Abstract
Laparoscopic adjustable gastric banding (LAGB) is considered to be a safe and effective method of weight loss and reduction of comorbidities associated with obesity. Despite its improved early safety profile compared with Roux-en-Y gastric bypass, patients with LAGB can manifest unique complications that must be recognized and managed appropriately to achieve good outcomes. This review will prepare the general surgeon to identify, diagnose and manage the common complications encountered in patients presenting following LAGB.
Abstract
On considère que le cerclage gastrique laparoscopique par anneau modulable constitue une méthode sûre et efficace pour la perte de poids et la réduction des comorbidités associées à l’obésité. Malgré son profil d’innocuité précoce amélioré comparativement à la dérivation gastrique de Roux-en-Y, les patients soumis à un cerclage gastrique peuvent présenter des complications particulières qu’il faut savoir reconnaître et corriger de manière appropriée pour obtenir de bons résultats. La présente synthèse préparera le chirurgien général à reconnaître, diagnostiquer et traiter les complications courantes chez les patients qui consultent après un cerclage gastrique laparoscopique.
It has recently been estimated that 1 in 10 premature deaths among Canadian adults aged 20–64 years is directly attributable to obesity.1 In addition to affecting personal health, the increased health risks associated with morbid obesity translate into an increased burden on the health care system. The cost of obesity in Canada has been conservatively estimated to be $2 billion a year or 2.4% of total health care expenditures in 1997.2
Nonsurgical management of obesity includes dietary changes and exercise, which can facilitate a 5%–10% weight loss.3–5 Unfortunately, once patients stop a weight loss program, weight gain typically occurs.2 Pharmacologic management of obesity is not much better than diet and exercise. The limited long-term success of behavioural and drug therapies in patients with severe obesity has led to a renewed interest in bariatric (obesity) surgery in Canada.6
Options for surgical management of morbid obesity include restrictive (adjustable gastric banding, vertical band gastroplasty), restrictive/resective (sleeve gastrectomy), restrictive/malabsorptive (Roux-en-Y gastric bypass, biliopancreatic diversion with duodenal switch) and purely malabsorptive (duodenal switch) options. Of the various available options, restrictive surgical techniques have been a mainstay of treatment for morbid obesity for several decades. In this light, adjustable gastric banding represents one of the more frequently performed bariatric operations in morbidly obese patients.7 Laparoscopic adjustable gastric banding (LAGB) is considered to be a safe and effective method of weight loss and reduction of comorbidities associated with obesity.8 Despite its improved early safety profile compared with Roux-en-Y gastric bypass, patients with LAGB can manifest unique complications that are distinctive to the LAGB and require a specific process for assessment and management.
Pouch enlargement, band slip, band erosion, port-site infections and port breakage represent the complications most commonly associated with LAGB. This manuscript will review these complications and the most appropriate method of assessment and management.
Discussion
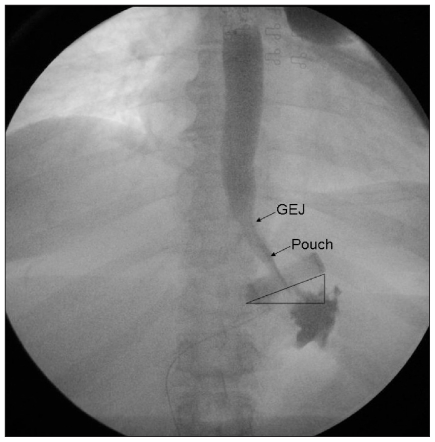
A normal image of the upper abdomen after LAGB placement is demonstrated in Figure 1. The band is placed just below the gastresophageal junction. The pouch is appropriately sized to 50–80 mL. The most appropriate placement of the band is at an approximately 45° angle toward the left shoulder with the medial aspect of the band juxta-posed to the left pedicle of the vertebra.
Fig. 1.
Radiograph showing a normal image of the upper abdomen after LAGB placement. GEJ = gastroesophageal junction.
Pouch enlargement
Pouch enlargement (type-III prolapse) is diagnosed when dilation of the proximal gastric pouch is present with or without change in the angle of the band and in the absence of signs of obstruction.9 The lower esophagus may or may not be dilated. Pouch enlargement is a pressure-related phenomenon that may be surgically induced by band overinflation or overeating with resulting high pressure in the pouch.9,10
Symptoms of pouch enlargement include lack of satiety, heartburn, regurgitation and occasional chest pain. The diagnosis is made with an upper gastrointestinal series (Fig. 2).
Fig. 2.
Radiograph of pouch enlargement.
