Abstract
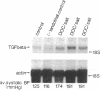
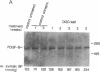
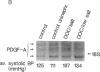
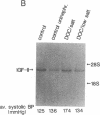
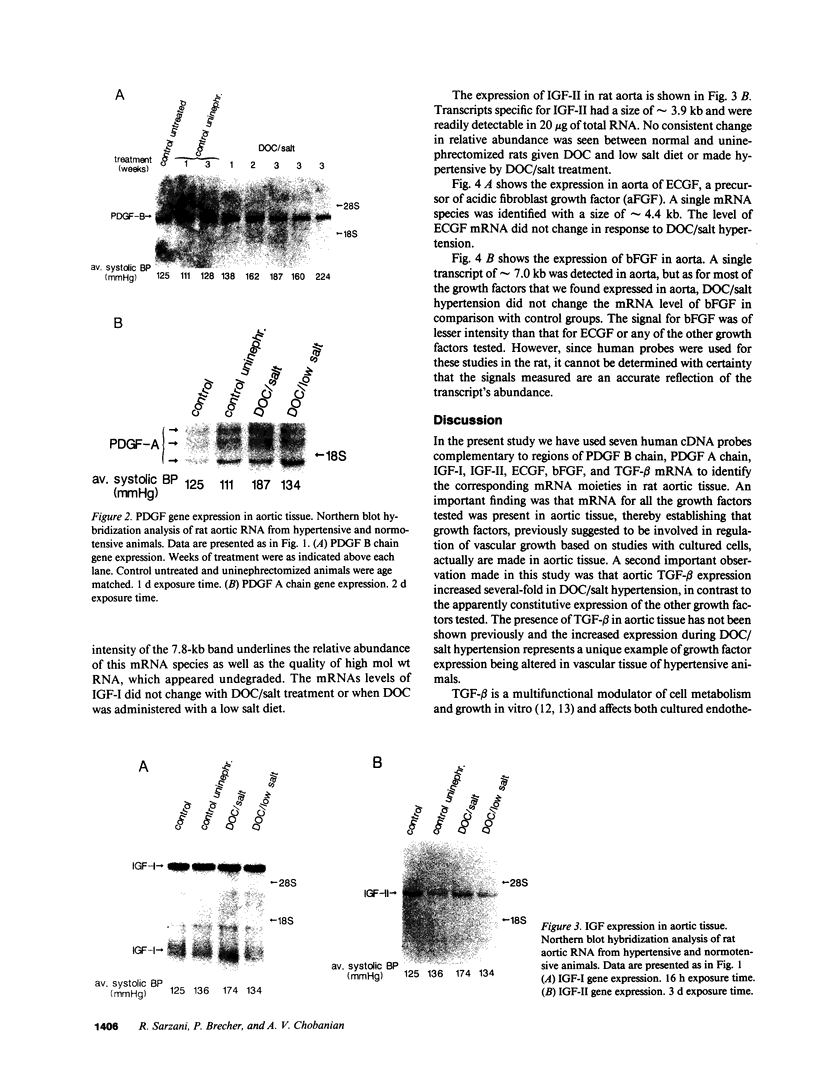
Hypertension causes biochemical and morphological changes in the vessel wall by unknown mechanisms. Locally produced substances may have a role in mediating these vascular changes. We have studied the expression of platelet-derived growth factor (PDGF) B chain and PDGF A chain, insulin-like growth factor (IGF)-I and IGF-II, endothelial cell growth factor (ECGF), basic fibroblast growth factor (bFGF), and transforming growth factor-beta (TGF-beta) in aortic tissue from normotensive rats and rats made hypertensive by deoxycorticosterone (DOC)/salt treatment. Using Northern blotting, we found that genes for each of these growth factors were transcriptionally active in the aorta of both normotensive and hypertensive rats. TGF-beta aortic mRNA levels increased up to threefold as a result of DOC/salt hypertension. In contrast, no major changes in the expression of either PDGF chain, IGF-I or II, ECGF, or bFGF were detectable. The results indicate that at least seven genes coding for growth factors that were shown previously to influence growth and function of vascular cells in vitro, are expressed in rat aorta in vivo. These findings support the hypothesis that synthesis and release of growth factors in the arterial wall are involved in autocrine and/or paracrine regulatory mechanisms. In addition, the increased expression of TGF-beta in vivo may have a role in mediating the aortic changes induced by hypertension.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Barrett T. B., Benditt E. P. sis (platelet-derived growth factor B chain) gene transcript levels are elevated in human atherosclerotic lesions compared to normal artery. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1099–1103. doi: 10.1073/pnas.84.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B., Lind P., Urdea M. S., Eddy R., Shows T. B., Philpott K., Mellor A. L. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986 Apr 24;320(6064):695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Bond J. F., Farmer S. R. Regulation of tubulin and actin mRNA production in rat brain: expression of a new beta-tubulin mRNA with development. Mol Cell Biol. 1983 Aug;3(8):1333–1342. doi: 10.1128/mcb.3.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. K., Hoshi H., McKeehan W. L. Transforming growth factor type beta specifically stimulates synthesis of proteoglycan in human adult arterial smooth muscle cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5287–5291. doi: 10.1073/pnas.84.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chobanian A. V. The influence of hypertension and other hemodynamic factors in atherogenesis. Prog Cardiovasc Dis. 1983 Nov-Dec;26(3):177–196. doi: 10.1016/0033-0620(83)90005-1. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Haaparanta T., Neufeld G. Basic fibroblast growth factor: expression in cultured bovine vascular smooth muscle cells. Eur J Cell Biol. 1988 Apr;46(1):144–151. [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Schweigerer L., Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987 May;8(2):95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- Haudenschild C. C., Prescott M. F., Chobanian A. V. Effects of hypertension and its reversal on aortic intima lesions of the rat. Hypertension. 1980 Jan-Feb;2(1):33–44. doi: 10.1161/01.hyp.2.1.33. [DOI] [PubMed] [Google Scholar]
