Abstract
Background
The National Nosocomial Infections Surveillance (NNIS) and Efficacy of Nosocomial Infection Control (SENIC) indexes are designed to develop control strategies and to reduce morbidity and mortality rates resulting from infections in surgical patients. We sought to assess the application of these indexes in patients under-going surgery for abdominal trauma and to develop an alternative model to predict surgical site infections (SSIs).
Methods
We conducted a prospective cohort study between November 2000 and March 2002. The main outcome measure was SSIs. We evaluated the variables included in the NNIS and SENIC indexes and some preoperative, intraoperative and postoperative variables that could be risk factors related to the development of SSIs. We performed multivariate analyses using a forward logistic regression method. Finally, we assessed infection risk prediction, comparing the estimated probabilities with actual occurrence using the areas under the receiver operating characteristic (ROC) curves.
Results
Overall, 614 patients underwent an exploratory laparotomy. Of these, 85 (13.8%) experienced deep incisional and organ/intra-abdominal SSIs. The independent variables associated with this complication were an Abdominal Trauma Index score greater than 24, abdominal contamination and admission to the intensive care unit. We proposed a model for predicting deep incisional and organ/intra-abdominal SSIs using these variables (alternative model). The areas under the ROC curves were compared using the estimated probabilities for this alternative model and for the NNIS and SENIC scores. The analysis revealed a greater area under the ROC curve for the alternative model. The NNIS and SENIC scores did not perform as well as the alternative model in patients with abdominal trauma.
Conclusion
The NNIS and SENIC indexes were inferior to the proposed alternative model for predicting SSIs in patients undergoing surgery for abdominal trauma.
Abstract
Contexte
Les indices du système national de surveillance des infections nosocomiales (National Nosocomial Infections Surveillance — NNIS) et d’efficacité du contrôle des infections nosocomiales (Efficacy of Nosocomial Infection Control —SENIC) sont conçus pour aider à élaborer des stratégies de contrôle et réduire les taux de morbidité et de mortalité causés par les infections chez les patients en chirurgie. Nous avons cherché à évaluer l’application de ces indices chez les patients qui subissent une intervention chirurgicale pour un traumatisme abdominal et à créer un modèle de rechange pour prédire les infections des sites chirurgicaux (ISC).
Méthodes
Nous avons procédé à une étude de cohorte prospective entre novembre 2000 et mars 2002. Les ISC ont constitué la principale mesure de résultats. Nous avons évalué les variables incluses dans les indices NNIS et SENIC et des variables préopératoires, périopératoires et postopératoires qui pourraient constituer des facteurs de risque reliés à l’apparition des ISC. Nous avons procédé à des analyses multivariées en utilisant une méthode de régression logistique prospective. Nous avons enfin évalué la prédiction du risque d’infection, en comparant les probabilités calculées aux occurrences réelles, en utilisant les aires couvertes par les courbes des caractéristiques de fonctionnement du récepteur (receiver operating characteristic — ROC).
Résultats
Dans l’ensemble, 614 patients ont subi une laparotomie exploratoire et 85 (13,8 %) d’entre eux ont été atteints d’une ISC au niveau de l’incision profonde et des organes ou à l’intérieur de l’abdomen. Les variables indépendantes associées à cette complication étaient les suivantes : indice du traumatisme abdominal de plus de 24, contamination abdominale et admission aux soins intensifs. Nous avons proposé un modèle de prédiction des ISC des incisions profondes et des organes ou de l’intérieur de l’abdomen en utilisant ces variables (modèle de rechange). Nous avons comparé les zones qui se trouvaient sous les courbes ROC en utilisant les probabilités calculées pour ce modèle de rechange et pour les indices NNIS et SENIC. L’analyse a révélé que l’aire qui se trouvait sous la courbe ROC était plus étendue dans le cas du modèle de rechange. Les scores NNIS et SENIC n’ont pas donné d’aussi bons résultats que le modèle de rechange chez les patients atteints d’un traumatisme abdominal.
Conclusion
Les indices NNIS et SENIC ont donné de moins bons résultats que le modèle de rechange proposé pour prédire les ISC chez les patients qui subissent une intervention chirurgicale pour un traumatisme à l’abdomen.