Nonoperative treatment includes complete band deflation, low-calorie diet, re-enforcement of portion size and follow-up contrast study in 4–6 weeks. If the band position and the pouch size return to normal, then the band can be incrementally reinflated. A study by Moser and colleagues9 demonstrated that this conservative approach to pouch enlargement was successful in up to 77% of patients. Conservative treatment is considered unsuccessful when the pouch fails to recover its original size after 8–10 weeks. In this circumstance, surgical treatment with either band removal or replacement is indicated.
Band slip
Band slip (Table 1) may be defined as cephalad prolapse of the body of the stomach or caudal movement of the band. O’Brien and Dixon11 reported a band slip rate of less than 5%. Interestingly, they reported 125 episodes of band slip (25%) in their first 500 patients using the perigastric approach (accessing the right crus perigastrically) and only 28 episodes (4.8%) in the last 600 patients after adoption of the pars flaccida technique (accessing the right crus through the pars flaccida). Other published literature report an incidence of slip of 1%–22%.10–15
Table 1.
Band slip classification
| Type | Definition | Mechanism | Etiology | Management |
|---|---|---|---|---|
| I | Anterior slip | Downward migration of band | Insufficient anterior fixation | Surgical |
| II | Posterior slip | Posterior stomach wall herniates through band | Perigastric approach | Surgical |
| III | Pouch enlargement | Pouch dilation | Tight band or overeating | Band deflation, re-education |
| IV | Immediate postoperative prolapse | Band placed too low on the stomach | Inappropriate low band placement | Surgical |
| V | Type I or II with gastric necrosis | Band slip with pouch ischemia | Acute pouch dilation | Surgical |
Since the cross-sectional area of the stomach is larger at the body than at the level of the angle of His (normal band position), complete obstruction of the stomach can occur when the band slips. Band slip can be posterior or anterior, depending on whether the anterior or posterior region of the stomach herniates through the band.
Anterior slip (type-I prolapse)
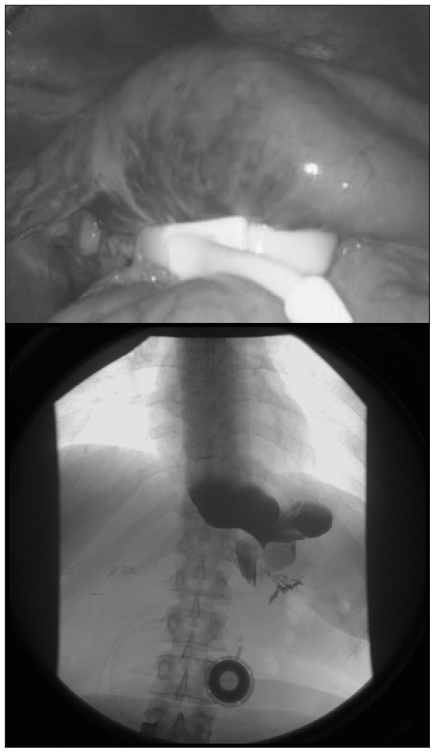
Anterior slip results from upward migration of the anterior wall of the stomach through the band. This can be due to insufficient anterior fixation and disruption of the fixation sutures. The second cause may be related to increased pressure in the pouch due to early solid food, vomiting, overeating or early (< 4 wk) band fill (Fig. 3).
Fig. 3.
Anterior slip as seen (top) intraoperatively and (bottom) on radiography.
Posterior slip (type-II prolapse)
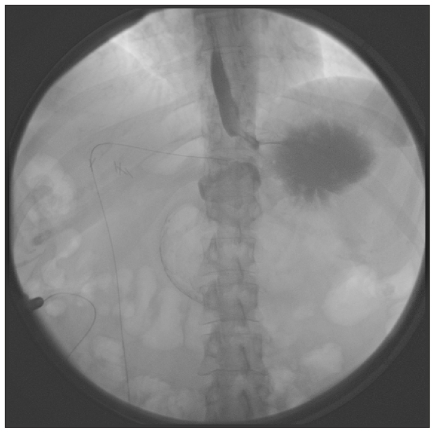
Posterior slip is defined as a herniation of the posterior wall of the stomach through the band. This is usually related to the surgical technique but is less frequent now with adoption of the pars flaccida approach instead of the perigastric approach (Fig. 4).
Fig. 4.
Radiograph showing posterior slip.
In both types of slip, the patient usually presents with dysphagia, vomiting, regurgitation and food intolerance. The diagnosis is made by upper gastrointestinal series. Complications related to band slip include gastric perforation, necrosis of the slipped stomach (type-V prolapse), upper gastrointestinal bleeding and aspiration pneumonia.
