Abstract
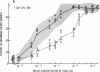
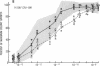
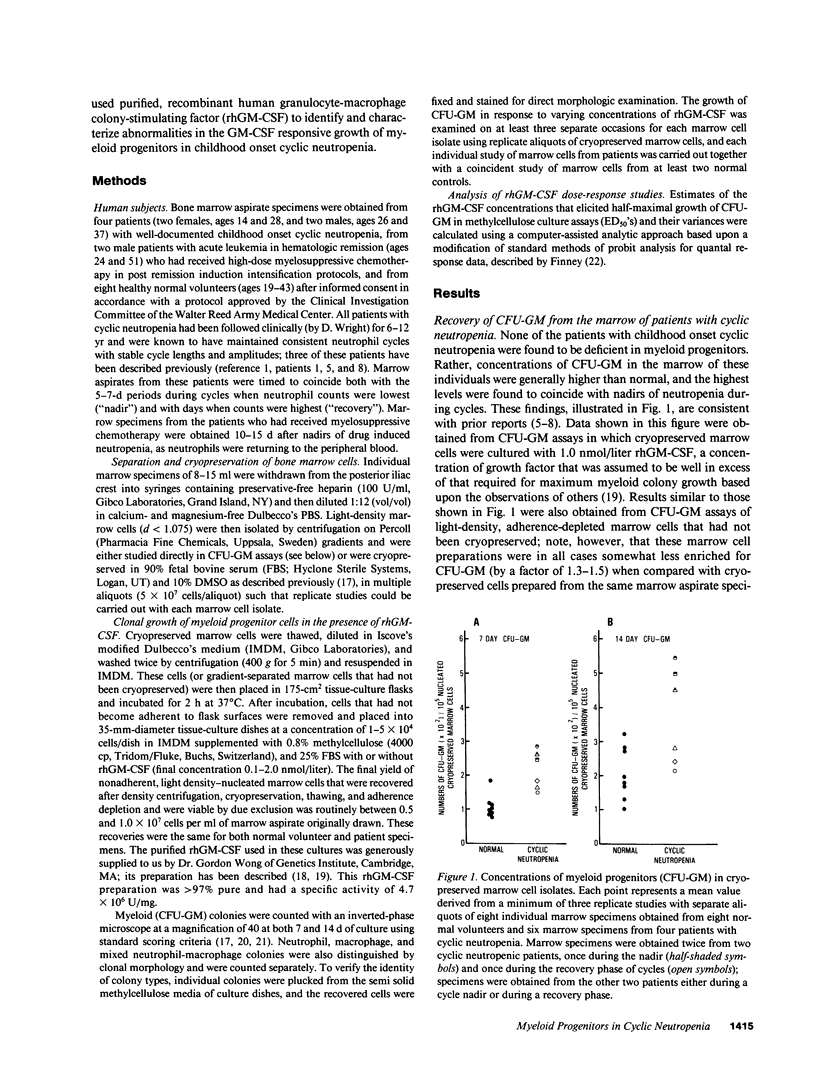
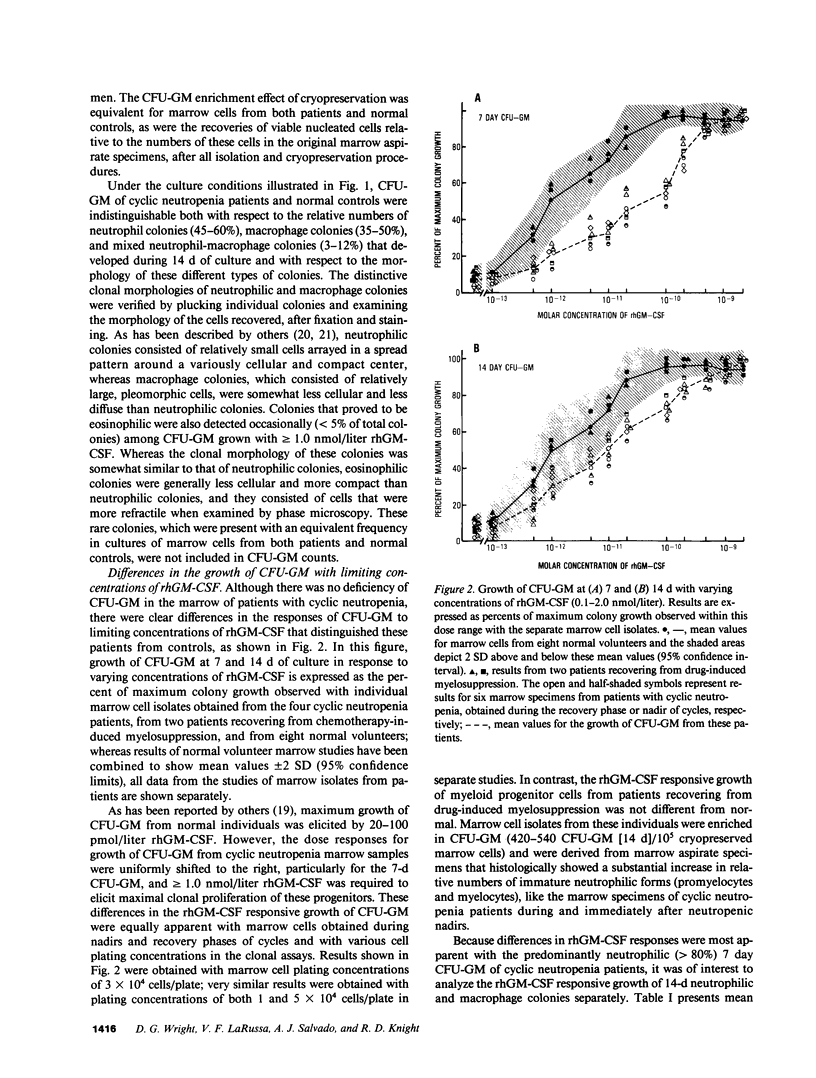
Granulocyte-macrophage progenitors (CFU-GM) from four patients with childhood onset cyclic neutropenia demonstrated abnormal in vitro proliferative responses to purified, recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) when examined in detailed dose-response studies. Marrow aspirate specimens were obtained for these studies from cyclic neutropenia patients (both during neutropenic nadirs and during recovery phases of cycles), from leukemia patients in remission who had received myelosuppressive chemotherapy, and from healthy normal volunteers. Nucleated marrow cells were then isolated by density-gradient centrifugation and cryopreserved to permit studies of CFU-GM from patients and controls to be carried out at the same time and in replicate. Maximum clonal growth of CFU-GM from normal subjects and from individuals recovering from drug-induced myelosuppression was elicited by 20-100 pmol/liter rhGM-CSF, and the CSF concentrations that induced half-maximal responses (ED50) were between 1.0 and 3.0 pmol/liter. In contrast, maximum growth of CFU-GM from the cyclic neutropenia patients required greater than or equal to 1.0 nmol/liter rhGM-CSF and ED50's were greater than 30.0 pmol/liter. These abnormalities in the GM-CSF responsive growth of myeloid progenitors were independent of cycle time and were most apparent with the predominantly neutrophilic 7-d CFU-GM. Moreover, differences in the growth of 14-d CFU-GM could be attributed mostly if not entirely to differences in the generation of neutrophilic colonies. These findings indicate that childhood onset cyclic neutropenia is associated with an underlying disturbance in the GM-CSF responsive growth of myeloid progenitors committed to neutrophilic differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broxmeyer H. E., DeSousa M., Smithyman A., Ralph P., Hamilton J., Kurland J. I., Bognacki J. Specificity and modulation of the action of lactoferrin, a negative feedback regulator of myelopoiesis. Blood. 1980 Feb;55(2):324–333. [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Dresch C., Thevenieau D., Castro-Malaspina H., Faille A. Cell kinetics in human cyclic neutropenia. Scand J Haematol. 1977 Jul;19(1):14–24. doi: 10.1111/j.1600-0609.1977.tb02713.x. [DOI] [PubMed] [Google Scholar]
