Graphical abstract
Keywords: NMR spectroscopy, Membrane-mimetic, Paramagnetic relaxation enhancement, hGHSR-1a, Ghrelin, Octanoylation
Abstract
The peptide hormone ghrelin, which is the natural ligand of the membrane-bound growth hormone secretagogue receptor (GHS-R), regulates overall body and cell growth, energy homeostasis, carbohydrate, protein and lipid metabolism and water electrolyte balance. It contains an O-acyl linked octanoyl group on Ser3 and is the only peptide known to contain such a modification. Using solution state NMR spectroscopy and ultrafiltration we found that human ghrelin binds to membrane-mimetic environments via its octanoyl group as well as the aromatic moiety of Phe4. Relaxation enhancements in a paramagnetic environment reveal that both the octanoyl group on Ser3 and the aromatic group on Phe4 are inserted deep into the hydrophobic core of phosphocholine assemblies while the remaining peptide is freely mobile in solution. In contrast, no binding was observed for des-octanoyl ghrelin. Thus, the octanoyl chain, together with the Phe4 aromatic group of ghrelin, functions as a membrane anchor. Our results are in parallel with the previous finding that a bulky hydrophobic group on Ser3 and Phe4 of ghrelin are necessary for its function and thus indicate that membrane-binding is essential for ghrelin function.
1. Introduction
The peptide hormone ghrelin, which was first found in rat and human stomachs, consists of 28 residues and contains an unusual octanoyl modification at Ser3.1–3 It was discovered using the orphan receptor strategy about 10 years ago as the natural ligand for GHS-R (growth hormone secretagogue receptor), a G protein-coupled receptor.1 Ghrelin stimulates the release of growth hormone in the pituitary gland and the hypothalamus.1,4 The secreted growth hormone regulates overall body and cell growth, energy homeostasis, carbohydrate, protein and lipid metabolism, and water electrolyte balance.1,5 Thus, ghrelin functions orexigenic and increases food intake and body weight. This highly conserved peptide hormone has been found in mammals but also in other vertebrates, such as reptiles, amphibians, birds and fish.6 Molecular features located in the N-terminal region, in particular residues 1–4 of the peptide, were found to be critical for its function.1 A bulky hydrophobic group (e.g., an octanoyl or decanoyl fatty acid) on the third amino acid has been shown to be required for maximum activity.1 A peptide corresponding to the first four residues of ghrelin (GSS(n-octanoyl)F) could activate the ghrelin receptor, but not after removing also Phe4.7–9 In human cells, ghrelin is synthesized as part of the 117 residue precursor protein preproghrelin, which is then transformed into ghrelin by posttranslational modifications including proteolysis and acylation.1,2,10 The hydroxyl group of Ser3 is n-octanoylated which makes ghrelin the first known peptide or protein to be modified with octanoic acid.1,2,10 The acyl-modifying enzyme GOAT (ghrelin-O-acyltransferase), which catalyzes the modification of ghrelin has recently been identified.11,12 Acylation of peptides and proteins with long chain fatty acids is known and studied for a long time.13–15 While the majority of such modifications employ myristic- and palmitic acid, the acylation with the medium chain fatty acid octanoic acid was unknown before the discovery of ghrelin. This hydrophobic moiety has been proposed to act as an anchor at the human growth hormone secretagogue receptor (hGHSR-1a).16,17 While myristoyl and palmitoyl modifications on peptides have been frequently found to be involved in peptide-membrane interactions, the involvement of octanoyl chains in membrane or lipid binding has not been reported so far. In fact, a molecular modeling study found an interaction of ghrelin with membranes near its C-terminus and predicted the octanoyl chain to point away from the membrane.18 Ghrelin has been shown to be unstructured in aqueous environment at low pH (1.0–3.0)10 as expected for a small linear peptide, but shows some tendency for the formation of an α-helix as confirmed by CD spectroscopy.19 A molecular modeling study of ghrelin in the presence of a biological membrane predicted a loop between residues Arg15 and Ser18 to bind to the membrane.18 The octanoyl group has been proposed to be necessary for binding to the GHS receptor rather than for binding to membranes.17,18 A recent NMR study measuring chemical shift perturbations of ghrelin in the presence of GHS-R1a in living cells found indications for an interaction basically along the whole peptide chain.17 Interestingly, these shift perturbations were not found when des-ghrelin, a peptide identical to ghrelin but lacking the acylation of Ser3, was used in the same study. The presence of an α-helix between residues Gly8 and Lys20 in the bound state was postulated based on the chemical shift index (CSI).17 Considering the role of peptide attached myristoyl and palmitoyl chains as membrane-anchors and the membrane localization of the GHS-R receptor, we carried out an investigation of ghrelin and des-n-octanoyl-ghrelin (called des-ghrelin further on) in the presence of membrane-mimetics by NMR and ultrafiltration experiments.
