Abstract
The uptake and expression of extracellular DNA has been established as a mechanism for horizontal transfer of genes between bacterial species. Such transfer can support acquisition of advantageous elements, including determinants that affect the interactions between infectious organisms and their hosts. Here we show that erythrocyte-stage Plasmodium falciparum malaria parasites spontaneously take up DNA from the host cell cytoplasm into their nuclei. We have exploited this finding to produce levels of reporter expression in P.falciparum that are substantially improved over those obtained by electroporation protocols currently used to transfect malaria parasites. Parasites were transformed to a drug-resistant state when placed into cell culture with erythrocytes containing a plasmid encoding the human dihydrofolate reductase sequence. The findings reported here suggest that the malaria genome may be continually exposed to exogenous DNA from residual nuclear material in host erythrocytes.
INTRODUCTION
The uptake and incorporation of extracellular DNA by bacteria were demonstrated in the classical studies of Avery et al. (1), who showed that non-virulent Streptococcus pneumoniae can be transformed through the incorporation of DNA from a heat-killed virulent bacterial strain. This phenomenon of ‘natural transformation’ has been considered a characteristic of many prokaryotic organisms (2,3) and the molecular mechanisms by which bacteria take up and express exogenous DNA have been studied extensively (4). Eukaryotic cells have also been observed to spontaneously take up and express exogenous DNA by mechanisms involving endocytosis (5,6); however, their genetic modification in the laboratory has invariably required manipulation of the cells using a variety of experimental methods before efficient rates of transfection and transformation can be achieved. These methods include introduction of DNA across the cell membrane by the use of diethylaminoethyl-dextran (7), calcium phosphate (8), electroporation (9), liposome-fusion (10), microinjection (11), cation-liposomes (12) and polyamidoamine dendrimers (13). Once introduced into the cytoplasm, the transfected DNA localizes into small endosome-like vesicles and then enters the nucleus, from which it is expressed (14–17).
Erythrocyte-stage Plasmodium falciparum malaria parasites have been transfected by electroporation (18) and polyamidoamine dendrimers (19). During the erythrocyte stage, the parasite is surrounded by a parasitophorous vacuolar membrane while it grows and develops within the host cell. Transfection of these parasites requires that exogenous DNA cross three membranes to enter the cytoplasm of the parasite: the erythrocyte plasma membrane, the parasitophorous vacuolar membrane and the parasite plasma membrane. Whether the experimental process of transfection actually enables the DNA to move across these multiple membrane layers or instead provides the DNA with access to a molecular trafficking pathway within the infected cell has not been determined.
Here we show that P.falciparum parasites can spontaneously take up DNA from the cytoplasm of host erythrocytes. This allows transfection and production of reporter signals at substantially higher levels than direct methods of electroporation and has enabled transformation with a human gene sequence that confers drug resistance. The natural ability of malaria parasites to take up and express extracellular DNA offers avenues of investigation into parasite trafficking of molecules and has implications for possible horizontal transfer of host DNA into the genomes of malaria parasites.
MATERIALS AND METHODS
Plasmid constructs and DNA preparation
The plasmids pHLH-1 and pHD22Y were propagated in XL10-Gold competent cells (Stratagene) and prepared using Qiagen maxi prep columns. pHLH-1 includes a luciferase open reading frame sequence (luc) driven by the P.falciparum hrp3 promoter (18); pHD22Y uses this same plasmid construction to express the human dihydrofolate reductase sequence (dhfr) instead of luc (20). Linearized pHLH-1 was obtained by restriction digestion with BamHI and SmaI followed by dialysis using Centricon 100 centrifugal filter units (Millipore) and verification by gel electrophoresis.
Parasite cultivation and luciferase assays
Plasmodium falciparum clones 3D7 and Dd2 were cultivated according to standard procedures (21,22) at 5% hematocrit in RPMI 1640 supplemented with 0.5% Albumax I (Life Technologies), 0.25% sodium bicarbonate and 0.01 mg/ml gentamicin. Luciferase expression assays were performed as described previously (20). Parasites transformed with pHD22Y were selected in media containing 5 nM WR99210 (20). Resistant parasites appeared in culture between 16 and 21 days after application of the drug.