- Gasson J. C., Kaufman S. E., Weisbart R. H., Tomonaga M., Golde D. W. High-affinity binding of granulocyte-macrophage colony-stimulating factor to normal and leukemic human myeloid cells. Proc Natl Acad Sci U S A. 1986 Feb;83(3):669–673. doi: 10.1073/pnas.83.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg P. L., Bax J., Levin J., Andrews T. M. Alteration of colony-stimulating factor output, endotoxemia, and granulopoiesis in cyclic neutropenia. Am J Hematol. 1976;1(4):375–385. doi: 10.1002/ajh.2830010403. [DOI] [PubMed] [Google Scholar]
- Guerry D., 4th, Dale D. C., Omine M., Perry S., Wolff S. M. Periodic hematopoiesis in human cyclic neutropenia. J Clin Invest. 1973 Dec;52(12):3220–3230. doi: 10.1172/JCI107522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Jacobsen N., Broxmeyer H. E. Oscillations of granulocytic and megakaryocytic progenitor cell populations in cyclic neutropenia in man. Scand J Haematol. 1979 Jul;23(1):33–36. doi: 10.1111/j.1600-0609.1979.tb02850.x. [DOI] [PubMed] [Google Scholar]
- King-Smith E. A., Morley A. Computer simulation of granulopoiesis: normal and impaired granulopoiesis. Blood. 1970 Aug;36(2):254–262. [PubMed] [Google Scholar]
- Leary A. G., Ikebuchi K., Hirai Y., Wong G. G., Yang Y. C., Clark S. C., Ogawa M. Synergism between interleukin-6 and interleukin-3 in supporting proliferation of human hematopoietic stem cells: comparison with interleukin-1 alpha. Blood. 1988 Jun;71(6):1759–1763. [PubMed] [Google Scholar]
- Loughran T. P., Jr, Clark E. A., Price T. H., Hammond W. P. Adult-onset cyclic neutropenia is associated with increased large granular lymphocytes. Blood. 1986 Nov;68(5):1082–1087. [PubMed] [Google Scholar]
- Mackey M. C. Unified hypothesis for the origin of aplastic anemia and periodic hematopoiesis. Blood. 1978 May;51(5):941–956. [PubMed] [Google Scholar]