Type-IV prolapse
A type-IV prolapse is defined as an immediate postoperative prolapse and is usually due to placing the band too low on the stomach.
Band slip types (I, II, IV and V) are acute and always require surgical intervention. Laparoscopic removal or repositioning of the band is the preferred method of treatment. Pouch enlargement is a chronic complication that should be managed nonoperatively in the first instance, and surgical readjustment is reserved only for those patients in whom conservative treatment fails.
Operative technique
A video of the operative technique is available for review at www.capitalhealth.ca/CAMIS. Incisions from the previous surgery are used to access the abdomen. A 5-mm/10-mm visually guided trochar or an open technique is used to gain access to the abdominal cavity. Once the abdominal cavity is entered, the tubing leading from the subcutaneous port to the band can be easily identified and followed toward the band. The band buckle can be identified by placing traction on the tubing, and the fibrous capsule surrounding the buckle is incised. Dissection is then initiated on the anterior aspect of the band over the fibrous capsule, avoiding injury to the stomach wall. Careful dissection of the wrap is achieved using endoshears and hook cautery. Adhesions between the pouch and the surrounding tissues are taken down. Previous stitches may then be divided with the aim of releasing the wrap. Once the band is released, the pouch is gently pulled down through the band using an atraumatic grasper, and the band is repositioned around the stomach. Two or 3 stitches of 2/0 nonabsorbable suture are used to maintain the band in position by creating a new anterior wrap over the band. If the band is damaged during this procedure, replacement is necessary, and the preformed posterior tunnel can be used to reinsert the new band. Once the band is repositioned, the integrity of the stomach wall is tested aggressively with air insufflation using a gastroscope. The stomach is air-leak tested under lavage fluid and with methylene blue injection.
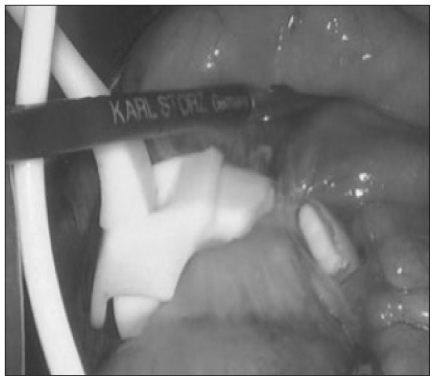
In situations of substantial prolapse where reduction is not possible or when evidence of intrabdominal infection is present, the most prudent management is removal of the gastric band. This can be accomplished by cutting the band to the anatomic left of the buckle and then pulling the band from the anatomic right of the retrogastric tunnel (Fig. 5). This direction is preferred to prevent dragging a larger edge of the band in the retrogastric space. After removal, the surgeon must ensure that the band has been completely removed to prevent a residual foreign-body reaction. A drain is left near the site of the band.
Fig. 5.
Intraoperative image showing the operative management of band slip, dividing the band to the anatomic left of the buckle.
A gastrografin study is performed on the first postoperative day to assess the position of the band and to rule out any leaks. A fluid diet is introduced on the same day, and patients may be discharged when tolerating fluids appropriately.
Band erosion
Band erosion is an uncommon complication of LAGB. In this scenario, the band gradually erodes through the stomach wall and into the gastric lumen. The incidence is less than 1%,11,16,17 with a reported prevalence varying from 0% to 11%.19 The etiology of band erosion may be the result of gastric-wall injury during band placement or tight anterior fixation, especially around the band buckle.19
A high index of suspicion is required for diagnosis of band erosion as most patients are asymptomatic. When symptomatic, complaints related to erosion include loss of restriction, nonspecific epigastric pain, gastrointestinal bleeding, intra-abdominal abscesses or port-site infection. The diagnosis is often made at the time of gastroscopy (Fig. 6).
Fig. 6.
Eroded band tubing. (Left) Note the eroded buckle at the 3 o’clock position and the change in the band colour owing to acid exposure. (Right) Note also the point of erosion at the 10 o’clock position.
The recommended treatment is complete removal of the eroded gastric band laparoscopically or via laparotomy. Removing a band that has eroded into the stomach can be fraught with difficulty owing to the extensive inflammatory response around the proximal stomach and left lobe of the liver. In addition, one must deal with the closure of a gastrotomy that results from opening the capsule around the eroded band. To circumvent these challenges, transgastric techniques have been proposed to facilitate band removal.20 Using distal transgastric ports, the band can be removed with a combined laparoscopic/endoscopic approach. It is surgically less arduous to operate and close a gastrotomy in normal gastric tissue than near an eroded band. In the case of acute gastric perforation, laparotomy with wide drainage is necessary.
