Abstract
Benign metastasizing leiomyomas are rare tumors, which are typically found in the lungs and, thus, might be confused with leiomyosarcomas. Further, it is not clear whether the term “benign metastasizing leiomyoma” is a misnomer and whether these lesions actually represent low-grade malignant tumors that have a low proliferation index. Micro-RNAs (miRNAs) are small noncoding RNAs, which repress translation. The altered expression of miRNAs has been strongly correlated with the malignant phenotype. In this study, the histologic features, Ki67 index, p53, bcl-2, and miRNA expression were studied in 15 leiomyosarcomas (11 primary lesions and 4 metastases), 8 leiomyomas, and 10 cases of benign metastasizing leiomyoma (9 pulmonary lesions and 1 primary uterine lesion). As expected, the Ki67 index for the benign metastasizing leiomyomas was equivalent to that for the leiomyomas and statistically less than that for the leiomyosarcomas. The mean index was 2.3% (range: 0.9% to 8.8%) for the leiomyomas and 3.4% (range: 0.7% to 8.1%) for the benign metastasizing leiomyomas compared with 28.6% (range: 14.4% to 62.0%) for the leiomyosarcomas (P < 0.025). The miRNA, miR-221, which has been associated with a variety of cancers, was detected by in situ hybridization in 13/15 leiomyosarcomas, 0/8 leiomyomas, and 0/10 benign metastasizing leiomyomas. In conclusion, benign metastasizing leiomyomas are indeed most likely benign lesions, and up-regulation of miR-221 expression is an accurate way to differentiate leiomyosarcoma from benign metastasizing leiomyoma.
Keywords: micro-RNA, in situ hybridization, LNA probes, benign metastasizing leiomyoma
Benign metastasizing leiomyomas represent a rare disease, which is typically diagnosed in younger women. It is characterized by the appearance of relatively small lung nodules many years after the removal of a histologically unremarkable uterus.1–10 In one study, the mean time period from the hysterectomy to the appearance of the lung lesions was 15 years.5 The pulmonary nodules show an unequivocal smooth muscle phenotype with a low proliferation index, as expected from their slow growth; the usual estrogen/progesterone receptor positivity suggests their uterine origin.5 Several different hypotheses have been put forth to explain the phenomenon of benign metastasizing leiomyomas. Some investigators have postulated that these lesions actually represent low-grade leiomyosarcomas.3–5 Another theory is that the pulmonary lesions represent the intravascular spread of a benign uterine leiomyoma. This theory is equivalent to a theory of the origin of endometriosis at distant sites such as the lung; it also postulates that benign metastasizing leiomyomas and intravascular leiomyomatosis are related entities.2,6 Another proposal is that the extrauterine lesions of benign metastasizing leiomyomas represent independent and multifocal smooth muscle proliferations.1 The latter theory has been disputed by several groups that have shown, using methods such as variable lengths of the polymorphic CGA repeat and comparative genomic-hybridization and X-chromosome-inactivation analyses, that the pulmonary and uterine lesions in benign metastasizing leiomyomas are indeed clonal.8,9 Further, Nucci et al7 recently showed a chromosomal aberration (19q and 22q terminal deletion), which seems to be characteristic of benign metastasizing leiomyomas. In sum, it now seems most likely that benign metastasizing leiomyomas represent clonal proliferations of lesions that are either benign or represent low-grade malignant processes.