Generally, structural studies of small membrane-bound polypeptides are not possible by X-ray crystallography due to their inherent flexibility, but can be carried out by liquid NMR spectroscopy, provided that the system under study is below the NMR size limit to avoid excessive signal broadening. Thus, small membrane-mimetic systems (typically micelles) have to be used.20–22 For the present study we used dodecyl-phosphocholine micelles, which are frequently used as a membrane-mimetic for NMR investigations. The zwitterionic DPC structurally resembles the structural organization of biological membranes.21,22 It can well preserve the 3D structure of bound peptides21,23,24 and proteins25 as well as the catalytic activity of membrane-bound enzymes,26,27 and it is commercially available in perdeuterated form. The binding of peptides to micelles can be monitored by relaxation enhancements in a paramagnetic environment.28–31 This approach yields the orientation and immersion depth without chemical modification of the system under investigation. Here, we show that ghrelin binds to membrane-mimetic systems (micelles and small unilamellar vesicles) via insertion of its octanoyl chain and also the aromatic moiety of Phe4 into the hydrophobic environment. Most of the peptide, including the C-terminal region, is basically unaffected by the membrane environment and stays unstructured in solution. No indication of an interaction of des-ghrelin with membranes was found.
2. Material and methods
2.1. Materials
Human ghrelin [GSS(n-octanoyl)FLSPEHQRVQQRKESKKPPAKLQPR] and human des-ghrelin were purchased from EZBiolab Inc. (Carmel, IN, USA) with an approved purity (HPLC) of 98.60% and 97.62%, respectively. Perdeuterated dodecylphosphocholine (DPC-d38, also known as FOS-CHOLINE®-12-d38 ANAGRADE®) was obtained from Anatrace (Maumee, Ohio, USA). Gd(DTPA-BMA) was purified from the MRI contrast agent Omniscan™ (Nycomed) as previously described.30 All other chemicals were purchased from Sigma-Aldrich (St. Louis, USA) in the highest purity available.
2.2. NMR spectroscopy
For the NMR spectra 2.31 mg of ghrelin (MW 3370.9 g/mol) or 2.50 mg des-ghrelin (MW 3244.7 g/mol) were dissolved in 50 mM potassium phosphate buffer (pH 5.0), 100 mM DPC-d38 and D2O were added to yield a H2O/D2O-ratio of 90/10. TOCSY and NOESY spectra were acquired at 298 K on a Varian Unity INOVA 600 MHz NMR spectrometer, equipped with a 5 mm HCN triple-resonance probe and z-axis gradients. All other NMR experiments were carried out on a Bruker Avance DRX 500 MHz NMR spectrometer with z-axis gradients using a 5 mm TXI probe. To obtain paramagnetic relaxation enhancements, the samples were titrated with Gd(DTPA-BMA) (stock solution of 30 mM) to final concentrations of 0.5, 1.0, 1.5 and 2.5 mM. The proton T1 relaxation times were obtained from a series of eight 2D TOCSY spectra with a saturation recovery sequence at the beginning and recovery delays of 100, 300, 500, 700, 1000, 1500, 2000 and 3000 ms between saturation and start of the TOCSY sequence. PREs were extracted from the slope of a straight line obtained by plotting the relaxation rate against the concentration of the paramagnetic agent Gd(DTPA-BMA). Spectra were processed using nmrPipe32 and analyzed by NMRView.33 Dissociation constants of ghrelin and octanoic acid bound to DPC micelles were determined by monitoring chemical shift changes of ghrelin or octanoic acid upon the stepwise addition of a 600 mM stock solution of DPC-d38. The DPC concentration was increased from 0 to 150 mM in steps of roughly 10 mM. From the observed mean chemical shifts the dissociation constants were calculated by least square fitting as described previously.34 Using the chemical shift titration data, the mole fraction partition coefficient
| (1) |
can be obtained. In this equation XA is the mole fraction of the peptide in the lipid phase and YA is the mole fraction of the peptide in the aqueous phase35:
| (2) |
where ηL and ηW are the moles of lipid and water and ηpL and ηpW are the moles of the peptide in the lipid and aqueous phase, respectively. Replacing ηpW by ηp − ηpL, with ηp being the total peptide concentration, and solving Eq. (1) for ηpL, we obtain:
| (3) |
The observed chemical shift of a peptide signal σobs as a function of ηpL is given by
| (4) |
where and are the chemical shifts of a peptide signal in the lipid and water phase, respectively. The mole fraction partition coefficient Kp can be obtained by non-linear least square fitting of Eq. (4) after replacing ηpL by Eq. (3).