Parasite transfection by direct electroporation
Transfection was performed as described previously (20). Briefly, cultures of ring-stage parasites at 5% parasitemia and 5% hematocrit were used. Cells from 0.5 ml of culture were washed once with 5 ml of incomplete cytomix (18), then combined with 50 µg of plasmid DNA to a total volume of 400 µl. The cells were transferred to a 0.2-cm cuvette and electroporated using a Bio-Rad Gene Pulser and conditions of 0.31 kV and 960 µF. Electroporated cells were washed once with complete media, then placed in a 5-ml culture at 5% hematocrit under standard conditions.
Parasite transfection by invasion of DNA-loaded erythrocytes
Stocks of erythrocytes were cleared of leukocytes by passage through a Sepacell R-500 column (Baxter Health Care). Erythrocytes were then washed three times with RPMI 1640 (Life Technologies), resuspended to 50% hematocrit and stored at 4°C. For plasmid DNA loading, 300 µl of processed erythrocytes were washed once with 5 ml of incomplete cytomix (18), then combined with 10–50 µg of either circular or linearized plasmid DNA to a total volume of 400 µl. The cells were transferred to a 0.2-cm cuvette, chilled on ice and electroporated using a Bio-Rad Gene Pulser and conditions of 0.31 kV and 960 µF. DNA-loaded erythrocytes were then washed with culture media and used immediately or stored in media at 4°C up to 48 h before use. Transfection was achieved by inoculating 4.5 ml of culture media containing DNA-loaded erythrocytes at 5% hematocrit with 0.5 ml of a standard parasite culture.
DNA extraction, plasmid rescue and Southern blot analysis
Genomic DNA was extracted from parasites (23) and Southern blotting and plasmid rescue experiments were performed as described previously (20). To ensure replication of plasmid DNA by parasites and that plasmid DNA detected on Southern blots was from parasites and not from DNA-loaded erythrocytes, transformed parasites were cultivated for 90 days under drug pressure in erythrocytes not loaded with plasmid prior to DNA extraction.
RESULTS
Uptake and expression of plasmid DNA by P.falciparum intraerythrocytic-stage parasites
The work in this report originated from a control experiment in which P.falciparum parasites were tested for luciferase activity after being allowed to invade human erythrocytes previously loaded with the plasmid pHLH-1. Levels of luciferase activity were detected that were proportional to the amount of DNA (5–100 µg) loaded into the erythrocytes. Expression was eliminated by treatment of parasites with 10 µg/ml cycloheximide (data not shown). Luciferase production from parasites that had been allowed to invade cells loaded with pHLH-1 was compared with that from parasitized erythrocytes directly electroporated in the presence of pHLH-1 DNA. Using equivalent amounts of DNA and initial numbers of parasites, we observed that parasite invasion of DNA-loaded cells consistently gave significantly higher expression levels than direct electroporation of parasitized erythrocytes (Fig. 1).
Figure 1.
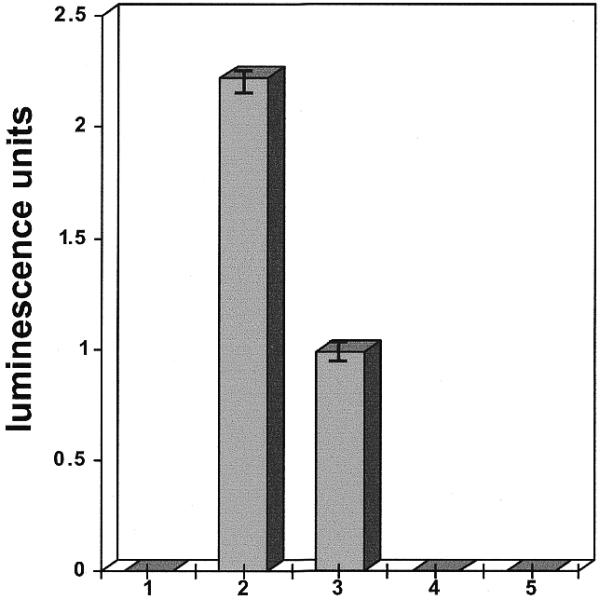
Luciferase expression levels from P.falciparum-infected erythrocytes in transfection experiments. The plasmid pHLH-1, containing the hrp3 promoter controlling a luciferase expression gene, was used in all experiments. Experiments were performed in triplicate; standard deviations are indicated by vertical bars. Luciferase levels were measured 72 h after transfection. 1, parasites cultivated without DNA; 2, parasites cultivated in red cells loaded with 50 µg supercoiled plasmid DNA; 3, parasites cultivated after electroporation in the presence of 50 µg supercoiled plasmid DNA; 4, parasites cultivated in media containing 50 µg plasmid DNA without electroporation; 5, uninfected erythrocytes loaded with 50 µg supercoiled plasmid DNA.