Surgical site infection (SSI) is one of the most common postoperative events,1 with a reported incidence of 37%.2,3 The high incidence of abdominal trauma requires knowledge not only of the management of trauma itself but also of the complications associated with it. Surgical site infections are frequent complications, and the incidence of organ/intra-abdominal SSIs ranges between 2% and 12%.1,3–6
Two models that assess certain patient risk factors and other intraoperative factors have been proposed to predict SSI risk in elective surgery: the National Nosocomial Infections Surveillance (NNIS) index7,8 and the Efficacy of Nosocomial Infection Control (SENIC) index.9,10 They are designed to develop control strategies and to reduce morbidity and mortality rates resulting from infections in surgical patients. Some studies have compared the 2 indexes and have shown them to be good predictors of SSIs, although the SENIC appears to have a higher predictive value than the NNIS.11,12
The NNIS index takes into account 3 risk factors, and each is awarded 1 point: contaminated or dirty-infected surgical wound, American Society of Anesthesiology (ASA) score greater than 2 and surgery duration longer than T (where T is defined as the 75th percentile of the average time for a surgical procedure). The SENIC index is calculated by awarding 1 point for each of the 4 risk factors: abdominal surgery, contaminated or dirty-infected surgical wound, more than 2 diagnoses at discharge and surgery longer than 2 hours.
In trauma patients, there are other intra-and postoperative patient-related factors associated with the occurrence of SSIs that ought to be considered when attempting to predict which patients may experience complications. Efforts to find specific scores that allow for effective prediction using the already established trauma severity scores or others used in critical care have failed because they have not included other risk factors associated with trauma patients. There are no reports to date about the assessment of the NNIS and SENIC scores in patients undergoing surgery for abdominal trauma.
Our objectives were to assess the application of the NNIS and SENIC indexes to patients undergoing surgery for abdominal trauma and to develop an alternative model to predict SSIs.
Methods
We conducted a prospective cohort study in patients over 12 years of age who underwent an exploratory laparotomy because of abdominal trauma at the Hospital San Vicente de Paúl, a level-1 trauma centre in Medellín, Colombia. The study was approved by the ethics committees of the University of Antioquia and the Hospital San Vicente de Paúl.
The main outcome was deep incisional and organ/intra-abdominal SSIs. We used the criteria developed by the Centers for Disease Control and Prevention and the National Nosocomial Infections Surveillance System to diagnose SSIs.13 Patients who presented with more than 1 SSI were considered as a single case.
The risk factors included in the assessment were age, sex, mechanism of injury, number of injured organs (according to International Classification of Diseases [ICD]-10 codes upon discharge), clinical status according to the ASA, time elapsed from trauma to surgical intervention, use of prophylactic antibiotics, revised trauma score, injury severity score (ISS), Abdominal Trauma Index (ATI) score, intraoperative hypotension (substance P < 90 mm Hg), blood transfusion, surgical duration, abdominal contamination (macroscopic contamination described by the surgeon in the operative description), type of wound (clean, clean-contaminated, contaminated or dirty-infected), damage-control surgery (bleeding-and contamination-control surgery, without specific repairs, lasting no more than 2 hours) and admission to the intensive care unit (ICU).
Surgical duration was defined according to the NNIS criterion that laparotomy is a 2-hour procedure. We used this time reference to dichotomize the variables present in this study.4 The discharge diagnoses were defined as the number of injured organs, each of which was assigned a code according to the ICD-10.
We excluded patients who were receiving antibiotic therapy at the time of injury, those with a known immunodeficiency, those who died within the first 48 hours after injury, those who had sustained burn injuries and those who had undergone surgery at another institution before admission to our hospital. Patients were followed for a period of 30 days after laparotomy.
The sample size was estimated with the goal of finding associations with a relative risk (RR) equal to or greater than 2.5, a 95% confidence interval (CI) significant at α = 0.05 and a power of 80%. With an incidence of deep incisional and organ/intra-abdominal SSIs estimated at 15%, the number of patients needed in the sample was 426.
Statistical analysis
We performed a bivariate analysis using the risk factors and the main outcome, and the results are reported as RRs and 95% CIs. We also performed a multivariate analysis using a forward logistic method. Calculations were performed using the Statistical Products and Service Solutions software (v. 14.0, SPSS Inc.). A receiver operating characteristic (ROC) curve was constructed using EPIDAT 3.1 OPS software to evaluate the accuracy of risk prediction comparing the calculated probabilities of SSI with the actual occurrence. We compared the performance of the alternative model with that of the NNIS and SENIC indexes using the area under the ROC curve to evaluate the accuracy of risk prediction.
Results
Over a 16-month period, 614 exploratory laparotomies were performed in patients with abdominal trauma who fulfilled our inclusion criteria. Of these, 85 (13.8%) patients experienced deep incisional and organ infection/intra-abdominal SSIs.
We included 553 (90.1%) male and 61 (9.9%) female patients with a mean age of 27.1 (range 12–71) years in the study. There were 556 (90.4%) penetrating injuries and 58 (9.4%) blunt trauma cases. Of the penetrating injuries, 197 (32%) were produced by stab wounds and 359 (58.4%) by firearms.