Micro-RNAs (miRNAs) are small, noncoding RNAs of around 18 to 25 nucleotides processed from larger hairpin precursors. MiRNAs help regulate cell processes by suppressing translation after binding to conserved sequences within the 3′ UTR of mRNA. Although this epigenetic event, much like the hypermethylation of gene promoters, might be important in embryogenesis and normal cell differentiation as has been established in some animal models,11 most of the attention with miRNAs has focused on their association with oncogenesis. As might be expected, documented targets of miRNAs include several important antioncogenes such as retinoblastoma 1 and the transforming growth factor, beta receptor II.12 Several hundred miRNAs have been identified; it is anticipated that many more will be discovered if they do indeed have a widespread role in regulating the activity of many diverse mRNAs.13 Altered expression of several miRNAs, including miR-15, miR-16, miR-221, and miR-376a, have been associated with a wide variety of malignant tumors.13–17
Most studies on miRNA expression have used Northern blotting, gene expression arrays, or reverse transcription-polymerase chain reaction; very few have used in situ hybridization analysis.13–17 The latter offers the advantage of localizing the miRNA to specific cell type(s). However, in situ hybridization detection is hindered when the probe/target complex is only around 20 bp,18 as is the case for miRNAs. Despite this, because the miRNAs can be markedly up-regulated with as many as thousands of copies per cell, in situ hybridization could detect these small molecules under the correct stringency conditions.18 The purpose of this study was to analyze a series of smooth muscle tumors of the uterus and lung, to determine the miRNA expression patterns and to correlate these with the histologic findings, immunohistochemical results, and clinical follow-up.
MATERIALS AND METHODS
Case Selection
All tissues were fixed in 10% buffered formalin. The 9 cases of benign metastasizing leiomyoma were obtained from the consult files of 2 of us (G.J.N. and S.S.). In each case, the pulmonary lesion was available for study; in one of these cases, the uterine lesion was also available for analysis for a total of 10 tissues. The 8 uterine leiomyoma were obtained consecutively from the surgical pathology files at the Ohio State University Medical Center, Department of Pathology. Similarly, the 11 cases of leiomyosarcoma of the uterus were consecutive cases; in 4 of these cases, the metastatic lesion (3 lung, 1 brain) was also available for analysis. In each case, strict adherence to the Institutional Review Board guidelines were followed. We were blinded to case identity when the miRNA in situ hybridization analysis was performed and analyzed. Routine histologic analysis and confirmation of the histologic diagnosis was performed by one of us (G.J.N.).
Immunohistochemistry
Immunohistochemical testing was performed using the Ventana Benchmark System (Ventana Medical Systems, Tuscon, AZ), according to the manufacturer’s recommendations. Expression of Ki67, p53, and bcl-2 was carried out, each at 1:100 dilution with the antigen-retrieval CC1. Each tissue was also tested (and found positive) with the actin clone HHF-35, to document the smooth muscle nature of the proliferation.
In Situ Hybridization
Our protocol for the detection of RNAs by in situ hybridization has been published earlier.18 In brief, the tissue was deparaffinized, proteased (30 min in 2 mg/mL of pepsin), washed in sterile water, then in 100% ethanol, and air-dried. The sequence of the probes (Exiqon, Denmark) that contained the lock nucleic acid (LNA)-modified bases, with digoxigenin conjugated to the 5′ end, were (miR-221)—(5′) AGCTACATTGTCTGCTGGGTTTC; (miR-301)—(5′) CAGTGCAATAGTATTGTCAAAGC; (miR-376a)—(5′) ACGTGGATTTTCCTCTATGAT. A scrambled probe (a sequence of the lock nucleic acid-modified nucleotides labeled with digoxigenin, which did not correspond with any known miRNA sequence) was also used: (5′) TTCACAATGCGTTATCGGATGT. Given the small size of the miRNA probe, and the concomitant reduced melting temperature of the annealed cDNA complex, hybridization was done at 37°C overnight and followed by a low stringency wash in 0.2 × standard sodium citrate and 2% bovine serum albumin at 4°C for 10 minutes. The probe-target complex was seen owing to the action of alkaline phosphatase on chromogen nitroblue tetrazolium and bromochloroindolyl phosphate. Nuclear fast red served as the counterstain. The negative controls included omission of the probe, use of the probe on tissues known to be negative for the miRNA of interest by real-time solution phase reverse transcription-polymerase chain reaction, the scrambled probe, and the internal controls of cell types such as adipocytes and nerve bundles, which typically do not contain amplified miRNAs such as miR-221.