- Herrera V. L., Chobanian A. V., Ruiz-Opazo N. Isoform-specific modulation of Na+, K+-ATPase alpha-subunit gene expression in hypertension. Science. 1988 Jul 8;241(4862):221–223. doi: 10.1126/science.2838907. [DOI] [PubMed] [Google Scholar]
- Höppener J. W., Mosselman S., Roholl P. J., Lambrechts C., Slebos R. J., de Pagter-Holthuizen P., Lips C. J., Jansz H. S., Sussenbach J. S. Expression of insulin-like growth factor-I and -II genes in human smooth muscle tumours. EMBO J. 1988 May;7(5):1379–1385. doi: 10.1002/j.1460-2075.1988.tb02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen M., van Schaik F. M., van Tol H., Van den Brande J. L., Sussenbach J. S. Nucleotide sequences of cDNAs encoding precursors of human insulin-like growth factor II (IGF-II) and an IGF-II variant. FEBS Lett. 1985 Jan 7;179(2):243–246. doi: 10.1016/0014-5793(85)80527-5. [DOI] [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Lynch S. E., Nixon J. C., Colvin R. B., Antoniades H. N. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7696–7700. doi: 10.1073/pnas.84.21.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A. Beta-type transforming growth factor specifies organizational behavior in vascular smooth muscle cell cultures. J Cell Biol. 1987 Jul;105(1):465–471. doi: 10.1083/jcb.105.1.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Benditt E. P., Schwartz S. M. Expression and developmental control of platelet-derived growth factor A-chain and B-chain/Sis genes in rat aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1524–1528. doi: 10.1073/pnas.85.5.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill H. C., Jr, Carey K. D., McMahan C. A., Marinez Y. N., Cooper T. E., Mott G. E., Schwartz C. J. Effects of two forms of hypertension on atherosclerosis in the hyperlipidemic baboon. Arteriosclerosis. 1985 Sep-Oct;5(5):481–493. doi: 10.1161/01.atv.5.5.481. [DOI] [PubMed] [Google Scholar]
- Pfeifle B., Boeder H., Ditschuneit H. Interaction of receptors for insulin-like growth factor I, platelet-derived growth factor, and fibroblast growth factor in rat aortic cells. Endocrinology. 1987 Jun;120(6):2251–2258. doi: 10.1210/endo-120-6-2251. [DOI] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Rotwein P. Two insulin-like growth factor I messenger RNAs are expressed in human liver. Proc Natl Acad Sci U S A. 1986 Jan;83(1):77–81. doi: 10.1073/pnas.83.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzani R., Claffey K. P., Chobanian A. V., Brecher P. Hypertension induces tissue-specific gene suppression of a fatty acid binding protein in rat aorta. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7777–7781. doi: 10.1073/pnas.85.20.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaras N. M., Sariban E., Pantazis P., Zetter B., Antoniades H. N. Human iliac artery endothelial cells express both genes encoding the chains of platelet-derived growth factor (PDGF) and synthesize PDGF-like mitogen. J Cell Physiol. 1987 Aug;132(2):376–380. doi: 10.1002/jcp.1041320228. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Peptide growth factors are multifunctional. Nature. 1988 Mar 17;332(6161):217–219. doi: 10.1038/332217a0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Roche N. S., Flanders K. C., Sporn M. B., Roberts A. B. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988 Jun 5;263(16):7741–7746. [PubMed] [Google Scholar]
- Winkles J. A., Friesel R., Burgess W. H., Howk R., Mehlman T., Weinstein R., Maciag T. Human vascular smooth muscle cells both express and respond to heparin-binding growth factor I (endothelial cell growth factor). Proc Natl Acad Sci U S A. 1987 Oct;84(20):7124–7128. doi: 10.1073/pnas.84.20.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]