Abstract
Background
Pancreatic adenocarcinoma (PA) is largely incurable, although recent progress has been made in the safety of surgery for PA and in adjuvant and palliative chemotherapy. The purpose of this study was to describe the management of PA in Ontario, Canada.
Methods
The Pathology Information Management System (PIMS), which uses electronic pathology reporting (E-path), was used to rapidly identify and recruit patients based on a pathologic diagnosis of PA between 2003 and 2006. Patients were mailed questionnaires for additional data.
Results
The patient participation rate was 26% (351 of 1325). Nonresponders were more likely to be older than 70 years (43% v. 28%, p < 0.001) and to have received treatment in nonacademic centres (53% v. 34%, p < 0.001). Fifty-four percent of responders underwent a potentially curative operation, and most (77%) were 70 years or younger (p = 0.03). Completed resections were documented in 83% of patients who underwent exploratory surgery with curative intent; 17% of patients had unresectable and/or metastatic disease at laparotomy. Of the completed resections, 24% were performed in nonacademic centres with a 32% positive margin rate; 76% were performed in academic centres with a 29% positive margin rate (p = 0.84). Resections with curative intent were less frequently aborted in academic centres (10% v. 33%, p < 0.001). Of the patients who responded to our questionnaire, 43% received chemotherapy and 7% participated in clinical trials.
Conclusion
Despite using PIMS and E-path, the response rate for this study was low (< 30%). Nonresponders were older and more commonly treated in nonacademic centres. Patients undergoing surgery in academic centres had higher resection rates. The rate of adjuvant and palliative chemotherapy was stage-dependent and low.
Abstract
Contexte
L’adénocarcinome du pancréas (AP) est en grande partie incurable, même si on a réalisé récemment des progrès au niveau de la sécurité de l’intervention chirurgicale contre l’AP et au niveau de la chimiothérapie d’appoint et palliative. Cette étude visait à décrire la prise en charge de l’AP en Ontario, au Canada.
Méthodes
Nous avons utilisé le Système de gestion de l’information en pathologie (SGIP), qui utilise le rapport électronique de pathologie (E-path), afin de trouver rapidement et de recruter des patients en fonction d’un diagnostic pathologique d’AP entre 2003 et 2006. Nous avons envoyé les questionnaires par la poste aux patients afin de réunir des données supplémentaires.
Résultats
Le taux de participation des patients a atteint 26 % (351 sur 1325). Les non-répondants étaient plus susceptibles d’avoir plus de 70 ans (43 % c. 28 %, p < 0,001) et d’avoir été traités dans des centres non universitaires (53 % c. 34 %, p < 0,001). Cinquante-quatre pour cent des répondants ont subi une intervention curative et la plupart (77 %) avaient 70 ans ou moins (p = 0,03). Les résections com-plétées ont été documentées chez 83 % des patients qui ont subi une intervention chirurgicale exploratoire à but curatif; 17 % des patients avaient une maladie irréséca-ble ou à métastases au moment de la laparotomie. Sur le total des résections com-plétées, 24 % ont été pratiquées dans des centres non universitaires et il y avait marge positive dans 32 % des cas, et 76 % ont été effectuées dans les centres universitaires et il y avait marge positive dans 29 % des cas (p = 0,84). Les résections à but curatif ont été interrompues moins souvent dans les centres universitaires (10 % c. 33 %, p < 0,001). Parmi les patients qui ont répondu à notre questionnaire, 43 % ont reçu de la chimiothérapie et 7 % ont participé à des essais cliniques.
Conclusion
Même si l’on a utilisé le SGIP et E-path, le taux de réponse était faible dans le cadre de cette étude (< 30 %). Les non-répondants étaient plus âgés et ont été traités plus souvent dans des centres non universitaires. Le taux de résection était plus élevé chez les patients qui ont subi leur intervention chirurgicale dans des centres uni-versitaires. Le taux de chimiothérapie d’appoint et palliative dépendait du stade de la maladie et a été faible.