RESULTS
Thirty-three tissues were available for study. These included 8 uterine leiomyomas, 11 uterine leiomyosarcomas [as well as 4 cases—lung (3) and brain (1)—in which the metastases were also available for study, for a total of 15 tissues], and 9 benign metastasizing leiomyomas in which the pulmonary lesion was studied; in one case, the uterine lesion was also available. The age range of the 8 women with leiomyoma was 32 to 54 (mean 47). This compared with an age range of 48 to 55 (mean 52) and 51 to 67 (mean 59), for the women with benign metastasizing leiomyomas and leiomyosarcomas, respectively. The ranges of the tumor sizes for the leiomyoma, benign metastasizing leiomyomas, and leiomyosarcomas were 1.0 to 9.1 cm (mean 4.2 cm), 0.2 to 5.0 cm (mean 1.5 cm), and 5.7 to 15.5 cm (mean 9.8 cm), respectively. The 9 women with benign metastasizing leiomyomas had 2 to 8 pulmonary nodules (mean 3) that had been detected 8 to 15 years after removal of the uterus; in each case, the pulmonary nodule was noted to be well defined; all but one were described as being homogeneous and rubbery; the other lesion was cystic.
Initially, the routine hematoxylin and eosin slides were reviewed, with emphasis on the mitotic index, nuclear atypia, presence of necrosis, and degree of cellularity. In each case, the initial diagnosis was confirmed. Specifically, the leiomyomas and benign metastasizing leiomyomas both showed a mitotic index that ranged from 0 to 2/10 high-power fields, compared with the leiomyosarcomas that showed a mitotic index that ranged from 8 to 24/10 high-power fields. None of the leiomyomas and benign metastasizing leiomyomas showed necrosis, and only one of each showed increased cellularity, but without substantial nuclear atypia. Each of the benign metastasizing leiomyomas was composed of only bland-appearing smooth muscle sharply demarcated from the adjacent pulmonary parenchyma, except for one that demonstrated a few epithelial inclusions. The leiomyosarcomas showed variable amounts of necrosis, and all were highly cellular with clear-cut nuclear atypia. Representative photographs of the histologic findings are presented in Figure 1.
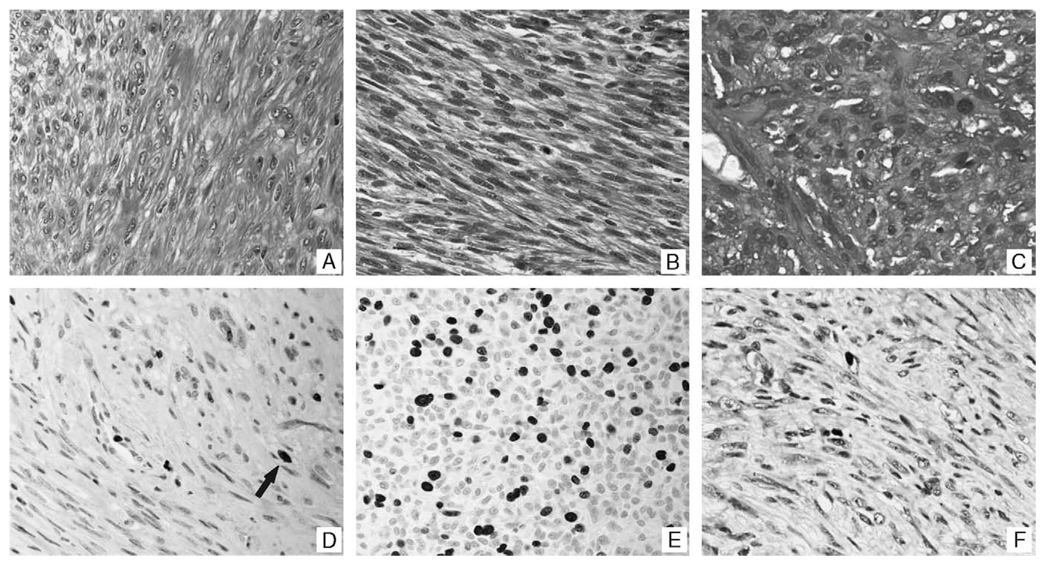
FIGURE 1.