2.3. Ultrafiltration
Centrifugal filter devices with a 10 kDa cut-off (Millipore, Billerica, USA) were loaded with ghrelin or des-acyl-ghrelin dissolved in buffer (50 mM potassium phosphate, 0.02% NaN3, pH 5.0). To investigate the binding of the peptides to lipid membranes, small unilamellar vesicles (SUVs) were added to the samples to a final concentration of 2 mM. The vesicles consisted of TCDA (10,12-tricosadiynoic acid, Sigma-Aldrich) and DMPC (Dimyristoylphosphatidylcholine, Avanti Lipids) in a molar ratio of 6.5:17.36 The lipids were dissolved and mixed in chloroform. After the solvent was evaporated, deionized water was added to the dried mixture until a total lipid concentration of 2.4 mM was reached. The solution was incubated at 80 °C for 10 min, and sonicated for another 10 min. Subsequently, the sample was filtered through a 0.45 μm polyethersulfone-membrane filter (Puradisc Syringe Filter, Whatman) and then irradiated with a UV lamp (∼245 nm) for 2 min as described by Charych et al.37 Ghrelin and des-acyl-ghrelin in the absence and presence of these SUVs were centrifuged in the filter devices at 27 °C for 10 min at 7000 g. The membrane-binding peptide toxin Fst was used as a positive control.38 Analysis of the filtrate and the supernatant was carried out with ninhydrin staining of samples loaded on TLC plates. Of each sample 2 μl were loaded on the plate which was sprayed with 0.2% ninhydrin in ethanol to detect amino acids.
3. Results
3.1. NMR spectroscopy
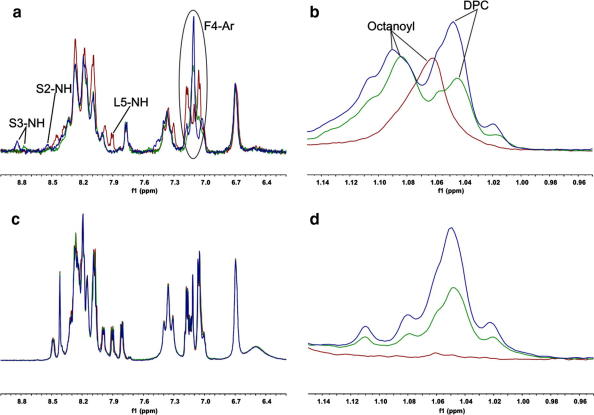
The one-dimensional 1H and two-dimensional TOCSY spectra of ghrelin in aqueous solution at pH 5.0 were quite similar to the NMR data previously reported at pH 1.010 and could therefore be assigned by comparison with these spectra. All signals were found in typical random coil regions (amide protons between 8.0 and 8.5 ppm and no methyl signals below 0.9 ppm). Des-ghrelin showed very similar resonance frequency distributions with a poor dispersion indicative of an intrinsically disordered conformation. Upon the addition of DPC-d38 several signals of ghrelin shifted significantly (see Fig. 1a and b). The largest shift was found for the amide proton of Ser3, which was shifted downfield to ∼8.9 ppm. Downfield shifts (to higher frequencies) were also found for Ser2-NH and Leu5-NH, while a series of shifts was recorded in the region containing the side chain aromatic signals of Phe4 (between 7.0 and 7.2 ppm). In the aliphatic region a chemical shift change was observed for the signal of the octanoyl alkyl chain (Fig. 1b), indicating direct interactions with the forming micelles. Aside from these signals most peaks of ghrelin retained their frequencies in the presence of the membrane-mimetic, implying that most residues of ghrelin remain in the aqueous phase. In contrast to that, the spectrum of des-ghrelin was virtually unaffected upon the addition of DPC (Fig. 1c and d). The appearing signals in the aliphatic region (Fig. 1d) were attributed to residual protonated DPC nuclei.
Figure 1.
One-dimensional 1H NMR spectra of ghrelin (a and b) and des-ghrelin (c and d), showing overlays of the aromatic and NH regions (a and c) and alkyl chain region (b and d) at 0 (red), 10 (green) and 20 mM (blue) DPC. Signals of ghrelin that shifted upon the addition of DPC are annotated.