Eighty-five patients experienced SSIs: 20 (23.5%) had only deep incisional SSIs, 8 (9.4%) experienced only organ/intra-abdominal SSIs and 57 (67.1%) had both types of SSIs. There were 14 deaths (2.28%).
Table 1 shows the frequency of deep incisional and organ/intra-abdominal SSIs according to the risk factors, the RRs and 95% CIs and p values. Table 2 shows the association of the individual NNIS and SENIC variables with SSIs.
Table 1.
Relation between SSIs and the studied risk factors of patients with abdominal trauma
| Risk factor | SSI; no. (%) | Total | RR (95% CI) | p value | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Sex | 0.80 (0.39–1.64) | 0.58 | |||
| Male | 75 (13.6) | 478 (86.4) | 553 | ||
| Female | 10 (16.4) | 51 (83.6) | 61 | ||
| Total | 85 (13.8) | 529 (86.2) | 614 | ||
| Age | 2.95 (0.89–9.81) | 0.08 | |||
| > 55 yr | 4 (30.8) | 9 (69.2) | 13 | ||
| ≤ 55 yr | 77 (13.1) | 511 (86.9) | 588 | ||
| Total | 81 (13.5) | 520 (86.5) | 601 | ||
| Mechanism of injury | 0.66 (0.33–1.33) | 0.23 | |||
| Penetrating | 74 (13.4) | 480 (86.6) | 554 | ||
| Blunt | 11 (19.0) | 47 (81.0) | 58 | ||
| Total | 85 (13.9) | 527 (86.1) | 612 | ||
| Time elapsed from trauma to surgery | 0.97 (0.59–1.61) | 1.00 | |||
| > 6 h | 25 (13.4) | 161 (86.6) | 186 | ||
| ≤ 6 h | 58 (13.8) | 363 (86.2) | 421 | ||
| Total | 83 (13.7) | 524 (86.3) | 607 | ||
| Use of antibiotics | 2.32 (1.28–4.18) | 0.010 | |||
| No | 18 (24.7) | 55 (75.3) | 73 | ||
| Yes | 67 (12.4) | 474 (87.6) | 541 | ||
| Total | 85 (13.8) | 529 (86.2) | 614 | ||
| Injury severity score | 1.50 (0.88–2.55) | 0.14 | |||
| > 20 | 22 (18.0) | 100 (82.0) | 122 | ||
| ≤ 20 | 63 (12.8) | 429 (87.2) | 492 | ||
| Total | 85 (13.8) | 529 (86.2) | 614 | ||
| Revised trauma score | 1.98 (1.21–3.25) | 0.008 | |||
| < 7.8 | 29 (20.7) | 111 (79.3) | 140 | ||
| ≥ 7.8 | 55 (11.7) | 417 (88.3) | 472 | ||
| Total | 84 (13.7) | 528 (86.3) | 612 | ||
| Abdominal trauma index score | 5.05 (3.09–8.26) | < 0.001 | |||
| ≥ 24 | 39 (33.9) | 76 (66.1) | 115 | ||
| < 24 | 46 (9.2) | 453 (90.8) | 499 | ||
| Total | 85 (13.8) | 529 (86.2) | 614 | ||
| Abdominal cavity contamination | 3.86 (2.26–6.67) | < 0.001 | |||
| Yes | 64 (21.0) | 241 (79.0) | 305 | ||
| No | 19 (6.4) | 278 (93.6) | 297 | ||
| Total | 83 (13.8) | 519 (86.2) | 602 | ||
| Transfused blood | 3.53 (2.20–5.69) | < 0.001 | |||
| Yes | 42 (26.2) | 118 (73.8) | 160 | ||
| No | 41 (9.2) | 407 (90.8) | 448 | ||
| Total | 83 (13.7) | 525 (86.3) | 608 | ||
| Intraoperative hypotension (SP < 90 more than 1 h) | 3.00 (1.82–4.94) | < 0.001 | |||
| Yes | 31 (26.7) | 85 (73.3) | 116 | ||
| No | 54 (10.8) | 444 (89.2) | 498 | ||
| Total | 85 (13.8) | 529 (86.2) | 614 | ||
| Damage control | 6.48 (1.59–26.43) | 0.016 | |||
| Yes | 4 (50.0) | 4 (50.0) | 8 | ||
| No | 81 (13.4) | 525 (86.6) | 606 | ||
| Total | 85 (13.8) | 529 (86.2) | 614 | ||
| Admision to ICU | 9.42 (5.51–16.11) | < 0.001 | |||
| Yes | 37 (48.1) | 40 (51.9) | 77 | ||
| No | 48 (8.9) | 489 (91.1) | 537 | ||
| Total | 85 (13.8) | 529 (86.2) | 614 | ||
CI = confidence interval; ICU = intensive care unit; RR = relative risk; SP = substance P; SSI = surgical site infection.