Histologic and immunohistochemical correlates of uterine smooth muscle tumors and their pulmonary counterparts. Panel A is a representative histologic section of a leiomyoma included in this study. The microscopic features are similar to that observed in the uterus (not shown) and lung (panel B), in a woman who had a benign metastasizing leiomyoma that was noted 15 years after the removal of her uterus. Panel C, in comparison, shows the histologic features of a leiomyosarcoma that had metastasized to the lung; note the marked nuclear atypia, disorganized growth pattern, and mitotic figures. Panels D and E show representative areas of the Ki67 data for a leiomyoma (D, arrow) and leiomyosarcoma (E), respectively. The Ki67 results for the pulmonary component of the benign metastasizing leiomyomas (panel F) was equivalent to that for the leiomyomas.
In the next part of the study, expressions of p53 and bcl-2, as well as of the Ki67 index, were studied for each lesion. The data is presented in Table 1. Note that the Ki67 index was statistically equivalent for the leiomyomas and benign metastasizing leiomyomas, consistent with the low mitotic index of each type of lesion. The rate of around 3% is statistically equivalent to that of well differentiated, slow-growing cancers, such as certain types of thyroid cancer.19 In comparison, the Ki67 index for the leiomyosarcomas increased 10-fold. None of the 18 leiomyomas/benign metastasizing leiomyomas were p53–positive, compared with 9/15 of the leiomyosarcomas. Bcl-2 was detected in all the lesions studied, except for 1 of the benign metastasizing leiomyomas. Each of the 33 lesions was positive for smooth muscle actin, confirming their smooth muscle origin. In 4 cases of leiomyosarcomas of the uterus, a metastasis (3—lung, 1—brain) was also available for study. In these 4 sets of cases, the p53 results were identical in both the primary lesion and the metastasis. In 3 of the 4 cases, the Ki67 index was also equivalent, defined by showing less than 10% variation between the uterine and the lung lesion. However, in one case, the Ki67 index was 17.2 for uterine cancer and 37.7 in the lung metastasis. Representative photographs of the immunohistochemical results are provided in Figure 2.
TABLE 1.
Immunohistochemical Analyses of the Uterine Smooth Muscle Lesions and Their Associated Pulmonary Manifestations