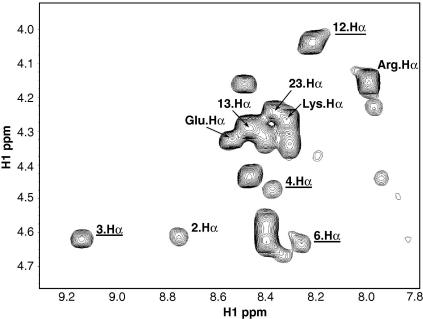
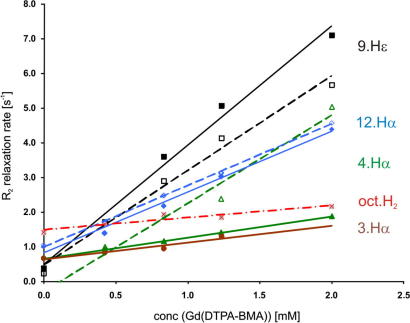
We examined the detailed mode of interaction with the micelles by determining relaxation enhancements in a paramagnetic environment. For this purpose the water soluble, inert paramagnetic agent Gd(DTPA-BMA)39 was added to the samples, resulting in an environment comparable to a paramagnetic solvent.30,39,40 Solvent exposed residues will therefore show high PRE values, while the combined PRE, integrated over the whole paramagnetic environment, decreases with 1/r3 (r being the distance to the closest paramagnetic center) for nuclei further inside the micelle.30,31 To extract PREs we used series of 2D saturation recovery TOCSY spectra obtained at different Gd(DTPA-BMA) concentrations to determine enhancements of T1 times. Only well-resolved, clearly assigned signals in the two-dimensional spectra of ghrelin and des-ghrelin were used to extract PREs (see Fig. 2). The PREs correspond to the slopes of lines obtained by least square fitting of longitudinal relaxation rates R1 as a function of the Gd(DTPA-BMA) concentration (Fig. 3). In ghrelin high PREs (>1.0) were found for signals belonging to residues Gly6, His9, Val12 and Ala23. Much lower values were recorded for Phe4 and in particular for the octanoyl side chain. The protons on C3 and C2 of the octanoyl chain had the lowest PREs. Other protons of the octanoyl chain could not be analyzed due to overlap with residual signals of DPC. Ser3 and Phe4 showed rather low PREs for the protons 3·Hα, 4·Hα and especially 4.Hδ, a proton located in the aromatic ring. The low values observed for the alkyl chain and the aromatic moiety can only be explained by their positioning deep inside the micelle. In contrast, all experimental PREs of des-ghrelin were very high, indicating free accessibility of the protons to the solvent. The PRE of 4·Hα is relatively low for ghrelin, but much higher for des-ghrelin. In order to estimate the contribution of the octanoyl moiety to the membrane-binding, we determined the dissociation constants Kd, mole fraction partition coefficients Kp and unitary Gibbs free energies of ghrelin and also of free octanoic acid bound to DPC micelles by NMR titration experiments. Differences in chemical shifts could be observed upon the addition of DPC to solutions of 1.0 mM octanoic acid or 1.14 mM ghrelin. The dissociation constants were obtained by least square fitting of observed chemical shifts as a function of DPC concentration as previously described.34 Assuming an aggregation number of 54 for DPC micelles20 and a 1:1 (ligand/micelle) complex of ghrelin and octanoic acid bound to DPC micelles, dissociation constants of 6 μM and <0.1 μM, were obtained for octanoic acid and ghrelin, respectively. Due to the inaccuracy of NMR-derived dissociation constants in case of relatively tight binding only an upper limit can be derived for ghrelin. The mole fraction partition coefficients are 8.1 × 106 and 1.7 × 106 and is −9.5 kcal/mol and −8.6 kcal/mol for ghrelin and octanoic acid, respectively.
Figure 2.
Fingerprint region of a two-dimensional TOCSY spectrum of ghrelin at 600 MHz. The most significant downfield shift is seen for Ser3. Signals which could be used for PRE determinations, because they are not overlapped and gave a correlation coefficient close to 1 in the least square fitting, are underlined.
Figure 3.
Transverse relaxation rates plotted as a function of Gd(DTPA-BMA) concentration for several signals of ghrelin (full line) and des-ghrelin (dashed line). The signal of the octanoyl chain of ghrelin is depicted by a dash-dotted line. The steeper the line the higher the PRE and thus the closer to the solvent the proton is located.
3.2. Ultrafiltration
Acylated ghrelin with a molecular weight of 3371 Da can easily pass a 10 kDa cutoff ultrafiltration membrane. After adding 2 mM TCDA-DMPC vesicles no peptide was found in the flow through solution, but only in the retentate as detected by staining with ninhydrin. With a size of 100–200 nm, these vesicles could not pass the filter membrane with pore sizes of approximately 5 nm. The des-acyl form of ghrelin on the other hand was able to pass the filter in the presence and absence of liposomes in the buffer.
4. Discussion
4.1. NMR spectroscopy
As previously reported, ghrelin has no well defined secondary structure in aqueous solution at low pH,10 which is quite typical for small linear peptides. NMR spectra of ghrelin at pH 5.0 are quite similar to the reported acidic data and thus indicated that even at moderate pH ghrelin is intrinsically disordered, at least on the NMR time scale. However, short-lived structured conformers as found by molecular modeling and CD spectroscopy18,19 cannot be excluded. The resonance frequencies of ghrelin, which are close to random coil values, were partially becoming better dispersed upon the addition of DPC, indicating interaction with this micelle-forming zwitterionic detergent. Larger shifts were found for Ser2-NH, Ser3-NH, octanoyl-aliphatics, Phe4-aromatics and Leu5-NH while most other signals were virtually unaffected by the addition of DPC. These chemical shift differences are a strong indication of binding to the micelles via the hydrophobic part of ghrelin, encompassing the octanoyl side-chain on Ser3 and the following Phe4. Due to the continuous shifting of NMR signals of ghrelin upon the addition of DPC there must be exchange between free and micelle-bound peptide. Therefore, binding cannot be considered a static process of ghrelin being attached to the micelles 100% of the time but is a dynamic, reversible process with the relative amounts of free and bound peptide being determined by the binding strength. Since the NMR signals of free and bound peptide are averaged the residence time in each of these states has to be short on the NMR time scale, that is, <10 ms.