Microscopic observation showed that direct electroporation of parasitized erythrocytes caused a 50–70% reduction in viable forms, whereas the use of cells loaded with pHLH-1 produced no detectable effect on parasite viability. When luciferase measurements were normalized to account for changes in parasitemia levels, both methods of transfection were found to yield similar average levels of expression per parasite. Parasites showed no luciferase expression in control infections of erythrocytes not loaded with plasmid DNA. Inclusion of the plasmid DNA in the culture medium rather than in loaded erythrocytes and DNA-loaded erythrocytes not infected with parasites also yielded no reporter activity.
Uptake and expression of linear DNA
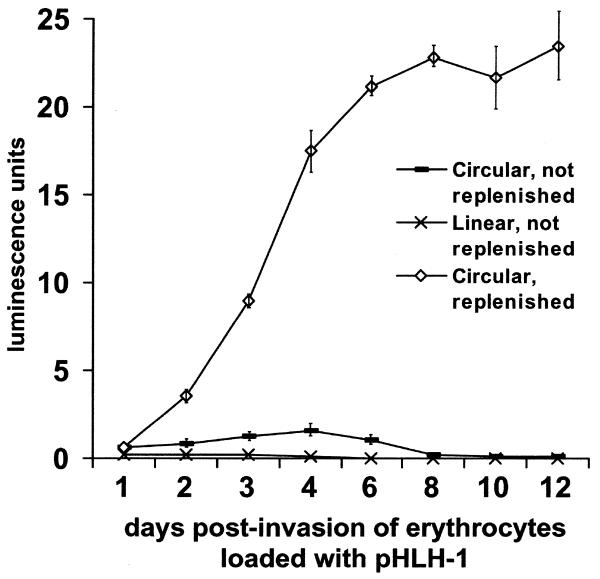
To compare the efficiency of expression from supercoiled versus linear forms of DNA, we placed P.falciparum parasites in culture with erythrocytes that had been previously loaded with equivalent amounts of circular pHLH-1 or its linearized form as a BamHI/SmaI-restricted fragment. One day after initiation of the experiment, parasites expressing luc from linear DNA yielded a luciferase signal ∼50% of that from parasites expressing luc from supercoiled DNA (Fig. 2). The signal from parasites that had taken up linear DNA increased slightly on the second and third days and subsequently decreased to near zero on the fourth day. In contrast, the signal from parasites expressing luc from circular DNA rose markedly through the first 4 days and persisted for more than a week.
Figure 2.
Luciferase expression levels from infected erythrocytes after transfection and uptake of plasmid DNA. Parasites were initially allowed to infect erythrocytes that had been loaded with 10 µg of either linearized or circular, supercoiled pHLH-1. Subsequently, one half of the total culture volume was harvested daily for luciferase measurements and replaced either with an equal volume of culture media containing uninfected erythrocytes that had not been loaded with plasmid DNA (linear and circular, not replenished) or with erythrocytes that had been loaded with circular plasmid DNA (circular, replenished). Parasitemias were maintained at a constant level of 5–7%. Experiments were performed in triplicate; standard deviations are indicated by vertical bars.
Maintenance of luciferase expression in culture by continuous supplementation with DNA-loaded erythrocytes
Expansion of culture volume and corresponding depletion of DNA-loaded erythrocytes from transfected cultures correlated with a decline in luc expression. We therefore performed experiments in which cultures were continuously maintained with erythrocytes loaded with pHLH-1. After four generations (8 days) of parasite multiplication, samples from these cultures showed levels of luc expression up to 10-fold higher than the maximum achieved from cultures with a single addition of DNA-loaded erythrocytes (Fig. 2) and up to 20-fold higher than that achieved by electroporating infected erythrocytes. This high level of reporter expression could be maintained indefinitely and was stable as long as the culture was supplemented with erythrocytes loaded with pHLH-1.