Table 2.
Relation between SSIs and individual risk factors on the NNIS and SENIC indexes
| Risk factor | SSI; no. (%) | Total | RR (95% CI) | p value | |
|---|---|---|---|---|---|
| Yes | No | ||||
| ASA score | 2.29 (1.42–3.70) | < 0.001 | |||
| 3–5 | 56 (18.8) | 242 (81.2) | 298 | ||
| 1–2 | 29 (9.2) | 287 (90.8) | 316 | ||
| Total | 85 (13.8) | 529 (86.2) | 614 | ||
| Surgical time | 2. 40 (1.48–3.89) | < 0.001 | |||
| > 2 h | 57 (19.1) | 242 (80.9) | 299 | ||
| ≤ 2 h | 28 (8.9) | 285 (91.1) | 313 | ||
| Total | 85 (13.9) | 527 (86.1) | 612 | ||
| Type of wound | 3.69 (2.11–6.46) | < 0.001 | |||
| Contaminated or dirty and infected | 68 (19.8) | 275 (80.2) | 343 | ||
| Clean or clean-contaminated | 17 (6.3) | 254 (93.7) | 271 | ||
| Total | 85 (13.8) | 529 (86.2) | 614 | ||
| No. of diagnoses upon discharge | 2.80 (1.75–4.48) | < 0.001 | |||
| ≥ 3 | 41 (23.7) | 132 (76.3) | 173 | ||
| 1–2 | 44 (10.0) | 397 (90.0) | 441 | ||
| Total | 85 (13.8) | 529 (86.2) | 614 | ||
Sex, age, the mechanism of injury, the time between trauma and surgery and the ISS did not appear to be associated with the development of SSIs (all p > 0.05). Bivariate logistic analysis showed a significant association between the development of SSIs and the number of injured organs, the ASA clinical condition, the use of prophylactic antibiotics, the revised trauma score, intraoperative hypotension, blood transfusion, duration of surgery, the ATI score, cavity contamination, type of wound, damage-control surgery and admission to the ICU.
We estimated NNIS and SENIC scores, and we compared the incidence of SSIs with that reported in the October 2004 NNIS System Report. Table 3 shows infection probabilities according to the NNIS and SENIC scores. The lowest value that could be obtained on the SENIC index was 2 points considering that all patients had sustained abdominal trauma and had undergone laparotomy.
Table 3.
Probability of SSIs according to NNIS and SENIC index scores: comparison with NNIS report 200410
| Index score | SSI; no. (%) | Total | Probability of SSI in NNIS report 2004,10 RR (95% CI) | |
|---|---|---|---|---|
| Yes | No. | |||
| NNIS | ||||
| 0 | 8 (10.7) | 67 (89.3) | 75 | 1.71 (0–2.87) |
| 1 | 29 (11.8) | 216 (88.2) | 245 | 3.08 (1.14–6.70) |
| 2 | 41 (17.6) | 192 (82.4) | 233 | 4.71 (1.65–10.17) |
| 3 | 7 (11.5) | 54 (88.5) | 61 | 7.19 NR |
| SENIC | ||||
| 2 | 20 (15.3) | 111 (84.7) | 131 | — |
| 3 | 54 (14.6) | 316 (85.4) | 370 | — |
| 4 | 11 (9.7) | 102 (90.3) | 113 | — |
In the multivariate analysis, we used a forward selection logistic regression model. The variables selected for the model were those with a p < 0.25. An ATI score greater than 24, abdominal contamination and admission to the ICU were independent predictors of SSIs (Table 4). The same table summarizes the results of the logistic regression of the NNIS and SENIC variables. This model explains the 27% variability of the dependent variable (R2 Nagelkerke = 0.27).
Table 4.