Pancreatic adenocarcinoma (PA) is one of few cancers with a mortality rate approaching 100%. It is the fourth leading cause of cancer death in Ontario, which has a population of about 13 million.1 In 2009, PA was diagnosed in about 1290 people, and 1380 people died of this disease (the apparent greater number of deaths, compared with incident cases, is related to underreporting of incident cases owing to lack of pathology reports required by the Ontario Cancer Registry for registration of incident cases).1 Surgical resection is currently the only curative treatment option available, but owing to late presentation of this disease, only 15%–20% of patients are candidates for curative surgery.2,3
Most cases of PA are metastatic at presentation. Metastatic PA is a rapidly progressive and inevitably fatal disease with an average survival of less than 6 months.4 Improvements in the technical aspects of surgical management, advances in adjuvant treatment and regionalization of treatment have all played roles in improving long-term survival of patients treated with curative intent.5,6 Owing to a high locoregional recurrence rate, adjuvant chemotherapy should be considered in all patients after curative resection, regardless of margin and nodal status,7,8 and patients with incurable disease may be offered palliative chemotherapy.8
Owing to the high fatality rate, epidemiologic studies of PA are challenging and limited by difficulties contacting living patients.9 Since PA is rapidly fatal (fatality rate = 95% at 5 yr), it is of great importance to identify and contact all patients shortly after diagnosis. An innovative approach to rapidly identify patients with PA across the province of Ontario, the electronic pathology reporting system (E-path), was implemented to provide the fastest source of cancer information. The Ontario Cancer Registry (OCR) uses the Pathology Information Management System (PIMS), which relies on E-path and supports rapid computerized case ascertainment.10 The E-path system is a centralized database used to collect electronic pathology information from Ontario laboratories processing tumour specimens. E-path not only provides more timely pathology reporting compared with paper pathology reports, but also has been shown to increase the completeness of reporting. This is especially important in studies of patients with a rapidly progressive disease such as PA. In the E-path system, pathology reports arrive from each laboratory electronically and are queued in a database for on-screen review by health record technicians. This electronic transfer of reports occurs daily for most of the laboratories, or weekly for some of the smaller-volume laboratories. If the health record technicians determine that a pathology report contains reportable findings, the report is coded and consolidated with the OCR database. Reports of specific cancers, such as PA, are filtered and printed for review by study personnel.10
Little is known about the system-wide management of highly fatal cancers such as PA. Although administrative databases, such as the OCR, Canadian Institute for Health Information (CIHI) Discharge Abstract Database and others, are useful in providing information on patients with cancer in Ontario, more sophisticated, population-based efforts are necessary to study the epidemiology, management and outcome of PA in greater detail. The Ontario Pancreas Cancer Study (OPCS) began on Apr. 1, 2003, and is an ongoing prospective case–control study. The primary objective of the OPCS is to collect data about genetic and epidemiologic factors involved in the etiology of PA. The OPCS is also 1 of 7 data collection sites that make up a multicentre, multidisciplinary Pancreatic Cancer Genetic Epidemiology (PACGENE) Consortium based out of the Mayo Clinic in Rochester, Minnesota, that aims to identify susceptibility genes in high-risk familial pancreatic cancer (FPC) kindreds.11
The goals of the present study were to use data from the OPCS to characterize the management of PA in Ontario and to describe the use of a survey to supplement cancer registry data.
Methods
Eligible patients included men and women of any age with a first primary, pathologically confirmed PA diagnosed in the province of Ontario, Canada, between Apr. 1, 2003, and Dec. 31, 2006. We identified patients using the OCR, which used the International Classification of Diseases for Oncology, third edition (ICD-O-3) to identify patients whose pathology reports met our ICD-O-3 site and histology code inclusion criteria. The Research Ethics Board of the Mount Sinai Hospital and the University Health Network approved our study protocol.
We asked the physician identified on the pathology report of each patient for confirmation of the PA diagnosis and for permission to contact the patient. Consent was obtained for each patient. Passive consent was assumed for patients with known PA if the physician had not replied after 2 weeks. In cases of ambiguous pathology reports, we did not contact the patients without first obtaining clarification of the diagnosis from the physician.
Patients were mailed a package including an introductory letter; consent forms; a family history questionnaire (FHQ) designed to obtain information about the family history of cancer, diabetes and pancreatitis; a personal history questionnaire (PHQ), including questions on environmental and epidemiologic exposures, such as medical history, medication use, dietary patterns, physical activity, reproductive history, chemical exposures and alcohol and tobacco consumption; and a clinical patient questionnaire (CPQ) that addressed treatment issues and knowledge of clinical trials in PA. A release form was included for patients to complete, allowing us to obtain medical records describing their surgical, adjuvant and palliative treatments. Within 2 weeks of the questionnaire mailing, we sent a follow-up postcard to patients who had not returned the package. A telephone call was made 2 weeks later to individuals who had not responded, and a second questionnaire package was mailed on request.