Acquisition of drug resistance by uptake of a human dhfr-expressing plasmid
Parasite uptake and transient expression of DNA from host erythrocytes suggested that this phenomenon could serve in experimental transformation to produce stable changes in phenotype. This possibility was investigated by cultivating parasites in erythrocytes loaded with pHD22Y plasmid DNA, which carries the human dhfr sequence and can confer resistance to such drugs as methotrexate and the antimalarial drugs pyrimethamine and WR99210 (20). Application of 5 nM WR99210 drug pressure to these cultures readily selected parasites fully resistant to the drug. Southern blots of DNA extracted from transformed parasites and plasmid rescue experiments (18,20) demonstrated that the population of transformed parasites carried episomes (Fig. 3). To ensure that drug resistance resulted from transfection with the plasmid and not from a mutation elsewhere in the genome, parasite cultures were grown for 90 days without drug. This resulted in loss of the plasmid and a corresponding reversion to WR99210 sensitivity (Fig. 3).
Figure 3.
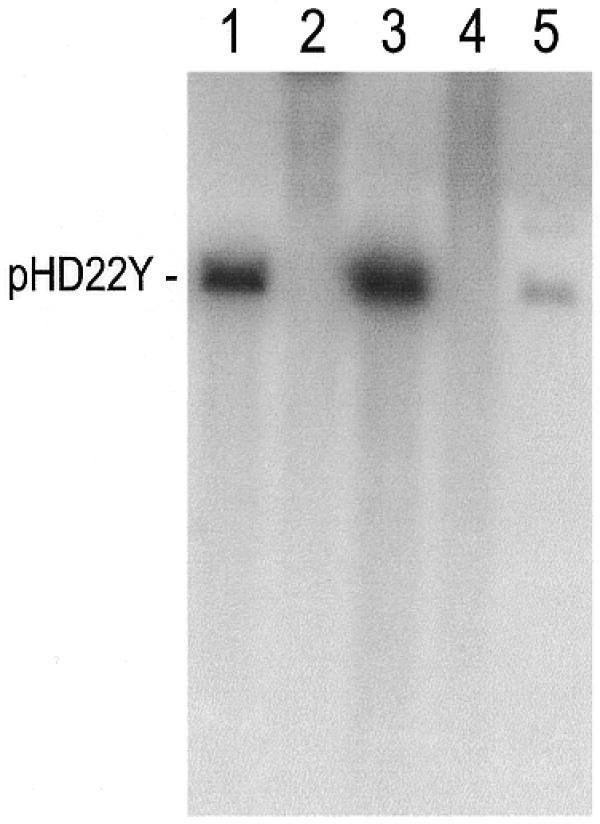
Southern blot showing the presence of plasmid DNA in parasites transformed by uptake and expression of the plasmid pHD22Y from DNA-loaded erythrocytes. The DNA in each lane was digested with EcoRI prior to gel electrophoresis and Southern blotting. The blot was probed with a pBluescript fragment labeled with [32P]dCTP. 1, pHD22Y linearized by digestion with EcoRI; 2, genomic DNA extracted from untransformed parasites sensitive to the antimalarial drug WR99210; 3, genomic DNA extracted from drug resistant parasites transformed by uptake of pHD22Y from DNA-loaded erythrocytes (transformed parasites were cultivated under drug pressure for 90 days in unloaded erythrocytes prior to DNA extraction); 4, genomic DNA extracted from transformed parasites cultivated for 90 days without drug pressure, demonstrating loss of the plasmid. Parasites that had lost the plasmid were once again sensitive to WR99210; 5, linearized plasmid DNA rescued from WR99210-resistant parasites.
DISCUSSION
Successful transfection of erythrocyte-stage parasites requires that three membranes be crossed by the plasmid DNA prior to its trafficking into the parasite nucleus. Two protocols for transfection have previously been reported: direct electroporation of infected cells (18) and use of polyamidoamine dendrimers (19). How these procedures successfully deliver plasmid DNA through these multiple membrane layers has been unclear. Our observations that parasites spontaneously import DNA across the parasitophorous vacuolar membrane from the erythrocyte cytoplasm and then transport it into the parasite nucleus may explain the reported success of these different methods. Both procedures may simply introduce DNA into the erythrocyte cytoplasm from which it is taken up by the malaria parasite.
Transfection of parasites by exposure to DNA-loaded erythrocytes has several advantages over previously described protocols. First, because the transfected parasites are not exposed to the trauma of electroporation, their viability is greatly improved, leading to higher levels of reporter gene expression and more rapid selection of stably transformed parasites. Second, transgene expression levels can be increased up to 20-fold by continuous supplementation of cultures with DNA-loaded erythrocytes. Third, growth of parasites in the presence of DNA-loaded erythrocytes allows the continuous expression of transgenes without the need for selectable markers. This is particularly valuable considering that the instability of P.falciparum DNA in Escherichia coli has greatly hindered the ability to make large constructs containing both transgene expression cassettes and selectable markers. Because of these advantages, we now routinely employ this approach to transfection over previous protocols from our laboratory (18,20). Improvements in the DNA loading of erythrocytes or its stabilization during uptake and transport may allow further increases in the efficiency of P.falciparum transformation.