Logistic regression analysis of risk factors for SSIs in the alternative model and NNIS and SENIC indexes
| Index, variable | β | p value | RR (95% CI) |
|---|---|---|---|
| Alternative model | |||
| ATI | 0.993 | 0.001 | 2.70 (1.51–4.84) |
| Contamination | 1.170 | < 0.001 | 3.22 (1.74–5.98) |
| Admission to ICU | 1.811 | < 0.001 | 6.12 (3.35–11.16) |
| NNIS index | |||
| Type of wound | 1.173 | < 0.001 | 3.23 (1.82–5.73) |
| ASA | 0.742 | 0.003 | 2.10 (1.28–3.44) |
| Surgical time | 0.566 | 0.028 | 1.76 (1.06–2.92) |
| SENIC index | |||
| Type of wound | 1.104 | < 0.001 | 3.02 (1.70–5.37) |
| No. of injured organs | 0.755 | 0.002 | 2.13 (1.31–3.47) |
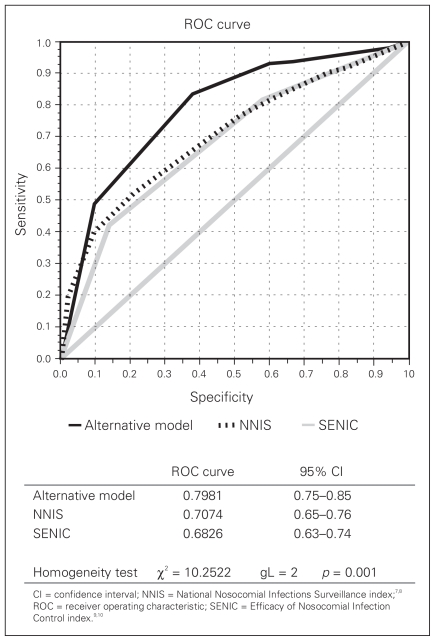
Figure 1 compares the ROC curves derived from the probabilities estimated for the alternative model and for the NNIS and SENIC scores. The area under the curve was larger for the alternative model. The difference between areas was statistically significant (p = 0.001).
Fig. 1.
Receiver operating characteristic (ROC) curve for the calculated risk in alternative and Efficacy of Nosocomial Infection Control (SENIC) and National Nosocomial Infections Surveillance (NNIS) models. CI = confidence interval.
Discussion
Surgical site infection is the most frequently reported complication in surgical patients, accounting for 14%–16% of all nosocomial infections.14 Surveillance and feedback programs for surgeons can help reduce SSI rates by an additional 35%–50%.15
Two infection risk models have been proposed to predict SSIs in patients undergoing elective surgery: the NNIS12 and the SENIC16,17 indexes. Some studies that have used the same databases for score comparison have shown that both indexes are good predictors of SSIs,18 pneumonia and hospital deaths, although the SENIC appears to have a better predictive value than the NNIS given that abdominal surgery, a risk factor included in the SENIC, is associated with a substantially greater risk of sepsis than other types of surgery.19
The sensitivity of a nosocomial infection surveillance system may be assessed on the basis of its ability to detect an infection. A Chilean study20 showed a sensitivity of 94.3% and a specificity of 97% for the NNIS, both consistent with reports by other authors.21
In patients undergoing trauma surgery, the incidence of infection can be as high as 37%. Of that, up to 76% corresponds to nosocomial infection, 22% to injury-associated infections and 2% are infections that were already present at the time of trauma. The rate of organ/intra-abdominal SSIs can be as high as 12%.22–24 Among the patients included in the present study, there were 85 SSIs (13.8%), a value that is comparable to that reported in the literature.1,8,22
Identifying independent risk factors for infection in trauma patients is a difficult task. However, in multivariate analyses, older age, type of trauma (blunt or penetrating), the presence of shock, the number of affected organs, unconsciousness, high ISS scores, prolonged mechanical ventilation, wound classification, use of prophylactic antibiotics,25–28 spinal cord injury, the requirement for mechanical ventilation, the use of central catheters, multiple transfusions and several surgical procedures have been reported to be substantial risk factors for infection in trauma patients. Undergoing a surgical procedure in the first 24 hours after admission offers a protective benefit against the development of an infection.29
In the present study, we found no association between age, sex, type of trauma, the time between the injury and surgery and SSIs.
In abdominal trauma, risk factors for SSIs other than those already mentioned include injury severity, systemic inflammatory response syndrome and degree of physiologic alteration, as measured by scores such as the revised trauma score and the Acute Physiology and Chronic Health Evaluation (APACHE II), the magnitude of the anatomic injuries assessed by the ISS, the ATI and the Trauma and Injury Severity Score (TRISS).30 Concomitant disease, obesity, diabetes mellitus, neoplasms, cirrhosis, prolonged hypotension, transfusion, previous splenectomy, use of drains, packing and stomas have also been associated with infection in patients with abdominal trauma.5
In the present study, we found a clear association between contamination of the abdominal cavity (understood as any type of contamination owing to hollow viscus injuries) and SSIs, especially considering that some of these patients received surgery more than 6 hours after sustaining their injuries and that their wounds were classified as dirty. However, this variable has not been considered in other risk prediction studies — not even in nontrauma surgery. On the other hand, as shown for elective surgery, the use of prophylactic antibiotics prevented the development of SSIs, although the association was not significant.
Intraoperative shock showed a clear relation with the development of SSIs. It has been well established that shock, through a number of physiopathological mechanisms, contributes to the development of any type of nosocomial infection. These data support what has been thoroughly reviewed in the literature regarding the importance of patient reanimation, but this time relates to the prevention of SSIs.