On receipt of the medical records, we reviewed the notes to extract data on specimen pathology (e.g., specimen type, histology, differentiation, regional lymph nodes, number of nodes removed, number of positive nodes, tumour–node–metastasis [TNM] staging, size of the tumour, margins and angiolymphatic and perineural invasion); curative procedures (e.g., distal or total resections, Whipple, pancreatectomy, vascular resection); palliative procedures (e.g., gastrojejunostomy, choledocho/hepatico-jejunostomy); chemotherapy and radiation treatments; and recurrence of PA (e.g., site, date of recurrence, pathology obtained).
We defined surgery with curative intent as any resection intended to remove the entire tumour with pathologically negative margins; this included both completed and aborted resections. We defined aborted surgery with curative intent as an intraoperative decision that the tumour was unresectable with no attempt to remove the tumour in its entirety. Completed surgery with curative intent included all operations in which a removal of the tumour was successful; pathologically, these resections may have had either positive or negative margins.
Academic institutions included all major hospitals affiliated with the 5 general surgery residency programs in the province (e.g., Hamilton Health Sciences Centre, Sunny-brook Health Sciences Centre, University Health Network, Mount Sinai Hospital, The Ottawa Hospital, London Health Sciences Centre and Kingston General Hospital), whereas nonacademic centres were not formally affiliated with any university or residency program.
Staging of PA was conducted using the TNM classification of the Union for International Cancer Control (UICC; 6th edition, 2002).12 The staging classification incorporated all medical reports generated within 3 months before and after the earliest pathologic diagnosis. Specific rules for consistency of staging classification were followed and are outlined in Appendix 1, available at www.cma.ca/cjs.
Statistical analysis
We used the χ2 test to compare the distribution of selected categorical variables between responders and nonresponders. The following variables were not available for nonresponders and hence were not compared: detailed specimen pathology; surgical treatment, including palliative procedures; stage of PA; chemotherapy and radiation use; and clinical trial enrolment. This analysis was also used to compare the distribution of surgical, chemotherapeutic and clinical trial variables between responders aged 70 years or younger and those older than 70 years, and between academic and nonacademic institutions. We performed a Fisher exact test in the case of a small sample size. Reported p values are 2-sided, and p < 0.05 indicates statistical significance. All analyses were conducted using SAS, version 9.1.
Results
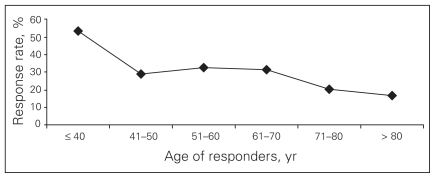
From Apr. 1, 2003, to Dec. 31, 2006, we identified 1325 eligible patients based on a pathologic diagnosis of PA (94% had E-path reports). Of these, 351 (26%) patients enrolled in the study. The remaining 974 (74%) patients were classified as nonresponders. Of these 974 patients, 375 (39%) refused participation because they were too ill, and 428 (44%) died before enrolment. Table 1 shows selected characteristics of responders and nonresponders. A greater percentage of nonresponders were older than 70 years of age (43% v. 28%, p < 0.001). Specifically, responders were significantly more likely to be 70 years of age or younger compared with nonresponders (odds ratio [OR] 1.82, 95% confidence interval [CI] 1.39–2.39, data not shown). A graphic depiction of the inverse relation between age and response rate is provided in Figure 1: 54% of patients aged 40 years or younger responded compared with 18% of patients over age 80 years. Responders were also significantly more likely to be treated at an academic hospital than nonresponders (66% v. 47%, p < 0.001).
Table 1.
Characteristics of patients with pancreatic adenocarcinoma, by responders and nonresponders, in Ontario, Canada, in 2003–2006
| Characteristic | Group; no. (%)* | p value | |
|---|---|---|---|
| Responders, n = 351 | Nonresponders, n = 974 | ||
| Age, yr | |||
| ≤ 70 | 252 (72) | 554 (57) | < 0.001 |
| > 70 | 99 (28) | 420 (43) | |
| Sex | |||
| Male | 191 (54) | 525 (54) | 0.86 |
| Female | 160 (46) | 449 (46) | |
| Treatment hospital | |||
| Academic† | 231 (66) | 459 (47) | < 0.001 |
| Nonacademic | 120 (34) | 515 (53) | |
Numbers may not add to total owing to missing values.