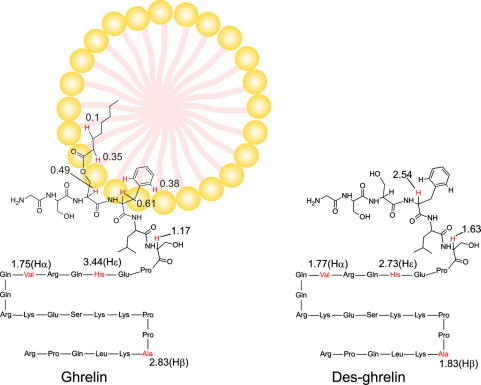
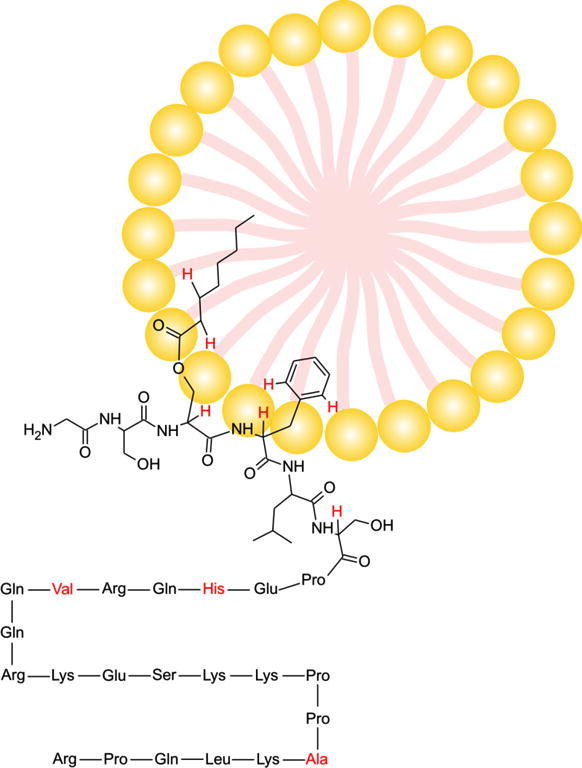
Posttranslationally introduced myristoyl and palmitoyl groups on peptides have been known to mediate peptide-membrane interactions.13 However, ghrelin is the only known peptide with an octanoyl chain and the potential role of this comparably short alkyl chain as a membrane anchor has not been shown previously. In fact, a theoretical study found that the octanoyl group does not interact with membranes, but instead points into the aqueous phase.18 However, the importance of this chain in attaching ghrelin to the micelle is seen by the absence of chemical shift changes in des-ghrelin upon the addition of DPC. Apparently, removing the octanoyl chain also abolishes micelle interactions. The nearby hydrophobic phenylalanine is not sufficient for membrane attachment although it likely strengthens the binding of ghrelin to membranes via additional hydrophobic interactions. More quantitative information about the interaction with DPC micelles was obtained through relaxation enhancements in a paramagnetic environment. Most residues of ghrelin for which unambiguous PREs could be obtained showed very high PREs indicative of their location outside the micelle, in the aqueous phase. The lowest PREs were found for the protons on C3 and C2 of the octanoyl chain which implies that they are furthest away from the paramagnetic solvent and thus most deeply buried inside the micelle. In addition, the side chain aromatic Hδ signal of Phe4 also showed a rather low PRE, which can only be explained by its insertion into the micelle. A schematic depiction of ghrelin and des-ghrelin with the experimental PREs, indicating the positioning of ghrelin relative to the micelle is shown in Figure 4. An exact positioning of the immersed nuclei within the micelle is prevented by the irregular shape of the DPC micelles with most of ghrelin outside. The undefined surface prevents the direct translation of PREs to immersion depths.28,30,31,40 On the other hand the much higher PREs found for des-ghrelin are in accordance with the absence of chemical shift differences and thus clearly corroborate that des-ghrelin does not interact with DPC micelles. The ghrelin receptor is homologous to the motilin receptor and a homology has also been found between ghrelin and motilin by amino acid sequence alignment. Therefore, ghrelin and motilin have been described as being structurally and functionally related.2,41 However, motilin forms a well-structured α-helix in membrane-mimetics,42 and thus a similarity is not seen beyond the sequence. To confirm the importance of the octanoyl chain for membrane-binding, we employed another technique as well as another membrane-mimetic. We used ultrafiltration with 10 kDa molecular weight cutoff membranes and TCDA-DMPC vesicles. Although SUVs have intrinsically a high number of defect states and therefore a different partitioning behavior compared to micelles, the results qualitatively parallel the ones from the micelle studies. The presence of the small unilamellar vesicles prevented ghrelin but not des-ghrelin from traversing the filter membrane.
