several miRNAs. In several instances, we did not observe altered expression of genomically co-localized miRNAs. For example, even though miR-25, miR-106b, and miR-93 form a co-transcribed miRNA cluster on chromosome 7, only miR-106b was found to be significantly misexpressed in this study. This observation implies that selective alteration in processing or degradation of certain miRNAs, as opposed to aberrant transcriptional regulation, may underlie their altered expression. This does not necessarily imply pathology within the miRNA processing or degradation pathways; if one or more regulated target genes have altered expression due to illness, the altered miRNA levels could reflect reactive changes brought about by normal regulatory mechanisms. However, hemizygous deletion of DGCR8, an RNA binding protein intimately involved in miRNA processing, has recently been shown to decrease the levels of at least 25 mature miRNAs in the prefrontal cortex and hippocampus of mice (22), 8 of which overlap with our set of misexpressed miRNAs. This same study demonstrated compelling schizophrenia-like behaviors associated with DGCR8 haploinsufficiency. In humans, hemizygous deletion of the 22q11.2 genomic region that harbors DGCR8 causes DiGeorge syndrome, a severe genetic disorder marked by facial dysmorphology, deficits in learning, attention, cognitive function, and emotional behavior, as well as a marked propensity to develop schizophrenia. While these converging lines of evidence are compelling, further investigation is needed to establish a causal link between primary genetic mutations at the 22q11.2 locus, global changes in human miRNA expression profiles, and increased susceptibility to psychotic illness.
Upon examining our list of significantly misexpressed miRNAs, it may appear at first that our results correlate poorly with previous expression studies. For example, Perkins et. al. also performed expression analysis on Brodmann area 9 of the prefrontal cortex, and yet none of the 16 featured miRNAs overlap with our findings. Several factors could account for these differences. First, genetic heterogeneity and diagnostic instability have consistently thwarted efforts to replicate findings among genetic association studies of schizophrenia, and these factors could certainly account for random differences between our small sample sets. Second, while Perkins et. al. used tissue samples that were group-matched for age, gender, post-mortem interval and hemisphere, they did not control for these and many other covariates with the same degree of statistical rigor as in the present study. Importantly, 4 of the 15 miRNAs (miR-92, -29a, 29b, 30a-5p) found to be under-expressed by Perkins et. al. also showed a “positive effect” of psychiatric diagnosis (posterior probability >75%) here, and with the same directional effect. The reason why these and other miRNAs failed to indicate a “strong effect” of diagnosis becomes evident when delving deeper into our BMA results. Three miRNAs (miR-29a, -29b, 30a-5p) showed a positive effect of brain pH, five miRNAs (miR-106b, -92, 29a, -29b, -29c) showed a positive effect of storage time, and three miRNAs (miR-29b, -7, and -212) showed a very strong effect (posterior probability >99.9%) of subject age. Only by examining these and other covariates simultaneously were we able to clearly distinguish the influence of psychiatric phenotype on miRNA expression levels. The other important question to address is why have others not replicated our set of featured miRNAs? While the aforementioned factors may play a part here as well, we believe that the implementation of the most sensitive and accurate available technology allowed us to measure and quantify miRNAs that exceeded the limit of detection in previous studies. Most of the misexpressed miRNAs in our study are present at low to moderate levels in the prefrontal cortex, and may not have been detected using microarrays or other high-throughput approaches for initial screening. Finally, anatomically and functionally different brain regions have been analyzed by other studies (18, 28, 29), so genuine differences in regional expression patterns would be expected to be observed in these comparisons.
In conclusion, the findings of this study support a role for miRNAs in schizophrenia and bipolar disorder. We employed technical and statistical methods to control for factors that often confound post-mortem expression studies, yet some important caveats remain. All tissue samples originated from a single anatomically defined region of the adult prefrontal cortex, thus providing only a single spatiotemporal snapshot of miRNA expression profiles. Detailed functional characterization as well as expression profiling over a developmental time course may help to assign specific neurobiological roles to brain-expressed miRNAs, including those that are misexpessed in individuals with psychiatric illness.
Supplementary Material
Acknowledgments
This work was supported by grant R01 MH80429 from the National Institutes of Mental Health and the National Alliance for Research on Schizophrenia and Depression (NARSAD)/Staglin Family Music Festival Schizophrenia Research Award (LMB), T32 MH019957-10, the Ruth L. Kirschstein National Research Service Award (MPM), and K25 AA015346 from the National Institute on Alcohol Abuse and Alcoholism (SB). Brain specimens were donated by the Stanley Medical Research Institute Brain Collection courtesy of Drs. Michael B. Knable, E. Fuller Torrey, Maree J. Webster, and Robert H. Yolken.