| Histologic Diagnosis | p53* | Ki67 Index† | bcl-2* |
|---|---|---|---|
| Leiomyomas | 0/8 | 2.3 (0.9–8.8) | 8/8 |
| Leiomyosarcomas | 9/15 | 28.6 (14.4–62.0) | 12/12 |
| Benign metastasizing leiomyomas | 0/10 | 3.4 (0.7–8.1) | 5/6 |
p53 and bcl-2 scoring was defined as positive if at least 10% of the cells showed a clear-cut nuclear signal; in 4 of the benign metastasizing leiomyomas and 3 of the leiomyosarcomas, the bcl-2 test was not performed.
The Ki67 index was defined as the percentage of cells with a strong nuclear signal per 100 cells—at least 600 cells were counted per case.
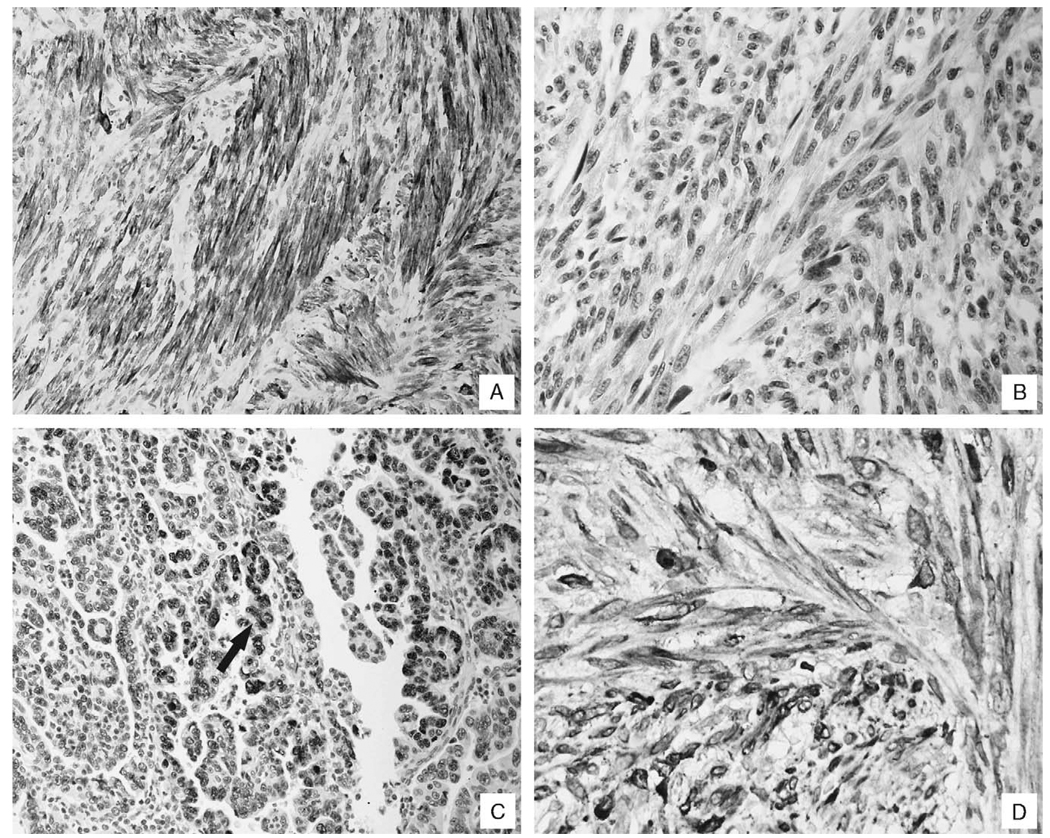
FIGURE 2.
Immunohistochemical correlates of uterine smooth muscle tumors and their pulmonary counterparts. Panel A shows a typical result for the HHF-35 immunohistochemical test for the pulmonary lesions of the benign metastasizing leiomyomas; the strong signal documents the smooth muscle origin of the lesion. Each of the benign metastasizing leiomyomas was negative for the p53 antigen (panel B). In contrast, note the nuclear-based signal, typical of most of the leiomyosarcomas (panel C, arrow). Panel D shows the strong signal for bcl-2, noted in the pulmonary lesion of the benign metastasizing leiomyoma, which is indicative of its uterine origin.
In the next part of the study, the expression pattern of miR-221 was analyzed by in situ hybridization. The results were as follows: 0/8 leiomyomas and 0/10 benign metastasizing leiomyomas were positive for miR-221. In comparison, 13/15 of the leiomyosarcomas were positive for miR-221. No signal was noted in the miR-221–positive cases if the probe was omitted or if a scrambled probe was used (Fig. 3). We then studied miR-301 and miR-376a, as these have been less strongly associated with malignant tumors, compared with miR-221. The data are presented in Table 2. Note that miR-221 was indeed, by far, the most common miR detected by in situ hybridization. MiR-301 was detected in 2 cases, including 1 that was negative for miR-221, and miR-376a was detected in only 1 case. Representative photographs of the miRNA data are provided in Figure 3. Note that the signal for miR-221 is evident in the malignant cells, but not in the adjacent normal cells (in the areas of fibrosis). Also note that the signal localizes to the cytoplasm and tends to concentrate around the nuclear membrane.
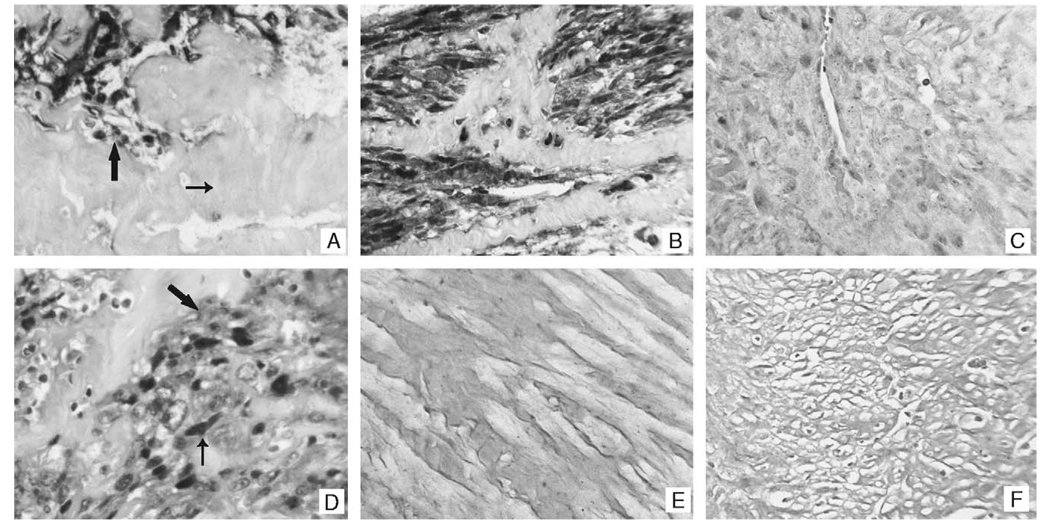
FIGURE 3.