Hamilton Health Sciences Centre, Sunnybrook Health Sciences Centre, University Health Network, Mount Sinai Hospital, The Ottawa Hospital, London Health Sciences Centre and Kingston General Hospital.
Fig. 1.
Relation between age of responders (years) and response rate (%).
Table 2 shows differences in treatment among the responders (n = 351). Of the 189 (54%) responders who underwent surgery with curative intent, 157 (83%) surgeries were completed, whereas 32 (17%) were aborted and a palliative operation performed. Adjuvant or palliative chemotherapy was administered to less than half of the responding patients. Most respondents were not enrolled in any clinical trials.
Table 2.
Management of the 351 responders with pancreatic adenocarcinoma in Ontario, Canada, in 2003–2006
| Management | No. (%)* |
|---|---|
| Surgery with curative intent† | 189 (54) |
| Completed | 157 (83) |
| Aborted | 32 (17) |
| No surgery with curative intent | 162 (46) |
| Chemotherapy‡ | 152 (43) |
| No chemotherapy | 199 (57) |
| Clinical trials | 26 (7) |
| No clinical trials | 325 (93) |
Numbers may not add to total owing to missing values.
Resections and aborted Whipple procedures.
Includes adjuvant and palliative chemotherapy.
A significantly greater proportion of patients aged 70 years or younger underwent surgery with curative intent than patients older than 70 years (58% v. 45%, p = 0.03; Table 3). There was no correlation between age and completion of surgery with curative intent, margin status, administration of chemotherapy or enrolment in clinical trials.
Table 3.
Management of the 351 responders with pancreatic adenocarcinoma dichotomized by age in Ontario, Canada, in 2003–2006
| Management | Age, yr; no. (%)* | p value | |
|---|---|---|---|
| ≤ 70 yr, n = 252 | > 70 yr, n = 99 | ||
| Surgery with curative intent† | 145 (58) | 44 (45) | 0.03 |
| No surgery with curative intent | 107 (42) | 54 (55) | |
| Curative surgery completed | 123 (85) | 34 1 (77) | 0.24 |
| Positive margins | 35 (29) | 10 (30) | 0.86 |
| Negative margins | 87 (71) | 23 (70) | |
| Curative surgery aborted | 22 (15) | 0 (23) | |
| Chemotherapy‡ | 117 (46) | 35 (35) | 0.07 |
| No chemotherapy | 135 (54) | 64 (65) | |
| Clinical trials | 21 (8) | 5 (5) | 0.30 |
| No clinical trials | 231 (92) | 93 (95) | |
Numbers may not add to total owing to missing values.
Resections and aborted Whipple procedures.
Includes adjuvant and palliative chemotherapy.
Table 4 shows the correlation of treatment site with management of PA responders. Surgeries with curative intent performed in academic centres were statistically more likely to be completed than those performed in nonacademic centres (90% v. 67%, p < 0.001). There was no statistical difference between treatment in academic or nonacademic centres with respect to margin status, enrolment in clinical trials or use of any form of chemotherapy.
Table 4.
Management of the 351 responders with pancreatic adenocarcinoma dichotomized by treatment centre in Ontario, Canada, in 2003–2006
| Management | Centre; no. (%)* | p value | |
|---|---|---|---|
| Academic, n = 231 | Nonacademic, n = 120 | ||
| Surgery with curative intent† | 132 (57) | 57 (48) | 0.09 |
| No surgery with curative intent | 99 (43) | 63 (52) | |
| Curative surgery completed | 119 (90) | 38 (67) | < 0.001 |
| Positive margins | 35 (29) | 12 (32) | 0.84 |
| Negative margins | 84 (71) | 26 (68) | |
| Curative surgery aborted | 13 (10) | 19 (33) | |
| Chemotherapy‡ | 101 (44) | 51 (43) | 0.83 |
| No chemotherapy | 130 (56) | 69 (57) | |
| Clinical trials | 21 (9) | 5 (4) | 0.09 |
| No clinical trials | 210 (91) | 115 (96) | |
Numbers may not add to total owing to missing values.