Blood transfusion, a variable widely discussed in prior research studies as having an association with the development of multiple infectious and noninfectious complications, was shown to have a clear association with the development of SSIs in the present study.
Damage-control surgery, reinterventions and nutrition are also risk factors for the presence of postoperative infection. Many of these factors overlap and interact in such a way that it is difficult to determine the actual impact of each.5,31
Damage-control surgery was performed in 50 of 614 patients in this study. Those patients had a higher incidence of SSIs, showing an associated risk that is clearly against the use of such a strategy. As this strategy is the only available life-saving measure for these trauma patients, it makes the management of SSIs and other complications a challenge for the surgical team.
In a study conducted in Thailand,32 a higher association with postoperative infection in trauma patients was found for the New Injury Severity Score (NISS; odds ratio [OR] 1.79, 95% CI 1.55–2.05), followed by the ISS (OR 1.65, 95% CI 1.42–1.92), the revised trauma score (OR 1.64, 95% CI 1.43–1.88) and the TRISS (OR 1.32, 95% CI 1.14–1.52).32
Croce and colleagues30 evaluated the relation between the ISS and the ATI with the development of infectious complications. An ISS greater than 16 or an ATI score greater than 25 were clearly associated with the development of such complications.
The present study found an association between the development of SSIs and the revised trauma and ATI scores. An association trend was found for the ISS but it was not statistically significant.
For elective surgery, several studies have examined the role of the NNIS and SENIC variables and have assessed their individual associations with SSIs.19,33–36
In the abdominal trauma patients included in the present study, the frequency of SSIs determined for patients with SENIC scores of 2, 3 and 4 was 15.3%, 14.6% and 9.7%, respectively (with the understanding that there would be no patients with fewer than 2 points because all of them underwent abdominal surgery). For NNIS scores of 0, 1, 2 and 3 points, these frequencies were 10.7%, 11.8%, 17.6% and 11.5%, respectively (Table 3).
According to the report of the NNIS System in 2004,4 SSI rates for the laparotomy procedure, discriminated by score, were as follows: 0 points, 1.71%; 1 point, 3.08%; 2 points, 4.71%; and 3 points, 7.19%. Compared with the rates obtained for the 614 patients, it is clear that the frequency of SSI is higher among trauma patients, probably as a result of the number of risk factors involved in this patient group.
As with the studies mentioned previously, when the association of the different variables in both scores was estimated in a dichotomized arrangement, the bivariate analysis revealed RRs in the following order of importance: type of wound (RR 3.7, 95% CI 2.11–6.45), number of diagnoses at discharge (RR 2.8, 95% CI 1.75–4.47), duration of surgery (RR 2.4, 95% CI 1.4–3.8) and ASA score (RR 2.3, 95% CI 1.4–3.7).
In the logistic regression model, an ATI score greater than 24, contamination of the abdominal cavity and admission into the ICU were independently associated with the risk of SSIs.
Limitations
There are other risk factors described in the literature (e.g., glycemic control oxygenation levels, core body temperature) that were not taken into account in the present study; these factors could have influenced our results to a certain extent.
In this study, we generated ROC curves to compare the predictive value of the alternative model with that of the NNIS and SENIC indexes. The probability of SSI was estimated using the logistic function of the models compared with the actual occurrence of the event. The analysis showed a larger area under the ROC curve for the alternative model, with an estimated value of 0.7981 (95% CI 0.75–0.85), reflecting a good predictive ability. The performance of the NNIS and SENIC indexes in trauma was not as good in this group of abdominal trauma patients as that of the proposed alternative model or their own performance in elective surgery samples.
Conclusion
Until now, a model that was able to predict the development of SSIs accurately in trauma patients had not been found. The NNIS and SENIC indexes do not perform well in abdominal trauma patients, in whom different factors not included in the indexes play an important part in the development of SSIs as well as nosocomial infection. Among these factors are previous health status, injuries due to the trauma that may involve multiple organs and systems, complications and the treatment itself.
The variables that showed an independent association with SSI incidence were ATI score, admission to the ICU and abdominal cavity contamination. An alternative index that contains these variables has a better predictive capacity for SSI development; however, although this model predicted 87% of the events in our study, it only explained 27% of the SSI variability. Thus, other undertermined risk factors are involved in the development of SSIs.
For the alternative model to be widely applicable to the trauma population, it would need to be validated with a larger data set.
Some risk factors for SSI are out of the trauma surgeon’s control; there may be additional perioperative variables not described in the present study that could reduce the incidence of intra-abdominal infections. There is an obvious need for more studies of the incidence and variables for developing SSIs in trauma patients to determine modifiable risk factors that can be influenced to reduce infectious complications.