Figure 4.
Schematic diagrams of ghrelin and des-ghrelin indicating their location relative to the micelles as derived from PREs. The numbers are the experimental PREs in [s−1 mM−1].
We believe that ghrelin is attached to the membrane to facilitate interactions with its specific membrane-bound target, the GHS-R receptor. Insertion into the membrane reduces the problem of finding its target from a three-dimensional (freely soluble) to a two-dimensional (gliding in the membrane) search. Previously, myristoyl and especially palmitoyl groups have been described to function as membrane-anchors. The long hydrophobic chains of palmitoyl and myristoyl groups bind relatively strongly to hydrophobic membrane interiors. The dissociation constants of octanoic acid and ghrelin bound to DPC micelles were determined by NMR chemical shift titration experiments. Assuming a binding stochiometry of 1:1 (peptide/micelle), dissociation constants of 6 μM (octanoic acid) and <0.1 μM (ghrelin) were found. However, it should be taken into consideration that more than one molecule of octanoic acid or ghrelin may bind per micelle and thus these dissociation constants should be interpreted cautiously.
Independent of the binding stochiometry, the mole fraction partition coefficients and unitary Gibbs free energies of ghrelin and octanoic acid binding to DPC micelles were determined. A relatively high value of = −9.5 kcal/mol, indicative of tight binding was found for ghrelin. Considering that the free energy for the transfer of a free C8 fatty acid from aqueous into a hydrophobic environment is just −1.5 kcal/mol43 and according to the Wimley/White scale44 a phenylalanine side chain contributes another −1.1 kcal/mol our experimental finding could indicate that additional interactions, for example, between the peptide backbone and the polar micelle surface might strengthen the binding or might be related, at least partially, to the used DPC environment. For octanoic acid we found = −8.6 kcal/mol, which is probably related to the pH of 5 which was used for our studies. The reduced pH was selected to account for the occurrence of ghrelin in often acidic environments. At this pH a larger proportion of octanoic acid is present in neutral form, for which much stronger binding has previously been reported for free fatty acids.45 Nevertheless, it is clear that binding of an octanoyl chain alone does not account for the comparatively tight binding observed for ghrelin. Thus, the much stronger binding of ghrelin apparently also depends on the insertion of the hydrophobic aromatic group of Phe4. The importance of both the Ser3 octanoyl group and Phe4 for binding to membrane-mimetics is a remarking similarity to the previously observed role of these two residues in ghrelin function.7–9 Therefore, the binding to biological membranes might be required for ghrelin function. The interaction with the GHS receptor might either depend on ghrelin binding to membrane in order to more easily find its target or the binding to the receptor could involve simultaneous attachment of ghrelin to the membrane. The necessity of two groups for membrane-binding is in accordance with similar findings of other groups, which have shown that two groups are necessary for stable attachment of various proteins and peptides to membranes.45–49
5. Conclusions
In conclusion, we have found that the peptide hormone ghrelin binds to membrane-mimetics via its octanoyl chain attached to Ser3. The binding is enhanced by additional hydrophobic interactions of the aromatic side-chain of Phe4. Elimination of the octanoyl chain is sufficient to prevent membrane attachment as shown by solution NMR and ultrafiltration. Relaxation enhancements in a paramagnetic environment were used to show the deep immersion of the octanoyl alkyl chain and aromatic ring of Phe4. Thus, the octanoyl group is not only involved in binding to GHS-R but also functions as a membrane anchor and can therefore more easily direct ghrelin to its receptor. The fundamental role of the octanoyl chain on Ser3 and the aromatic group of Phe4 in membrane-binding strikingly resembles the importance of these two residues for ghrelin function, suggesting a functional importance of ghrelin binding to membranes.
Acknowledgements
Financial support by the Austrian Science Foundation (Fonds zur Förderung der wissenschaftlichen Forschung, FWF) under project number 20020 to K.Z. is gratefully acknowledged.
Contributor Information
Jörg Großauer, Email: joerggrossauer@gmail.com.
Simone Kosol, Email: simonekosol@yahoo.de.
Evelyne Schrank, Email: evelyne.schrank@edu.uni-graz.at.
Klaus Zangger, Email: klaus.zangger@uni-graz.at.