Resections and aborted Whipple procedures.
Includes adjuvant and palliative chemotherapy.
At the time of diagnosis, TNM classification was used to stage all patients (Appendix 1). Treatment plans were shown to be different based on stage of disease (Table 5). All of the patients with stage-I and -II tumours underwent surgery with curative intent; these surgeries were completed in all but 1 patient with stage-II tumours. Stage-III and -IV cancers were identified based on postoperative investigations or intraoperatively; thus, 17 of 60 (28%) patients with stage-III tumours and 19 of 78 (24%) patients with stage-IV tumours underwent an attempted surgery with curative intent because metastatic disease was not evident preoperatively. None of the patients with stage-III tumours had completed curative resections; whereas 9 of 19 (47%) patients with stage-IV tumours had completed curative resections as metastases became evident within 3 months postoperatively. Of note, completion of surgery with curative intent only applies to stage-IV tumours that were diagnosed postoperatively as, by definition, stage-III tumours were unresectable owing to invasion into adjacent structures. Patients with stage-I tumours received chemotherapy less often than patients with stage-II, -III or -IV tumours (p < 0.001). When stratified by both treatment site and stage, chemotherapy use was significantly different between academic (45%) and nonacademic centres (22%) for patients with stage-II PA (p = 0.05).
Table 5.
Management of the 351 responders with pancreatic adenocarcinoma stratified by stage and treatment centre in Ontario, Canada, in 2003–2006
| Management | Stage; treatment hospital; no. (%)* |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage I |
Stage II |
Stage III |
Stage IV |
|||||||||
| Academic | Nonacademic | p value | Academic | Nonacademic | p value | Academic | Nonacademic | p value | Academic | Nonacademic | p value | |
| Curative surgery† | 15 (100) | 9 (100) | NA | 83 (100) | 23 (100) | NA | 5 (14) | 12 (52) | 0.001 | 12 (26) | 7 (23) | 0.77 |
| No curative surgery | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA | 32 (86) | 11 (48) | NA | 35 (74) | 24 (77) | NA |
| Curative surgery completed | 15 (100) | 9 (100) | NA | 83 (100) | 22 (96) | 0.22 | 0 (0) | 0 (0) | NA | 7 (58) | 2 (29) | 0.35 |
| Curative surgery aborted | 0 (0) | 0 (0) | NA | 0 (0) | 1 (4) | NA | 5 (100) | 12 (100) | NA | 5 (42) | 5 (71) | NA |
| Chemotherapy‡ | 3 (20) | 0 (0) | 0.27 | 37 (45) | 5 (22) | 0.05 | 22 (59) | 13 (57) | 0.82 | 24 (51) | 20 (65) | 0.24 |
| No chemotherapy | 12 (80) | 9 (100) | NA | 46 (55) | 18 (78) | NA | 15 (41) | 10 (43) | NA | 23 (49) | 11 (35) | NA |
| Clinical trials | 1 (9) | 0 (0) | 1.0 | 10 (19) | 1 (9) | 0.67 | 2 (8) | 2 (12) | 1.0 | 4 (12) | 2 (9) | 1.0 |
| No clinical trials | 10 (91) | 4 (100) | NA | 43 (81) | 10 (91) | NA | 23 (92) | 15 (88) | NA | 29 (88) | 20 (91) | NA |
NA = not applicable.
Numbers may not add to total owing to missing values.
Resections and aborted Whipple procedures.
Includes adjuvant and palliative chemotherapy.
Table 6 shows the management of unresectable PA (stage-III and -IV tumours) and demonstrates a statistically significant correlation between the incidence of attempted surgery with curative intent and academic institution for these patients. The significance is a result of the differences among patients with stage-III tumours between academic and nonacademic centres (Table 5). The correlation between treatment centre and surgery completion almost approached significance with a success rate of 41% in academic centres compared with 11% in nonacademic centres (p = 0.06). There was no difference in the use of chemotherapy or enrolment in clinical trials between academic and nonacademic centres.
Table 6.