MiR-221–expression patterns in the uterine smooth muscle tumors and their pulmonary counterparts. Panel A contains a representative example of miR-221 detection in a leiomyosarcoma of the uterus. Note the strong signal in the tumor cells (large arrow) and the lack of a signal in the adjacent fibrous tissue (small arrow). A similar pattern was evident in the lesions showing pulmonary metastasis (panel B). The signal was lost in the leiomyosarcoma if the probe was omitted (not shown) or if a scrambled lock nucleic acid probe was used (panel C). Panel D shows the miR-221 signal in the leiomyosarcomas at higher magnification; note that the signal is present in the cytoplasm (large arrow) and tends to concentrate around the nucleus (small arrow). MiR-221 was not detected by in situ hybridization in any of the leiomyomas (panel E) or benign metastasizing leiomyomas (panel F).
TABLE 2.
MiR Expression Patterns for the Smooth Muscle Lesions of the Uterus/Benign Metastasizing Leiomyomas
| Histologic Diagnosis | miR-221 | miR-301 | miR-376a |
|---|---|---|---|
| Leiomyoma | 0/8 | 0/8 | 0/8 |
| Benign metastasizing leiomyomas | 0/10 | 0/10 | 0/10 |
| Leiomyosarcomas | 13/15 | 2/15 | 1/15 |
DISCUSSION
The primary finding of this study is that uterine leiomyomas and benign metastasizing leiomyomas are similar in their immunohistochemical profiles (p53-negative, bcl-2–positive, and a low proliferation index) and in their lack of detectable expression of miR-221, miR-301, and miR-376a. In contrast, uterine leiomyosarcomas and their metastases show frequent p53 expression, a much greater proliferation index that is statistically significant, and a high rate of marked up-regulation of miR-221. The much lower frequency of up-regulation of miR-301 and miR-376a underscores the importance of miR-221 in carcinogenesis, which has been found to be increased in a variety of malignant tumors.12,14–17
Considerable debate has taken place regarding the origin and the correct classification of benign metastasizing leiomyomas. Some investigators have concluded that benign metastasizing leiomyomas are actually low-grade leiomyosarcomas.3–5 Others have postulated that the pulmonary lesions of benign metastasizing leiomyomas represent multifocal smooth muscle proliferation, in women with uterine leiomyomas.1 The other theory is that benign metastasizing leiomyomas are benign uterine lesions that are capable of entering the vascular system and being deposited at sites such as the lungs; although, the lesions in the lungs might take up to 20 years to grow large enough to be detectable.2,6 The demonstration, in this and other studies,20 that benign metastasizing leiomyomas are often bcl-2–positive, which is typical of uterine leiomyomas, does not favor the multiorgan smooth muscle proliferation theory. More importantly, several groups have shown that the uterine lesions and associated benign metastasizing leiomyomas are clonal proliferations8,9: this strongly suggests that the latter are either truly benign processes that have entered the vascular system (as it is generally recognized as one mechanism by which endometriosis can be found at distant sites) or low-grade sarcomas. The Ki67 index cannot differentiate between these 2 possibilities, as slow-growing well differentiated cancers, such as those that occur in the thyroid or prostate, can have Ki67 indexes equivalent to or even less than that noted in this study for uterine leiomyomas.19