The uptake and expression of plasmid DNA described here may provide insights into the import and trafficking of host molecules by malaria parasites. Plasmodium falciparum parasites carry out extensive endocytosis of hemoglobin and other constituents of the erythrocyte cytoplasm during development from ring stage to schizont. DNA residing in the host erythrocyte cytoplasm thus could be incorporated along with these other molecules and transported into the parasite cytoplasm from where it is targeted to the nucleus. This can be compared to the transport of transfected DNA in mammalian cells, which moves through an endosomal/lysosomal pathway to the nucleus (14–17). It is tempting to speculate that the parasite uptake pathway may be related to mechanisms involved in the high rates of ingestion of host cell hemoglobin during the trophozoite and schizont phases of intraerythrocytic growth. Another possibility is that DNA uptake may be related to hijacking of host cell components for essential metabolic functions by the parasite. We note that malaria parasites have been reported to import and utilize host cell δ-aminolevulinate dehydrase and perhaps other enzymes from the heme biosynthesis pathway (24).
The spontaneous uptake and expression of exogenous DNA by malaria parasites in culture suggests that parasites have the ability to actively take up DNA during a natural infection. Malaria parasites may often be exposed to human DNA even though erythrocytes typically extrude their nuclei early in development. Erythrocytes carrying residual nuclear material (Howell–Jolly bodies) occur in the circulation of normal individuals and are prominent in individuals with thalassemia disorders or sickle cell anemia (25). The prevalence of these conditions in regions with high levels of malaria transmission (26,27) might provide opportunity for host DNA uptake. Although initial data from genome sequencing has identified several sequences that are unusual for unicellular organisms, leading to the suggestion that they may have been acquired by horizontal transfer sometime during the evolutionary lineage of the parasite’s genome (28), it is not clear whether there has been natural incorporation of human DNA into the P.falciparum genome. It remains possible that protective mechanisms may act to shield the parasite genome from insertion of foreign sequences. Analysis of the increasing amounts of data becoming available from chromosome sequencing projects should help to further evaluate these possibilities.
Acknowledgments
ACKNOWLEDGEMENTS
The authors thank Drs Jane M.-R. Carlton and John C. Wootton for discussions, and Brenda Rae Marshall for editing of the manuscript.
References
- 1.Avery O.T., McLeod,C.M. and McCarty,M. (1944) Studies on the chemical nature of the substance inducing transformation of pneumococcal types. J. Exp. Med., 79, 137–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redfield R.J., Schrag,M.R. and Dean,A.M. (1997) The evolution of bacterial transformation: sex with poor relations. Genetics, 146, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vellai T. and Vida,G. (1999) The origin of eukaryotes: the difference between prokaryotic and eukaryotic cells. Proc. R. Soc. Lond. B. Biol. Sci., 266, 1571–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmen R. and Hellingwerf,K.J. (1997) Uptake and processing of DNA by Acinetobacter calcoaceticus—a review. Gene, 192, 179–190. [DOI] [PubMed] [Google Scholar]
- 5.de Jonge A.J. and Bootsma,D. (1984) Chromosome and DNA-mediated gene transfer in cultured mammalian cells. Int. Rev. Cytol., 92, 132–158. [PubMed] [Google Scholar]
- 6.Bennett R.M. (1993) As nature intended? The uptake of DNA and oligonucleotides by eukaryotic cells. Antisense Res. Dev., 3, 235–241. [DOI] [PubMed] [Google Scholar]
- 7.McCutchan J.H. and Pagano,J.S. (1968) Enhancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J. Natl Cancer Inst., 41, 351–357. [PubMed] [Google Scholar]
- 8.Loyter A., Scangos,G., Juricek,D., Keene,D. and Ruddle,F.H. (1982) Mechanisms of DNA entry into mammalian cells. II. Phagocytosis of calcium phosphate DNA co-precipitate visualized by electron microscopy. Exp. Cell Res., 139, 223–234. [DOI] [PubMed] [Google Scholar]