Management of 138 patients with unresectable pancreatic cancer (stage III and IV) in academic versus nonacademic centres in Ontario, Canada, in 2003–2006
| Management | Centre; no. (%)* | p value | |
|---|---|---|---|
| Academic, n = 84 | Nonacademic, n = 54 | ||
| Surgery with curative intent† | 17 (20) | 19 (35) | 0.05 |
| No surgery with curative intent | 67 (80) | 35 (65) | NA |
| Curative surgery completed | 7 (41) | 2 (11) | 0.06 |
| Curative surgery aborted | 10 (59) | 17 (89) | NA |
| Chemotherapy‡ | 46 (55) | 33 (61) | 0.46 |
| No chemotherapy | 38 (45) | 21 (39) | NA |
| Clinical trials | 6 (10) | 4 (10) | 1.0 |
| No clinical trials | 52 (90) | 35 (90) | NA |
NA = not applicable.
Numbers may not add to total owing to missing values.
Resections and aborted Whipple procedures.
Includes adjuvant and palliative chemotherapy.
Discussion
Surgical resection continues to be the only potentially curative treatment for PA. Recent evidence also demonstrates a survival advantage for adjuvant therapy after resection.7,8 Palliative chemotherapy and bypass surgery have been shown to be beneficial in improving the quality of life of selected patients with incurable disease.13 The present study uses a prospective population-based registry to capture patients at the time of diagnosis and consequently follow the management of their PA.
The OPCS was designed as a population-based registry of incident cases of PA in Ontario for epidemiologic, genetic and health services and outcomes research. Even with the use of E-path reporting and rapid case ascertainment, the overall response rate in the OPCS has never exceeded 35%, which is consistent with other attempts to obtain data through population-based registries of PA with the use of questionnaires.14 Despite the low response rate, we sought to characterize responders and nonresponders and use the OPCS to describe the management of PA in our registry of patients in Ontario. Our experience and observations underscore the need for further prospective, population-based studies of PA with emphasis on data collection from patients, physicians and existing administrative databases in the province. Our study simultaneously highlights the practical obstacles to progress, such as the rapid identification of patients with newly diagnosed PA.
We found that nonresponders were significantly older and more likely to have received treatment in a nonacademic centre than responders. This inverse relation between age and enrolment in prospective studies has been previously shown in cancer trials.15 Increasing age has been correlated with less aggressive management, which has been shown to be a barrier to participation in these studies owing to patient apathy and a sense of helplessness.16 Patients treated in nonacademic centres were less likely to respond to our recruitment strategy, possibly demonstrating the potential influence of an academic environment on the attitudes of these patients. In the province of Ontario, academic centres are geographically situated in the larger urban centres, and it has been shown that rural patients are less likely to participate in cancer research.16 However, it is also plausible that patients who are more likely to participate in research studies may be more likely to initially seek medical attention at an academic centre.
In our registry, patients aged 70 years and younger were more likely to undergo surgery with curative intent than those older than 70 years. Older patients are more likely to have medical comorbidities that would preclude surgery, and this was not accounted for in this study. However, the small number of patients unfit for the anesthetic or operative risk would likely not account for the statistical significance between these groups. Such a difference may be owing to physician education and attitudes toward aggressive surgical treatment in elderly patients and a tendency for physicians to be more aggressive in the treatment of younger patients.17 Other studies have also reported that older patients refuse pancreatic surgery more often than younger patients;18 however, this may be owing in part to the reluctance of surgeons to operate on older patients.
There is an ongoing trend toward centralization of management of PA in high-volume institutions owing to lower perioperative surgical morbidity and mortality.6,19,20 Our study examined the differences in the management of PA between academic and nonacademic centres and revealed that, within our registry, the number of aborted surgeries with curative intent was significantly higher in nonacademic centres than academic centres. However, when surgery with curative intent was completed, there was no significant difference in the percentage of positive margins between these 2 groups. This suggests that, although the decision to abort versus complete curative resection may have varied between academic and nonacademic centres, once the decision was made to complete a curative resection, surgical technique to acquire negative margins did not vary. This may indicate that a more aggressive approach to completion of surgery with curative intent is taken in academic centres with a higher threshold for aborting an operation with curative intent. However, it is also possible that the quality of preoperative staging evaluation and radiographic studies (e.g., pancreas protocol computed tomography, endoscopic ultrasonography) may be higher at academic centres; as a result, patients with technically unresectable disease may be identified preoperatively more often at academic centres, lowering the likelihood that an operative procedure would need to be aborted based on unexpected intraoperative findings.
