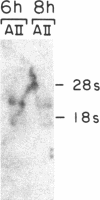
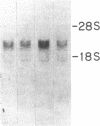
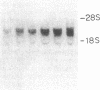
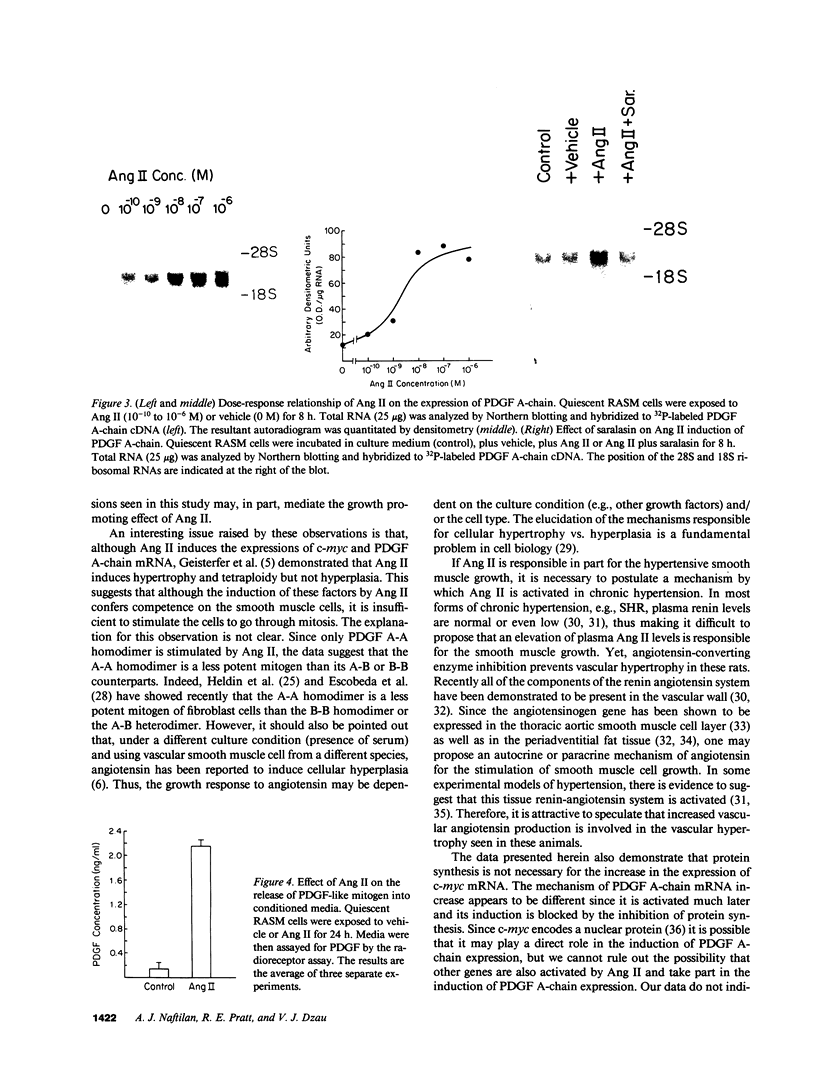
Abstract
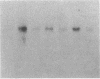
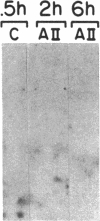
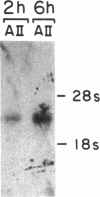
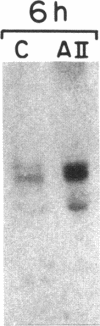
Recently, angiotensin II (Ang II) has been shown to cause hypertrophy of cultured quiescent rat aortic smooth muscle (RASM) cells. This observation along with the demonstration of angiotensinogen mRNA in the vessel wall has led us to postulate a role for vascular angiotensin in hypertensive blood vessel hypertrophy. To investigate further the possible molecular mechanisms, we examined the effect of Ang II on the expression of two genes known to be involved with cellular growth response. Near-confluent RASM cells were made quiescent by 48-h exposure to a defined serum-free medium. Ang II (10(-6) to 10(-11) M) resulted in an induction of the protooncogene c-myc mRNA within 30 min which persisted for 6 h. Interestingly, 6 h after the addition of Ang II, platelet-derived growth factor (PDGF) A-chain mRNA expression was elevated, peaked in 9 h, and persisted for 11 h. This was accompanied with a 15-20-fold increase in PDGF concentration in the culture medium. These effects were dose-dependent and were blocked by saralasin. Whereas the inhibition of protein synthesis by cycloheximide resulted in a stabilization of c-myc mRNA, cycloheximide abolished the elevation of the PDGF A-chain mRNA. Taken together, our data show that exposure of RASM cells to Ang II results in the sequential activation of c-myc and PDGF A-chain mRNA expressions. This sequential activation of protooncogene and growth factor gene may be an important mechanism in angiotensin-induced smooth muscle growth and hypertrophy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asaad M. M., Antonaccio M. J. Vascular wall renin in spontaneously hypertensive rats. Potential relevance to hypertension maintenance and antihypertensive effect of captopril. Hypertension. 1982 Jul-Aug;4(4):487–493. doi: 10.1161/01.hyp.4.4.487. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B., Lind P., Urdea M. S., Eddy R., Shows T. B., Philpott K., Mellor A. L. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986 Apr 24;320(6064):695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Campbell-Boswell M., Robertson A. L., Jr Effects of angiotensin II and vasopressin on human smooth muscle cells in vitro. Exp Mol Pathol. 1981 Oct;35(2):265–276. doi: 10.1016/0014-4800(81)90066-6. [DOI] [PubMed] [Google Scholar]
- Campbell D. J., Habener J. F. Cellular localization of angiotensinogen gene expression in brown adipose tissue and mesentery: quantification of messenger ribonucleic acid abundance using hybridization in situ. Endocrinology. 1987 Nov;121(5):1616–1626. doi: 10.1210/endo-121-5-1616. [DOI] [PubMed] [Google Scholar]