Footnotes
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Craddock N, O’Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006 Jan;32(1):9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGuffin P. Nature and nurture interplay: schizophrenia. Psychiatr Prax. 2004 Nov;31(Suppl 2):S189–193. doi: 10.1055/s-2004-834565. [DOI] [PubMed] [Google Scholar]
- 3.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005 Jan 14;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 4.Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha V, Lindblad-Toh K, et al. Systematic discovery of regulatory motifs in human promoters and 3′ UTRs by comparison of several mammals. Nature. 2005 Mar 17;434(7031):338–345. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM, et al. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006 Sep 22;126(6):1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Kim VN, Nam JW. Genomics of microRNA. Trends Genet. 2006 Mar;22(3):165–173. doi: 10.1016/j.tig.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, et al. MicroRNA genes are transcribed by RNA polymerase II. Embo J. 2004 Oct 13;23(20):4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003 Sep 25;425(6956):415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 9.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003 Dec 15;17(24):3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grun D, Wang YL, Langenberger D, Gunsalus KC, Rajewsky N. microRNA target predictions across seven Drosophila species and comparison to mammalian targets. PLoS Comput Biol. 2005 Jun;1(1):e13. doi: 10.1371/journal.pcbi.0010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. Rna. 2003 Oct;9(10):1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5(3):R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P, et al. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 2004;5(9):R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smirnova L, Grafe A, Seiler A, Schumacher S, Nitsch R, Wulczyn FG. Regulation of miRNA expression during neural cell specification. Eur J Neurosci. 2005 Mar;21(6):1469–1477. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- 15.Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006 Jan 19;439(7074):283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 16.Cheng HY, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP, et al. microRNA modulation of circadian-clock period and entrainment. Neuron. 2007 Jun 7;54(5):813–829. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, et al. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8(2):R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beveridge NJ, Gardiner E, Carroll AP, Tooney PA, Cairns MJ. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol Psychiatry. 2009 Sep 1; doi: 10.1038/mp.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viallefont V, Raftery AE, Richardson S. Variable selection and Bayesian model averaging in case-control studies. Stat Med. 2001 Nov 15;20(21):3215–3230. doi: 10.1002/sim.976. [DOI] [PubMed] [Google Scholar]
- 20.van Buuren S, Karin Groothuis-Oudshoorn. Mice: Multivariate Imputation by Chained Equations. R package version 1.21. 2009. [Google Scholar]
- 21.Kass RE, Raftery AE. Bayes factors. Journal of the American Statistical Association. 1995;90:773–795. [Google Scholar]
- 22.Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008 Jun;40(6):751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33( 20):e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sethi P, Lukiw WJ. Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer’s disease temporal lobe neocortex. Neuroscience letters. 2009 Aug 7;459(2):100–104. doi: 10.1016/j.neulet.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 25.Soverchia L, Ubaldi M, Leonardi-Essmann F, Ciccocioppo R, Hardiman G. Microarrays--the challenge of preparing brain tissue samples. Addict Biol. 2005 Mar;10(1):5–13. doi: 10.1080/13556210412331327803. [DOI] [PubMed] [Google Scholar]
- 26.Harrison PJ, Heath PR, Eastwood SL, Burnet PW, McDonald B, Pearson RC. The relative importance of premortem acidosis and postmortem interval for human brain gene expression studies: selective mRNA vulnerability and comparison with their encoded proteins. Neurosci Lett. 1995 Nov 24;200(3):151–154. doi: 10.1016/0304-3940(95)12102-a. [DOI] [PubMed] [Google Scholar]
- 27.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005 Jul 15;65(14):6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 28.Beveridge NJ, Tooney PA, Carroll AP, Gardiner E, Bowden N, Scott RJ, et al. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum Mol Genet. 2008 Apr 15;17(8):1156–1168. doi: 10.1093/hmg/ddn005. [DOI] [PubMed] [Google Scholar]
- 29.Burmistrova OA, Goltsov AY, Abramova LI, Kaleda VG, Orlova VA, Rogaev EI. MicroRNA in schizophrenia: genetic and expression analysis of miR-130b (22q11) Biochemistry (Mosc) 2007 May;72(5):578–582. doi: 10.1134/s0006297907050161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.