- Meagher R. C., Salvado A. J., Wright D. G. An analysis of the multilineage production of human hematopoietic progenitors in long-term bone marrow culture: evidence that reactive oxygen intermediates derived from mature phagocytic cells have a role in limiting progenitor cell self-renewal. Blood. 1988 Jul;72(1):273–281. [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- Migliaccio G., Migliaccio A. R., Adamson J. W. In vitro differentiation of human granulocyte/macrophage and erythroid progenitors: comparative analysis of the influence of recombinant human erythropoietin, G-CSF, GM-CSF, and IL-3 in serum-supplemented and serum-deprived cultures. Blood. 1988 Jul;72(1):248–256. [PubMed] [Google Scholar]
- Miyajima A., Miyatake S., Schreurs J., De Vries J., Arai N., Yokota T., Arai K. Coordinate regulation of immune and inflammatory responses by T cell-derived lymphokines. FASEB J. 1988 Jun;2(9):2462–2473. doi: 10.1096/fasebj.2.9.2836253. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H., Chiyoda S., Miura Y., Takaku F., Asano S. Colony-forming cells in culture and colony-stimulating activity of the urine and the serum in a case of cyclic neutropenia. Tohoku J Exp Med. 1976 Aug;119(4):317–324. doi: 10.1620/tjem.119.317. [DOI] [PubMed] [Google Scholar]
- Moore M. A. Humoral regulation of granulopoiesis. Clin Haematol. 1979 Jun;8(2):287–309. [PubMed] [Google Scholar]
- Walker F., Nicola N. A., Metcalf D., Burgess A. W. Hierarchical down-modulation of hemopoietic growth factor receptors. Cell. 1985 Nov;43(1):269–276. doi: 10.1016/0092-8674(85)90032-7. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Wright D. G., Dale D. C., Fauci A. S., Wolff S. M. Human cyclic neutropenia: clinical review and long-term follow-up of patients. Medicine (Baltimore) 1981 Jan;60(1):1–13. doi: 10.1097/00005792-198101000-00001. [DOI] [PubMed] [Google Scholar]
- Wright D. G., Fauci A. S., Dale D. C., Wolff S. M. Correction of human cyclic neutropenia with prednisolone. N Engl J Med. 1978 Feb 9;298(6):295–300. doi: 10.1056/NEJM197802092980602. [DOI] [PubMed] [Google Scholar]
- von Schulthess G. K., Mazer N. A. Cyclic neutropenia (CN): a clue to the control of granulopoiesis. Blood. 1982 Jan;59(1):27–37. [PubMed] [Google Scholar]