- Cassis L. A., Lynch K. R., Peach M. J. Localization of angiotensinogen messenger RNA in rat aorta. Circ Res. 1988 Jun;62(6):1259–1262. doi: 10.1161/01.res.62.6.1259. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzau V. J. Significance of the vascular renin-angiotensin pathway. Hypertension. 1986 Jul;8(7):553–559. doi: 10.1161/01.hyp.8.7.553. [DOI] [PubMed] [Google Scholar]
- Escobedo J. A., Navankasatussas S., Cousens L. S., Coughlin S. R., Bell G. I., Williams L. T. A common PDGF receptor is activated by homodimeric A and B forms of PDGF. Science. 1988 Jun 10;240(4858):1532–1534. doi: 10.1126/science.2836953. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Folkow B., Hallbäck M., Lundgren Y., Sivertsson R., Weiss L. Importance of adaptive changes in vascular design for establishment of primary hypertension, studied in man and in spontaneously hypertensive rats. Circ Res. 1973 May 5;32(Suppl):2–16. doi: 10.1007/978-3-642-65441-1_23. [DOI] [PubMed] [Google Scholar]
- Geisterfer A. A., Peach M. J., Owens G. K. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988 Apr;62(4):749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Gunther S., Alexander R. W., Atkinson W. J., Gimbrone M. A., Jr Functional angiotensin II receptors in cultured vascular smooth muscle cells. J Cell Biol. 1982 Feb;92(2):289–298. doi: 10.1083/jcb.92.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Bäckström G., Ostman A., Hammacher A., Rönnstrand L., Rubin K., Nistér M., Westermark B. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. EMBO J. 1988 May;7(5):1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. T., Gopal T. V., Moulton A. D., Nienhuis A. W. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4794–4798. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y., Sunako M., Tsuda T., Fukuzaki H., Fukumoto Y., Takai Y. Angiotensin II induces expression of the c-fos gene through protein kinase C activation and calcium ion mobilization in cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1988 Jan 15;150(1):52–59. doi: 10.1016/0006-291x(88)90485-8. [DOI] [PubMed] [Google Scholar]
- Kindy M. S., Sonenshein G. E. Regulation of oncogene expression in cultured aortic smooth muscle cells. Post-transcriptional control of c-myc mRNA. J Biol Chem. 1986 Sep 25;261(27):12865–12868. [PubMed] [Google Scholar]
- Libby P., O'Brien K. V. Culture of quiescent arterial smooth muscle cells in a defined serum-free medium. J Cell Physiol. 1983 May;115(2):217–223. doi: 10.1002/jcp.1041150217. [DOI] [PubMed] [Google Scholar]