References and notes
- 1.Kojima M., Hiroshi Hosoda., Yukari Date., Masamitsu Nakazato., Hisayuki Matsuo., Kangawa K. Nature. 1999;402:656. [Google Scholar]
- 2.Kojima M., Kangawa K. Physiol. Rev. 2005;85:495. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 3.Tschöp M., Smiley D.L., Heiman M.L. Nature. 2000;407:908. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 4.Nakazato M., Murakami N., Date Y., Kojima M., Matsuo H., Kangawa K., Matsukura S. Nature. 2001;409:194. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 5.Ariyasu H., Takaya K., Tagami T., Ogawa Y., Hosoda K., Akamizu T., Suda M., Koh T., Natsui K., Toyooka S., Shirakami G., Usui T., Shimatsu A., Doi K., Hosoda H., Kojima M., Kangawa K., Nakao K. J. Clin. Endocrinol. Metab. 2001;86:4753. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 6.Kaiya H., Miyazato M., Kangawa K., Peter R.E., Unniappan S. Comp. Biochem. Physiol. a-Mol. Integr. Physiol. 2008;149:109. doi: 10.1016/j.cbpa.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Bednarek M.A., Feighner S.D., Pong S.S., McKee K.K., Hreniuk D.L., Silva M.V., Warren V.A., Howard A.D., Van der Ploeg L.H.Y., Heck J.V. J. Med. Chem. 2000;43:4370. doi: 10.1021/jm0001727. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto M., Hosoda H., Kitajima Y., Morozumi N., Minamitake Y., Tanaka S., Matsuo H., Kojima M., Hayashi Y., Kangawa K. Biochem. Biophys. Res. Commun. 2001;287:142. doi: 10.1006/bbrc.2001.5553. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto M., Kitajima Y., Iwanami T., Hayashi Y., Tanaka S., Minamitake Y., Hosoda H., Kojima M., Matsuo H., Kangawa K. Biochem. Biophys. Res. Commun. 2001;284:655. doi: 10.1006/bbrc.2001.5014. [DOI] [PubMed] [Google Scholar]
- 10.Elipe M.V.S., Bednarek M.A., Gao Y.-D. Biopolymers. 2001;59:489. doi: 10.1002/1097-0282(200112)59:7<489::AID-BIP1054>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez J.A., Solenberg P.J., Perkins D.R., Willency J.A., Knierman M.D., Jin Z., Witcher D.R., Luo S., Onyia J.E., Hale J.E. Proc. Natl. Acad. Sci. 2008;105:6320. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J., Brown M.S., Liang G., Grishin N.V., Goldstein J.L. Cell. 2008;132:387. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Resh M.D. Biochim. Biophys. Acta. 1999;1451:1. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 14.Schlesinger M.J., Magee A.I., Schmidt M.F.G. J. Biol. Chem. 1980;255:21. [PubMed] [Google Scholar]
- 15.Schmidt M.F.G., Bracha M., Schlesinger M.J. Proc. Natl. Acad. Sci. 1979;76:1687. doi: 10.1073/pnas.76.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kukol A. Vol. 77. Elsevier Academic Press Inc.; San Diego: 2008. (Vitamins and Hormones, Ghrelin). p 1. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Pastor M., De Capua A., Alvarez C.J., Diaz-Hernandez M.D., Jimenez-Barbero J., Casanueva F.F., Pazos Y. Bioorg. Med. Chem. 2010;18:1583. doi: 10.1016/j.bmc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Beevers A.J., Kukol A. J. Biomol. Struct. Dynam. 2006;23:357. doi: 10.1080/07391102.2006.10531231. [DOI] [PubMed] [Google Scholar]
- 19.Dehlin E., Liu J., Yun S.H., Fox E., Snyder S., Gineste C., Willingham L., Geysen M., Gaylinn B.D., Sando J.J. Peptides. 2008;29:904. doi: 10.1016/j.peptides.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Göbl C., Dulle M., Hohlweg W., Grossauer J., Falsone S.F., Glatter O., Zangger K. J. Phys. Chem. B. 2010;114:4717. doi: 10.1021/jp9114089. [DOI] [PubMed] [Google Scholar]
- 21.Kallick D.A., Tessmer M.R., Watts C.R., Li C.Y. J. Magn. Reson. B. 1995;109:60. doi: 10.1006/jmrb.1995.1146. [DOI] [PubMed] [Google Scholar]
- 22.Lauterwein J., Boesch C., Brown L.R., Wuethrich K. Biochim. Biophys. Acta, Biomembranes. 1979;556:244. doi: 10.1016/0005-2736(79)90046-4. [DOI] [PubMed] [Google Scholar]