All patients with stage-I or -II tumours for whom data was available underwent operations with curative intent, and all but 1 operation was completed. Bilimoria and colleagues18 found that 38.2% of their patients with stage-I or -II PA with no identifiable contraindication to surgery did not undergo surgical management. The likely difference in our database, in which all responders with stage-I or -II disease underwent surgical resection, is that our case ascertainment was limited to pathologic diagnosis and to questionnaire responders. As the usual management of clinically diagnosed early-stage PA is resection with no preoperative biopsy, the only pathology for these patients, which was our means of case ascertainment, was their surgical resection specimen. Owing to incomplete diagnosis of stage preoperatively, 36 (26%) patients with stage-III or -IV tumours had surgery with curative intent. There was no significant difference between the ability to diagnose unresectable tumours in academic compared with nonacademic centres. All surgeries with curative intent in patients with stage-III tumours were aborted in both academic and nonacademic centres, whereas the intraoperative decision to abort these surgeries in patients with stage-IV tumours was made more frequently in nonacademic centres than academic centres.
It has recently been shown that adjuvant chemotherapy should be offered to all patients with a diagnosis of PA.8 Although it is difficult to study the proportion of patients being offered chemotherapy, our analysis reveals that less than half of our patients received any adjuvant or palliative treatment, with no statistical differences demonstrated by either age or treatment site. This may be owing to our data collection having begun in 2003 before these guidelines were established. As stated, patient choice may also play a role; however, it is difficult to assess the proportion of patients being offered treatment and declining it. There was a significant difference in the use of chemotherapy based on stage of disease; patients undergoing curative surgical treatment for stage-I or -II tumours were less likely to receive chemotherapy as an adjunct to their treatment (13% and 40%, respectively) than those with stage-III or -IV tumours. Once again, this may be owing to both physician and patient knowledge and attitudes toward the role of chemotherapy.
Another finding in our study was the low enrolment in clinical trials across all patients, with no correlation between age or stage of disease and likelihood of enrolment. This may not be surprising, considering that most patients with cancer do not participate in clinical trials, and many patients with PA have poor performance status, limiting their eligibility for trials.
There are several limitations to our study. As PA is rapidly fatal, survival bias almost certainly affected our results. Although we used rapid case ascertainment to recruit patients, 44% of the patients died before enrolment. In addition, our response rate never exceeded 35%. Nonresponders may have had more aggressive disease and may have been less responsive to treatment. As a result, patients who underwent surgery may have been more eager to participate than patients with inoperable tumours. In fact, over 40% of our patients underwent surgery, which is double the number seen in the general PA population.2,3,11 Selection bias is also possible because we only recruited individuals with a pathologically confirmed diagnosis of PA. Over 50% (59% in 2002, Cancer Care Ontario: personal communication, 2006) of patients with PA in Ontario never have tissue confirmation while they are alive, and are only reported to the OCR from hospital records or death certificates.
Some patients may have been treated at more than 1 hospital and, although we used pathology reports and operative notes to classify the treatment centres, other decisions and treatment plans may have occurred elsewhere and were not accounted for in the analyses. Hospital records on chemotherapy were not always complete, although patient-acquired history was also used to determine use of chemotherapy. Of the 351 responders, only 269 had complete data from which to determine the stage of disease (i.e., in some patients a full work-up was not complete after diagnosis). Another limitation was the variation of synoptic pathology reporting, with different laboratories having varying thresholds for describing positive and negative margins.
Conclusions derived from the current data set must be interpreted in light of these limitations and may not be reflective of the entire population of patients with PA in Ontario. However, despite these limitations, to our knowledge this study provides the first overview of the management of PA in the province and the variables that may play a significant role in treatment strategies. Although the literature on current guidelines for the management of PA is quite thorough, very few studies examine the actual management strategies being used at a population level. We have demonstrated that even with rapid case ascertainment, response rates were low, and response rate was correlated with both age and treatment sites. We have also shown that age played a significant role in surgical management strategies and that treatment at an academic centre, although not significantly affecting margin status, correlated with rates of completed curative resections. The use of chemotherapy, either adjuvant or palliative, was low and varied depending on the stage of disease. This study has also demonstrated the need for further investigations into the epidemiologic limitations of collecting data in cancer populations with poor prognoses and the need to identify better methods to recruit patients shortly after diagnosis.