Intra-abdominal infection
Laparoscopic adjustable gastric band placement may predispose to an intra-abdominal infection or abscess. The LAGB as a foreign body may decrease the number of bacteria required to produce infection and form abscesses. When an abscess is present, the band should be removed, the area widely drained and the stomach tested for occult leaks.
Port-site infection
Port-site infections can be classified as early and late. Early infections will manifest with the cardinal signs of erythema, swelling and pain. These infections typically occur within the immediate postoperative period and may be reduced by the use of perioperative antibiotics. Early infection with cellulitis alone may be treated with oral antibiotics. If the response is inadequate, then intravenous antibiotic use is warranted. When the infection does not respond to intravenous antibiotics and is limited to the port, the port should be removed and the tubing knotted and left inside the abdomen. Once the local infection is resolved, a new port may be placed and tubing connected with laparoscopic guidance. Late port site infections are often caused by delayed band erosion with ascending infection. This usually manifests several months after surgery and can be associated with loss of restriction. These infections typically do not respond well to antibiotic treatment. If left undetected, band infection can evolve into potentially life-threatening intra-abdominal sepsis. Gastroscopy will confirm the diagnosis of band erosion. This complex clinical scenario is treated most expeditiously by removal of the band.
Port breakage
Breakage or damage of the port typically refers to leakage through a damaged port septum or tubing leading into the port. The use of a standard coring needle is strongly discouraged, and only Huber (noncoring) needles should be used to access the port. If port access is difficult or if the tubing connected to the port is at risk of perforation, then band adjustment under fluoroscopy is advised. Port breakage usually manifests as a slow leak with the loss of the injected fluid volume on aspiration and the absence of restriction. It can be difficult to identify the leak site but local exploration of the port site can confirm the diagnosis.
Leakage from the intra-abdominal tubing is more difficult to diagnose. Injection of dilute nonionic iodinated contrast into the port under fluoroscopy can help to identify the site of the leak. Another approach is to inject diluted methylene blue into the port under direct laparoscopic visualization of the tubing and the band. Port, tubing or band replacement is usually necessary depending on the site of the leakage and type of band used (Fig. 7).21
Fig. 7.
Tube disruption (arrow) following blunt abdominal trauma.
A summary of discussed complications is available in Table 2.
Table 2.
Band complications
| Complication | Rate | Management |
|---|---|---|
| Minor | ||
| Port leak | < 1% | Port replacement |
| Port displacement | 2.5%–6% | Fluoroscopy fill/readjustment |
| Minor port infection | < 1.8% | Antibiotics/temporary removal |
| Pouch enlargement | 12% | Deflation, re-education |
| Major | ||
| Band slip | < 5% | Surgical |
| Band erosion | < 1% | Removal |
| Late port or band infection | < 1% | Possible erosion |
| Intra-abdominal abscess | 0.1% | Possible erosion |
Conclusion
Laparoscopic adjustable gastric banding is considered to be safe with a medium-term efficacy that is comparable to a Roux-en-Y gastric bypass (excess weight loss of up to 60%).22,23 It has a lower overall and major complication rate24 than Roux-en-Y gastric bypass.
Given the accessibility of LAGB in private clinics in and outside of Canada, it is important for general surgeons to be aware of the common presentations of LAGB complications. A diagnosis may be made with a standardized approach, as presented in this manuscript. Depending on the experience of the surgeon with laparoscopic upper gastrointestinal surgery, they may be able to manage most acute and chronic LAGB-associated complications. Otherwise, laparotomy or referral to a bariatric surgeon is appropriate.
Footnotes
Competing interests: None declared for Drs. Eid, Birch, Sherman and Karmali. Dr. Sharma declares having received a speaker’s honorarium from Allergan.
Contributors: All authors designed the review, reviewed the article and approved its publication. Drs. Birch and Karmali acquired the data, which Drs. Eid, Birch, Sharma and Karmali analyzed.