Footnotes
Competing interests: None declared.
Contributors: Drs. Morales and Villegas designed the study. Drs. Morales and Escobar analyzed the data and wrote the article. All authors acquired data, reviewed the article and approved its publication.
References
- 1.Smyth ET. Surgical site infection surveillance. J Hosp Infect. 2000;45:173–84. doi: 10.1053/jhin.2000.0736. [DOI] [PubMed] [Google Scholar]
- 2.Berard F, Gandon J. Postoperative wound infections: the influence of ultraviolet irradiation of the operating room and of various other factors. Ann Surg. 1964;160(Suppl 2):1–192. [PubMed] [Google Scholar]
- 3.Weigelt JA, Haley RW, Seibert B. Factors influence the risk of wound infection in trauma patients. J Trauma. 1987;27:774–81. doi: 10.1097/00005373-198707000-00015. [DOI] [PubMed] [Google Scholar]
- 4.National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 to June 2004, issued October 2004. [accessed 2009 Aug. 1]. Available: www.cdc.gov/ncidod/hip/NNIS/2004NNISReport_AJIC.PDF. [DOI] [PubMed]
- 5.Morales CH, Villegas MI, Villavicencio R, et al. Intra-abdominal infection in patients with abdominal trauma. Arch Surg. 2004;139:1278–85. doi: 10.1001/archsurg.139.12.1278. [DOI] [PubMed] [Google Scholar]
- 6.Deitch EA, Livingston DH, Hauser CJ. Septic complications in the trauma patient. New Horizons. 1999;7:158–72. [Google Scholar]
- 7.Owens WD. ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49:239–43. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Culver DH, Horan TH, Gaynes RP. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am J Med. 1991;91(3B):152S–7S. doi: 10.1016/0002-9343(91)90361-z. [DOI] [PubMed] [Google Scholar]
- 9.Salemi C, Anderson D, Flores D. American Society of Anesthesiology scoring discrepancies affecting the National Nosocomial Infection Surveillance System: surgical site infection risk index rates. Infect Control Hosp Epidemiol. 1997;18:246–7. doi: 10.1086/647603. [DOI] [PubMed] [Google Scholar]
- 10.Haley RW, Culver DH, Morgan WM. Identifying patients at high risk of surgical wound infection: a simple multivariate index of patient susceptibility and wound contamination. Am J Epidemiol. 1985;121:206–15. doi: 10.1093/oxfordjournals.aje.a113991. [DOI] [PubMed] [Google Scholar]
- 11.Haynes SR, Fawler PGP. An assessment of the consistency of ASA physical status classification allocation. Anaesthesia. 1995;50:195–9. doi: 10.1111/j.1365-2044.1995.tb04554.x. [DOI] [PubMed] [Google Scholar]
- 12.Horan TC, Culver DH, Gaynes RP, et al. Nosocomial infections in surgical patients in the United States, January 1986–June 1992. National Nosocomial Infections Surveillance (NNIS) System. Infect Control Hosp Epidemiol. 1993;14:73–80. doi: 10.1086/646686. [DOI] [PubMed] [Google Scholar]
- 13.Horan TC, Gaynes RP, Martone WJ. Infect Control Hosp Epidemiol. Vol. 13. CDC; 1992. definitions of nosocomial surgical site infection, 1992: a modification of CDC definitions of surgical wound infection; pp. 606–8. [PubMed] [Google Scholar]
- 14.Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6:428–42. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campos ML, Cipriano ZM, Freitas PF. Suitability of the NNIS index for estimating surgical-site infection risk at a small university hospital in Brazil. Infect Control Hosp Epidemiol. 2001;22:268–72. doi: 10.1086/501898. [DOI] [PubMed] [Google Scholar]
- 16.Haley RW, Culver DH, Morgan WM. Identifying patients at high risk of surgical wound infection: a simple multivariate index of patient susceptibility and wound contamination. Am J Epidemiol. 1985;121:206–15. doi: 10.1093/oxfordjournals.aje.a113991. [DOI] [PubMed] [Google Scholar]
- 17.Special issue: The SENIC Project. Am J Epidemiol. 1980;111:465–653. [PubMed] [Google Scholar]
- 18.Archibald L, Gaynes R. Hospital-acquired infections in the United States. The importance of interhospital comparisons. Infect Dis Clin North Am. 1997;11:245–55. doi: 10.1016/s0891-5520(05)70354-8. [DOI] [PubMed] [Google Scholar]