Acknowledgments
This study was supported by grants from the National Institutes of Health (R01 CA97075, as part of the PACGENE consortium), the Lustgarten Foundation for Pancreatic Cancer Research and the Ontario Cancer Research Network. We acknowledge the Sam Minuk Cancer Genetics lab at Mount Sinai Hospital and the Pancreatic Cancer Canada foundation (www.pancreaticcancercanada.ca) for their continued support of research into the early detection of pancreatic cancer.
Footnotes
Competing interests: None declared.
Contributors: Ms. Borgida and Drs. Urbach, Moore, Cotterchio and Gallinger designed the study. Ms. Borgida and Ms. Rothemund and Drs. Ashamalla, Al-Sukhni, Cotterchio and Gallinger acquired the data, which Ms. Borgida and Drs. Cotterchio and Gallinger analyzed. Ms. Borgida and Drs. Ashamalla, Al-Sukhni, Cotterchio and Gallinger wrote the article, which Ms. Borgida, Ms. Rothenmund and Drs. Urbach, Moore and Gallinger critically reviewed. All authors approved publication of the article.
References
- 1.Canadian Cancer Society’s Steering Committee. Canadian Cancer Statistics 2009. Toronto (ON): Canadian Cancer Society; 2009. [Google Scholar]
- 2.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100313 patients diagnosed from 1985–1995 using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 3.Warshaw AL, Fernández-del Castillo. Pancreatic carcinoma. N Engl J Med. 1992;326:455–65. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 4.Nitecki SS, Sarr MG, Colby TV, et al. Long term survival after resection of ductal adenocarcinoma of the pancreas: Is it really improving. Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexakis N, Halloran C, Raraty M, et al. Current standards of surgery for pancreatic cancer. Br J Surg. 2004;91:1410–27. doi: 10.1002/bjs.4794. [DOI] [PubMed] [Google Scholar]
- 6.Simunovic M, To T, Theriault M, et al. Relation between hospital surgical volume and outcome for pancreatic resection for neoplasm in a publicly funded health care system. CMAJ. 1999;160:643–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Newman EA, Simeone DM, Mulholland M. Adjuvant treatment strategies for pancreatic cancer. J Gastrointest Surg. 2006;10:916–26. doi: 10.1016/j.gassur.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 9.Pawlik TM, Abdalla EK, Barnett CC, et al. Feasibility of a randomized trial for extended lymphadenectomy for pancreatic cancer. Arch Surg. 2005;140:584–9. doi: 10.1001/archsurg.140.6.584. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan T. Cancer registry project passes midterm exam. CAP TODAY. 2005 Dec; [Google Scholar]
- 11.Petersen GM, de Andrade M, Goggins M, et al. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2006;15:704–10. doi: 10.1158/1055-9965.EPI-05-0734. [DOI] [PubMed] [Google Scholar]
- 12.Sobin LH, Wittekind C, editors. UICC TNM classification of malignant tumours. 6th ed. New York (NY): Wiley-Liss; 2002. [Google Scholar]
- 13.Nuzzo G, Clemente G, Greco F, et al. Is the chronologic age a contra-indication for surgical palliation of unresectable periampullary neoplasms. J Surg Oncol. 2004;88:206–9. doi: 10.1002/jso.20147. [DOI] [PubMed] [Google Scholar]
- 14.Nkondjock A, Krewski D, Johnson KC, et al. Canadian cancer registries epidemiology research group. Specific fatty acid intake and the risk of pancreatic cancer in Canada. Br J Cancer. 2005;92:971–7. doi: 10.1038/sj.bjc.6602380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trial. JAMA. 2004;291:2720–6. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 16.Lai GY, Gary TL, Tilburt J, et al. Effectiveness of strategies to recruit underrepresented populations into cancer clinical trials. Clin Trials. 2006;3:133–41. doi: 10.1191/1740774506cn143oa. [DOI] [PubMed] [Google Scholar]
- 17.Townsley C, Pond GR, Peloza B, et al. Analysis for treatment practices for elderly cancer patients in Ontario, Canada. J Clin Oncol. 2005;23:3802–10. doi: 10.1200/JCO.2005.06.742. [DOI] [PubMed] [Google Scholar]
- 18.Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–80. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 20.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]