- 9.Potter H., Weir,L. and Leder,P. (1984) Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc. Natl Acad. Sci. USA, 81, 7161–7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cudd A., Labbe,H., Gervais,M. and Nicolau,C. (1984) Liposomes injected intravenously into mice associate with liver mitochondria. Biochim. Biophys. Acta, 774, 169–180. [DOI] [PubMed] [Google Scholar]
- 11.Capecchi M.R. (1980) High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell, 22, 479–488. [DOI] [PubMed] [Google Scholar]
- 12.Felgner P.L., Gadek,T.R., Holm,M., Roman,R., Chan,H.W., Wenz,M., Northrop,J.P., Ringold,G.M. and Danielsen,M. (1987) Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl Acad. Sci. USA, 84, 7413–7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang M.X., Redemann,C.T. and Szoka,F.C.J. (1996) In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug. Chem., 7, 703–714. [DOI] [PubMed] [Google Scholar]
- 14.Legendre J.Y. and Szoka,F.C.J. (1992) Delivery of plasmid DNA into mammalian cell lines using pH-sensitive liposomes: comparison with cationic liposomes. Pharm. Res., 9, 1235–1242. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe Y., Nomoto,H., Takezawa,R., Miyoshi,N. and Akaike,T. (1994) Highly efficient transfection into primary cultured mouse hepatocytes by use of cation-liposomes: an application for immunization. J. Biochem., 116, 1220–1226. [DOI] [PubMed] [Google Scholar]
- 16.Escriou V., Ciolina,C., Helbling-Leclerc,A., Wils,P. and Scherman,D. (1998) Cationic lipid-mediated gene transfer: analysis of cellular uptake and nuclear import of plasmid DNA. Cell Biol. Toxicol., 14, 95–104. [DOI] [PubMed] [Google Scholar]
- 17.Coonrod A., Li,F.Q. and Horwitz,M. (1997) On the mechanism of DNA transfection: efficient gene transfer without viruses. Gene Ther., 4, 1313–1321. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y., Sifri,C.D., Lei,H.-H., Su,X. and Wellems,T.E. (1995) Transfection of Plasmodium falciparum within human red blood cells. Proc. Natl Acad. Sci. USA, 92, 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mamoun C.B., Truong,R., Gluzman,I., Akopyants,N.S., Oksman,A. and Goldberg,D.E. (1999) Transfer of genes into Plasmodium falciparum by polyamidoamine dendrimers. Mol. Biochem. Parasitol., 103, 117–121. [DOI] [PubMed] [Google Scholar]
- 20.Fidock D.A. and Wellems,T.E. (1997) Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc. Natl Acad. Sci. USA, 94, 10931–10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haynes J.D., Diggs,C.L., Hines,F.A. and Desjardins,R.E. (1976) Culture of human malaria parasites Plasmodium falciparum. Nature, 263, 767–769. [DOI] [PubMed] [Google Scholar]
- 22.Trager W. and Jensen,J.B. (1976) Human malaria parasites in continuous culture. Science, 193, 673–675. [DOI] [PubMed] [Google Scholar]
- 23.Peterson D.S., Milhous,W.K. and Wellems,T.E. (1990) Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc. Natl Acad. Sci. USA, 87, 3018–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonday Z.Q., Taketani,S., Gupta,P.D. and Padmanaban,G. (1997) Heme biosynthesis by the malarial parasite. Import of delta-aminolevulinate dehydrase from the host red cell. J. Biol. Chem., 272, 21839–21846. [DOI] [PubMed] [Google Scholar]
- 25.Harmening D.M. (1992) Clinical Hematology and Fundamentals of Hemostasis. F.A. Davis Company, Philadelphia, PA.
- 26.Allison A.C. (1954) Protection afforded by sickle-cell trait against subtertian malarial infection. Br. Med. J., 1, 290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weatherall D.J. (1997) Thalassaemia and malaria, revisited. Ann. Trop. Med. Parasitol., 91, 885–890. [DOI] [PubMed] [Google Scholar]
- 28.Gardner M.J., Tettelin,H., Carucci,D.J., Cummings,L.M., Aravind,L., Koonin,E.V., Shallom,S., Mason,T., Yu,K., Fujii,C., Pederson,J., Shen,K., Jing,J., Aston,C., Lai,Z., Schwartz,D.C., Pertea,M., Salzberg,S., Zhou,L., Sutton,G.G., Clayton,R., White,O., Smith,H.O., Fraser,C.M., Adams,M.D., Venter,J.C. and Hoffman,S.L. (1998) Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum. Science, 282, 1126–1132. [DOI] [PubMed] [Google Scholar]