- Marx J. L. The fos gene as "master switch". Science. 1987 Aug 21;237(4817):854–856. doi: 10.1126/science.3039659. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Baandrup U., Gundersen H. J. Evidence for hyperplasia in mesenteric resistance vessels of spontaneously hypertensive rats using a three-dimensional disector. Circ Res. 1985 Nov;57(5):794–800. doi: 10.1161/01.res.57.5.794. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nilsson J., Sjölund M., Palmberg L., Thyberg J., Heldin C. H. Arterial smooth muscle cells in primary culture produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4418–4422. doi: 10.1073/pnas.82.13.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K., Murray J. M. Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):639–649. doi: 10.1128/mcb.7.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura T., Miyazaki M., Inagami T., Toda N. Vascular renin-angiotensin system in two-kidney, one clip hypertensive rats. Hypertension. 1986 Jul;8(7):560–565. doi: 10.1161/01.hyp.8.7.560. [DOI] [PubMed] [Google Scholar]
- Owens G. K. Influence of blood pressure on development of aortic medial smooth muscle hypertrophy in spontaneously hypertensive rats. Hypertension. 1987 Feb;9(2):178–187. doi: 10.1161/01.hyp.9.2.178. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Schwartz S. M. Alterations in vascular smooth muscle mass in the spontaneously hypertensive rat. Role of cellular hypertrophy, hyperploidy, and hyperplasia. Circ Res. 1982 Sep;51(3):280–289. doi: 10.1161/01.res.51.3.280. [DOI] [PubMed] [Google Scholar]
- Persson H., Leder P. Nuclear localization and DNA binding properties of a protein expressed by human c-myc oncogene. Science. 1984 Aug 17;225(4663):718–721. doi: 10.1126/science.6463648. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Devare S. G., Aaronson S. A. Molecular cloning of integrated simian sarcoma virus: genome organization of infectious DNA clones. Proc Natl Acad Sci U S A. 1981 May;78(5):2918–2922. doi: 10.1073/pnas.78.5.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejersen T., Betsholtz C., Sjölund M., Heldin C. H., Westermark B., Thyberg J. Rat skeletal myoblasts and arterial smooth muscle cells express the gene for the A chain but not the gene for the B chain (c-sis) of platelet-derived growth factor (PDGF) and produce a PDGF-like protein. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6844–6848. doi: 10.1073/pnas.83.18.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton L. W., Watt R., Marcu K. B. Translocation, breakage and truncated transcripts of c-myc oncogene in murine plasmacytomas. Nature. 1983 Jun 2;303(5916):401–406. doi: 10.1038/303401a0. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Sassone-Corsi P. Proto-oncogene fos: complex but versatile regulation. Cell. 1987 Nov 20;51(4):513–514. doi: 10.1016/0092-8674(87)90115-2. [DOI] [PubMed] [Google Scholar]
- Walker L. N., Bowen-Pope D. F., Ross R., Reidy M. A. Production of platelet-derived growth factor-like molecules by cultured arterial smooth muscle cells accompanies proliferation after arterial injury. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7311–7315. doi: 10.1073/pnas.83.19.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]