miRNAs are small sequences of RNA, which are capable of binding to the 3′ untranslated region of mRNAs, and which ultimately lead to reduced translation. Although the study of miRNAs is relatively new, it is clear that aberrant regulation of these molecules is characteristic of many cancers.12–17 The aberrant regulation might be the loss of the miR, such as miR-15 and miR-16, which is associated with an increase in the production of bcl-2 that can facilitate the evolution of chronic lymphocytic leukemia.13 More commonly, however, the aberrant regulation manifests itself as increased miRNA production, typical of miR-221 in pancreatic and other cancers.14,15,17 This is consistent with the observation in this study that miR-221 was found to be up-regulated in 87% of leiomyosarcomas. To our knowledge, this is the first study to document the up-regulation of any miRNA in uterine leiomyosarcomas. Importantly, we did not detect miR-221 (or miR-301/miR-376a) either in the 10 benign metastasizing leiomyomas or in the 8 leiomyomas studied. This suggests that benign metastasizing leiomyomas are indeed benign tumors. This statement must be tempered by the realization that there is little information on miRNA expression in low-grade malignant tumors, especially sarcomas. Moreover, our study did not address the loss of expression of any miRNAs, which can be found in malignant lesions as well. In this regard, a recent paper showed that 5 miRs (let-7, let-21, let-23b, let-29b, and let-197) were dysregulated in benign leiomyomas, suggesting that these molecules might play a role in the increased growth rate of these benign lesions, without conferring a malignant phenotype.21 Interestingly, this study noted that miR-221 was not dysregulated in benign leiomyoma.21
Uterine smooth muscle tumors provide an interesting format to study the role of miRNAs. Most of these lesions are benign, but can show a high proliferation index, especially in the context of pregnancy or with certain types of exogenous hormonal therapy. This per se does not seem to be associated with miR-221 dysregulation, as noted above. Another category of uterine smooth muscle tumor is the so-called smooth muscle tumor of uncertain malignant potential, typically marked by increased cellularity and variable degrees of nuclear atypia and mitotic activity. These lesions were not addressed in this study, as the focus was on benign metastasizing leiomyomas. However, the data with leiomyosarcomas suggests that special emphasis on certain miRNAs, especially miR-221, can be useful in this regard. Benign metastasizing leiomyomas are very slow-growing lesions that are able, in ways that are unclear, to enter the vascular system and get seeded at other sites, such as the lung, despite the fact that they do indeed seem to be benign. Finally, with regard to leiomyosarcomas, the strong association with miR-221 expression might help in better elucidating the specific pathways that are essential for the formation and maintenance of the malignant phenotype. This might in some way be related to the loss of 19q and 22q, in view of the recent observation by Nucci et al.7 The importance of such putative pathways is underscored by the data, which shows that increased miR-221 expression can be found in many diverse cancers, including pancreatic adenocarcinoma, glioblastoma multiforme, and chronic lymphocytic leukemia.14–17
ACKNOWLEDGMENTS
The authors appreciate the assistance of Dr Jeffrey Kneille. They are especially grateful to Dr Saul Suster for help in obtaining the specimens and, more importantly, in discussions regarding the identities of the benign metastasizing leiomyomas. They are very grateful to Ventana Medical Systems for providing the reagents for this study.
Supported by a grant from the Lewis Foundation (G.J.N.) and NCI grant CA114304 (T.D.S.).