- 19.Farinas-Alvarez C, Farinas MC, Prieto D, et al. Applicability of two surgical-site infection risk indices to risk of sepsis in surgical patients. Infect Control Hosp Epidemiol. 2000;21:633–8. doi: 10.1086/501705. [DOI] [PubMed] [Google Scholar]
- 20.Febre N, De Medeiros ES, Wey SB, et al. Is the epidemiological surveillance system of nosocomial infections recommended by the American CDC applicable in a Chilean hospital. Rev Med Chil. 2001;129:1379–86. [PubMed] [Google Scholar]
- 21.Emori TG, Edwards JR, Culver DH, et al. Accuracy of reporting nosocomial infections in intensive care unit patients to the national nosocomial infections surveillance system: a pilot study. Infect Control Hosp Epidemiol. 1998;19:308–16. doi: 10.1086/647820. [DOI] [PubMed] [Google Scholar]
- 22.Papia G, McLellan BA, El-Helou P, et al. Infection in hospitalized trauma patients: incidence, risk factors, and complications. J Trauma. 1999;47:923–33. doi: 10.1097/00005373-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Caplan ES, Hoyt N. Infection surveillance and control in the severely traumatized patient. Am J Med. 1981;70:638–40. doi: 10.1016/0002-9343(81)90587-8. [DOI] [PubMed] [Google Scholar]
- 24.Caplan ES, Hoyt NJ. Identification and treatment of infections in multiply traumatized patients. Am J Med. 1985;79(Suppl 1A):68–76. doi: 10.1016/0002-9343(85)90194-9. [DOI] [PubMed] [Google Scholar]
- 25.Baker JW, Deitch EA, Li M, et al. Hemorrhagic shock induces bacterial translocation from the gut. J Trauma. 1988;28:896–906. doi: 10.1097/00005373-198807000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Peitzman AB, Udekwu AO, Ochoa J, et al. Bacterial translocation in trauma patients. J Trauma. 1991;31:1083–7. [PubMed] [Google Scholar]
- 27.Nichols RL, Smith JW, Klein DB. Risk of infection after penetrating abdominal trauma. N Engl J Med. 1984;311:1065–70. doi: 10.1056/NEJM198410253111701. [DOI] [PubMed] [Google Scholar]
- 28.Weigelt JA, Haley RW, Seibert B. Factors which influence the risk of wound infection in trauma patients. J Trauma. 1987;27:774–81. doi: 10.1097/00005373-198707000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Cheadle WG. Risk factors for surgical site infection. Surg Infect (Larchmt) 2006;7(Suppl 1):S7–11. doi: 10.1089/sur.2006.7.s1-7. [DOI] [PubMed] [Google Scholar]
- 30.Croce MA, Fabian TC, Stewart RM, et al. Correlation of abdominal trauma index and injury severity score with abdominal septic complications in penetrating and blunt trauma. J Trauma. 1992;32:380–7. doi: 10.1097/00005373-199203000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Garibaldi RA, Cushing D, Lerer T. Risk factors for postoperative infection. Am J Med. 1991;91(Suppl 3B):158s–63s. doi: 10.1016/0002-9343(91)90362-2. [DOI] [PubMed] [Google Scholar]
- 32.Jamulitrat S, Narong MN, Thongpiyapoom S. Trauma severity scoring systems as predictors of nosocomial infection. Infect Control Hosp Epidemiol. 2002;23:268–73. doi: 10.1086/502047. [DOI] [PubMed] [Google Scholar]
- 33.Delgado-Rodriguez M, Martinez-Gallego G, Sillero-Arenas M, et al. Nosocomial infection in surgical patients: comparison of two measures of intrinsic patient risk. Infect Control Hosp Epidemiol. 1997;18:19–23. doi: 10.1086/647495. [DOI] [PubMed] [Google Scholar]
- 34.Fajardo-Rodríguez H, Quemba Gordillo J, Eslava Schmalbach J. Prediction scales and infection on surgical sites in 15625 surgeries. 2001–2003] Article in Spanish. Rev Salud Publica (Bogota) 2005;7:89–98. doi: 10.1590/s0124-00642005000100007. [DOI] [PubMed] [Google Scholar]
- 35.Inigo JJ, Bermejo B, Oronoz B, et al. Surgical site infection in general surgery: 5-year analysis and assessment of the National Nosocomial Infection Surveillance (NNIS) index [Article in Spanish] Cir Esp. 2006;79:224–30. doi: 10.1016/s0009-739x(06)70857-0. [DOI] [PubMed] [Google Scholar]
- 36.Vázquez-Aragón P, Cascales-Sánchez A, Lizan-García M, et al. Estudio prospectivo de la frecuencia de infección nosocomial y factores de riesgo en un servicio de cirugía general. Cir Esp. 2003;74:86–91. [Google Scholar]