- 23.Shenkarev Z.O., Nadezhdin K.D., Lyukmanova E.N., Sobol V.A., Skjeldal L., Arseniev A.S. J. Inorg. Biochem. 2008;102:1246. doi: 10.1016/j.jinorgbio.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Shou-Liang Dong S.A.C., Ferdinando Fiorino., Uwe Bertsch., Luis Moroder., Christian Renner. Peptide Sci. 2007;88:840. [Google Scholar]
- 25.Arora A., Tamm L.K. Curr. Opin. Struct. Biol. 2001;11:540. doi: 10.1016/s0959-440x(00)00246-3. [DOI] [PubMed] [Google Scholar]
- 26.Czerski L., Sanders C.R. Anal. Biochem. 2000;284:327. doi: 10.1006/abio.2000.4720. [DOI] [PubMed] [Google Scholar]
- 27.Vinogradova O., Sönnichsen F., Sanders C.R. J. Biomol. NMR. 1998;11:381. doi: 10.1023/a:1008289624496. [DOI] [PubMed] [Google Scholar]
- 28.Franzmann M., Otzen D., Wimmer R. Chembiochem. 2009;10:2339. doi: 10.1002/cbic.200900347. [DOI] [PubMed] [Google Scholar]
- 29.Kosol S., Zangger K. J. Struct. Biol. 2010;170:172. doi: 10.1016/j.jsb.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Respondek M., Madl T., Göbl C., Golser R., Zangger K. J. Am. Chem. Soc. 2007;129:5228. doi: 10.1021/ja069004f. [DOI] [PubMed] [Google Scholar]
- 31.Zangger K., Respondek M., Göbl C., Hohlweg W., Rasmussen K., Grampp G., Madl T. J. Phys. Chem. B. 2009;113:4400. doi: 10.1021/jp808501x. [DOI] [PubMed] [Google Scholar]
- 32.Delaglio F., Grzesiek S., Vuister G.W., Zhu G., Pfeifer J., Bax A. J. Biomol. NMR. 1995;6:277. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 33.Johnson B.A., Blevins R.A. J. Biomol. NMR. 1994;4:603. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- 34.Oettl K., Greilberger J., Zangger K., Haslinger E., Reibnegger G., Jürgens G. Biochem. Pharmacol. 2001;62:241. doi: 10.1016/s0006-2952(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 35.van Dael H., Ceuterickx P. Chem. Phys. Lipids. 1984;35:171. doi: 10.1016/0009-3084(84)90023-9. [DOI] [PubMed] [Google Scholar]
- 36.Kolusheva S., Shahal T., Jelinek R. Biochemistry. 2000;39:15851. doi: 10.1021/bi000570b. [DOI] [PubMed] [Google Scholar]
- 37.Charych D., Cheng Q., Reichert A., Kuziemko G., Stroh M., Nagy J.O., Spevak W., Stevens R.C. Chem. Biol. 1996;3:113. doi: 10.1016/s1074-5521(96)90287-2. [DOI] [PubMed] [Google Scholar]
- 38.Göbl, C.; Kosol, S.; Rückert, H. M.; Zangger, K. Biochemistry, in press. [DOI] [PMC free article] [PubMed]
- 39.Pintacuda G., Otting G. J. Am. Chem. Soc. 2002;124:372. doi: 10.1021/ja016985h. [DOI] [PubMed] [Google Scholar]
- 40.Madl T., Bermel W., Zangger K. Angew. Chem., Int. Ed. 2009;48:8259. doi: 10.1002/anie.200902561. [DOI] [PubMed] [Google Scholar]
- 41.Asakawa A., Inui A., Kaga T., Yuzuriha H., Nagata T., Ueno N., Makino S., Fujimiya M., Niijima A., Fujino M.A., Kasuga M. Gastroenterology. 2001;120:337. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 42.Jarvet J., Zdunek J., Damberg P., Graslund A. Biochemistry. 1997;36:8153. doi: 10.1021/bi970193b. [DOI] [PubMed] [Google Scholar]
- 43.Tanford C. John Wiley & Sons; New York: 1980. The Hydrophobic Effect: Formation of Micelles and Biological Membranes. [Google Scholar]
- 44.Wimley W.C., White S.H. Nat. Struct. Biol. 1996;3:842. doi: 10.1038/nsb1096-842. [DOI] [PubMed] [Google Scholar]
- 45.Peitzsch R.M., McLaughlin S. Biochemistry. 1993;32:10436. doi: 10.1021/bi00090a020. [DOI] [PubMed] [Google Scholar]
- 46.Brunsveld L., Waldmann H., Huster D. Biochim. Biophys. Acta. 2009;1788:273. doi: 10.1016/j.bbamem.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Gorfe A.A., Babakhani A., McCammon J.A. J. Am. Chem. Soc. 2007;129:12280. doi: 10.1021/ja073949v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shahinian S., Silvius J.R. Biochemistry. 1995;34:3813. doi: 10.1021/bi00011a039. [DOI] [PubMed] [Google Scholar]
- 49.Vogel A., Reuther G., Weise K., Triola G., Nikolaus J., Tan K.T., Nowak C., Herrmann A., Waldmann H., Winter R., Huster D. Angew. Chem., Int. Ed. 2009;48:8784. doi: 10.1002/anie.200903396. [DOI] [PubMed] [Google Scholar]