REFERENCES
- 1.Abell MR, Littler ER. Benign metastasizing uterine leiomyoma. Multiple lymph nodal metastases. Cancer. 1975;36:2206–2213. doi: 10.1002/cncr.2820360938. [DOI] [PubMed] [Google Scholar]
- 2.Canzonieri V, D’Amore ES, Bartoloni G, et al. Leiomyomatosis with vascular invasion. A unified pathogenesis regarding leiomyoma with vascular microinvasion, benign metastasizing leiomyoma and intravenous leiomyomatosis. Virchows Arch. 1994;425:541–545. doi: 10.1007/BF00197559. [DOI] [PubMed] [Google Scholar]
- 3.Esteban JM, Allen WM, Schaerf RH. Benign metastasizing leiomyoma of the uterus: histologic and immunohistochemical characterization of primary and metastatic lesions. Arch Pathol Lab Med. 1999;123:960–962. doi: 10.5858/1999-123-0960-BMLOTU. [DOI] [PubMed] [Google Scholar]
- 4.Jautzke G, Muller-Ruchholtz E, Thalmann U. Immunohistological detection of estrogen and progesterone receptors in multiple and well differentiated leiomyomatous lung tumors in women with uterine leiomyomas (so-called benign metastasizing leiomyomas). A report on 5 cases. Pathol Res Pract. 1996;192:215–223. doi: 10.1016/S0344-0338(96)80224-X. [DOI] [PubMed] [Google Scholar]
- 5.Kayser K, Zink S, Schneider T, et al. Benign metastasizing leiomyoma of the uterus: documentation of clinical, immunohistochemical and lectin-histochemical data of ten cases. Virchows Arch. 2000;437:284–292. doi: 10.1007/s004280000207. [DOI] [PubMed] [Google Scholar]
- 6.Koh DM, Burn PR, King DM. Benign metastasizing leiomyoma with intracaval leiomyomatosis. Br J Radio. 2000;73:435–437. doi: 10.1259/bjr.73.868.10844871. [DOI] [PubMed] [Google Scholar]
- 7.Nucci MR, Drapkin R, Cin PD, et al. Distinctive cytogenetic profile in benign metastasizing leiomyoma: pathogenic implications. Am J Surg Path. 2007;31:737–743. doi: 10.1097/01.pas.0000213414.15633.4e. [DOI] [PubMed] [Google Scholar]
- 8.Patton KT, Cheng L, Papavero V, et al. Benign metastasizing leiomyoma: clonality, telomere length and clinicopathologic analysis. Mod Pathol. 2006;19:130–140. doi: 10.1038/modpathol.3800504. [DOI] [PubMed] [Google Scholar]
- 9.Tietze L, Gunther K, Horbe A, et al. Benign metastasizing leiomyoma: a cytogenetically balanced but clonal disease. Hum Pathol. 2000;31:126–128. doi: 10.1016/s0046-8177(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 10.Wolff M, Kaye G, Silva F. Pulmonary metastases (with admixed epithelial elements) from smooth muscle neoplasms. Report of nine cases, including three males. Am J Surg Pathol. 1979;3:325–332. doi: 10.1097/00000478-197908000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kloosterman WP, Wienholds E, deBruijn E, et al. In situ detection of miRNAs in animal embryos using LNA-modified probes. Nat Methods. 2006;3:27–29. doi: 10.1038/nmeth843. [DOI] [PubMed] [Google Scholar]
- 12.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting bcl-2. Proc Natl Acad Sci USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calin GA, Ferracin M, Cimmino A, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 15.Ciafre SA, Galardi S, Mangiola A, et al. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005;334:1351–1358. doi: 10.1016/j.bbrc.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 16.He H, Jazdzewski K, Li W, et al. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA. 2005;102:19075–19080. doi: 10.1073/pnas.0509603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee EJ, Gusev Y, Jiang J, et al. Expression profiling identifies distinct microRNA signature in pancreatic cancer. Int J Cancer. 2007;120:1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuovo GJ. PCR In Situ Hybridization: Protocols and Applications. 3rd ed. Baltimore, Maryland: Lippincott Williams and Wilkins Press; 1997. [Google Scholar]
- 19.Saltman B, Singh B, Hedvat CV, et al. Patterns of expression of cell cycle/apoptosis genes along the spectrum of thyroid carcinoma progression. Surgery. 2006;140:899–905. doi: 10.1016/j.surg.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Suster S, Fisher C, Moran CA. The spectrum of bcl-2 oncoprotein reactivity in benign and malignant spindle cell tumors of soft tissue, skin, serosal surfaces and gastrointestinal tract. An immunohistochemical study of 380 cases. Am J Surg Pathol. 1998;22:863–872. doi: 10.1097/00000478-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Zhang X, Obijuru L, et al. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer. 2007;46:336–347. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]