References
- 1.Lau DC, Douketis JD, Morrison KM. Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. CMAJ. 2006;2007;176:S1–13. doi: 10.1503/cmaj.061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birmingham CL, Muller JL, Palepu A, et al. The cost of obesity in Canada. CMAJ. 1999;160:483–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon JB, O’Brien PE. Health outcomes of severely obese type 2 diabetic subjects 1 year after laparoscopic adjustable gastric banding. Diabetes Care. 2002;25:358–63. doi: 10.2337/diacare.25.2.358. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien PE, McPhail T, Chaston TB, et al. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16:1032–40. doi: 10.1381/096089206778026316. [DOI] [PubMed] [Google Scholar]
- 5.Fisher BL, Schauer P. Medical and surgical options in the treatment of severe obesity. Am J Surg. 2002;184:9S–16S. doi: 10.1016/s0002-9610(02)01173-x. [DOI] [PubMed] [Google Scholar]
- 6.Padwal RS. Characteristics of patients undergoing bariatric surgery in Canada. Obes Res. 2005;13:2052–4. doi: 10.1038/oby.2005.253. [DOI] [PubMed] [Google Scholar]
- 7.Oria HE. Gastric banding for morbid obesity. Eur J Gastroenterol Hepatol. 1999;11:105–14. doi: 10.1097/00042737-199902000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Chapman A, Kiroff G, Game P, et al. ASERNIP-S Report No 31. Adelaide (South Australia): ASERNIP-S; 2002. Systematic review of laparoscopic adjustable gastric banding in the treatment of obesity. [Google Scholar]
- 9.Moser F, Gorodner MV, Galvani CA, et al. Pouch enlargement and band slippage: two different entities. Surg Endosc. 2006;20:1021–9. doi: 10.1007/s00464-005-0269-4. [DOI] [PubMed] [Google Scholar]
- 10.Suter M. Laparoscopic band repositioning for pouch dilatation/slippage after gastric banding: disappointing results. Obes Surg. 2001;11:507–12. doi: 10.1381/096089201321209431. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien PE, Dixon JB. Weight loss and early and late complications —the international experience. Am J Surg. 2002;184(6B):42S–45S. doi: 10.1016/s0002-9610(02)01179-0. [DOI] [PubMed] [Google Scholar]
- 12.Fielding GA, Ren CJ. Laparoscopic adjustable gastric band. Surg Clin North Am. 2005;85:129–40. doi: 10.1016/j.suc.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Martikainen T, Pirinen E, Alhava E, et al. Long-term results, late complications, and quality of life in a series of adjustable gastric banding. Obes Surg. 2004;14:648–54. doi: 10.1381/096089204323093435. [DOI] [PubMed] [Google Scholar]
- 14.Sarker S, Herold K, Creech S, et al. Early and late complications following adjustable gastric banding. Am Surg. 2004;70:146–9. discussion 149–50. [PubMed] [Google Scholar]
- 15.Tran D, Rhoden DH, Cacchione RN, et al. Techniques for repair of gastric prolapse after laparoscopic gastric banding. J Laparoendosc Adv Surg Tech A. 2004;14:117–20. doi: 10.1089/109264204322973916. [DOI] [PubMed] [Google Scholar]
- 16.Niville E, Dams A, Vlasselaers J. Lap-Band erosion: incidence and treatment. Obes Surg. 2001;11:744–7. doi: 10.1381/09608920160558704. [DOI] [PubMed] [Google Scholar]
- 17.Msika S. Surgery for morbid obesity: 2. Complications. Results of a technologic evaluation by the ANAES. J Chir (Paris) 2003;140:4–21. [PubMed] [Google Scholar]
- 18.Silecchia G, Restuccia A, Elmore U, et al. Laparoscopic adjustable silicone gastric banding: prospective evaluation of intragastric migration of the lap-band. Surg Laparosc Endosc Percutan Tech. 2001;11:229–34. doi: 10.1097/00129689-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Abeid S, Szold A. Laparoscopic management of Lap-Band erosion. Obes Surg. 2001;11:87–9. doi: 10.1381/096089201321454169. [DOI] [PubMed] [Google Scholar]
- 20.Basa NR, Dutson E, Lewis C, et al. Laparoscopic transgastric removal of eroded adjustable band: a novel approach. Surg Obes Relat Dis. 2008;4:194–7. doi: 10.1016/j.soard.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Mehanna MJ, Birjawi G, Moukaddam HA, et al. Complications of adjustable gastric banding, a radiological pictorial review. AJR. 2006;186:522–34. doi: 10.2214/AJR.04.0655. [DOI] [PubMed] [Google Scholar]
- 22.Weiner R, Blanco-Engert R, Weiner S, et al. Outcome after laparoscopic adjustable gastric banding — 8 years experience. Obes Surg. 2003;13:427–34. doi: 10.1381/096089203765887787. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien PE, Dixon JB, Brown W, et al. The laparoscopic adjustable gastric band (Lap-Band): a prospective study of medium-term effects on weight, health and quality of life. Obes Surg. 2002;12:652–60. doi: 10.1381/096089202321019639. [DOI] [PubMed] [Google Scholar]
- 24.Chapman AE, Kiroff G, Game P, et al. Laparoscopic adjustable gastric banding in the treatment of obesity: a systematic literature review. Surgery. 2004;135:326–51. doi: 10.1016/S0039-6060(03)00392-1. [DOI] [PubMed] [Google Scholar]