Abstract
OBJECTIVE
To examine the relationship between magnitude of weight loss and changes in urinary incontinence frequency.
METHODS
Overweight and obese women (N=338) with 10 or more urinary incontinence episodes per week were assigned randomly to an intensive 6-month behavioral weight loss program followed immediately by a 12-month weight maintenance program (intervention; n=226) or to a structured education program (control; n=112). The intervention and control groups were combined to examine the effects of the magnitude of weight loss on changes in urinary incontinence assessed by 7-day voiding diary, pad test, and self-reported satisfaction with change in urinary incontinence.
RESULTS
Compared with participants who gained weight (reference), those who lost 5% to less than 10% or 10% or more of their body weight had significantly greater percent reductions in urinary incontinence episodes and were more likely to achieve at least a 70% reduction in the frequency of total and urge urinary incontinence episodes at 6, 12, and 18 months. Satisfaction was also related to magnitude of weight loss; approximately 75% of women who lost 5% to less than 10% of their body weight reported being moderately or very satisfied with their changes in urine leakage.
CONCLUSION
Weight losses between 5% and 10% of body weight were sufficient for significant urinary incontinence benefits. Thus, weight loss should be considered as initial treatment for incontinence in overweight and obese women.
Urinary incontinence is an important health problem for women, affecting over 13 million women in the United States, and has a profound adverse effect on quality of life.1–4 Depending on the type of incontinence, bladder muscle training, pharmacologic treatments, and surgery may be considered.5–7 Obesity has been shown to be a strong risk factor for incontinence1,8–10 and several studies have suggested that weight loss may reduce the frequency of urinary incontinence.11–13 We recently reported results of a clinical trial, the Program to Reduce Incontinence by Diet and Exercise (PRIDE), showing that overweight and obese women with urinary incontinence randomized to lifestyle intervention had greater improvements in urinary incontinence than women in the control group at 6 and 12 months, especially for stress incontinence14 (Wing RR, West DS, Grady D, Creasman JM, Richter HE, Myers D, et al. Effect of weight loss on urinary incontinence in overweight and obese women: results at 12 and 18 months. J Urol. In press). Differences in urinary incontinence were not significant at 18 months, but women in the lifestyle group continued to report greater satisfaction with changes in incontinence (Wing RR. In press).
To encourage weight loss as a treatment approach for incontinence, it is important to estimate the magnitude of weight loss needed to improve urinary incontinence and produce satisfaction with urinary incontinence changes. Thus, this article extends our previous study by looking at the improvements in urinary incontinence that occurred with specific amounts of weight loss in PRIDE participants. A number of clinical guidelines stress that overweight and obese patients should be given the goal of losing 5–10% of their body weight, because these goals are feasible and likely to achieve health benefits. Modest weight losses of 5–10% of initial body weight have been shown to be effective in reducing blood pressure and prevention of noninsulin-dependent diabetes mellitus.15–18 In this article, we examine the effects of losses of 5–10% of body weight on urinary incontinence among women in the PRIDE study.
MATERIALS AND METHODS
A total of 338 participants were recruited in approximately equal numbers in Providence, RI, and in Birmingham, AL, between July 2004 and April 2006. Eligibility criteria included being at least 30 years of age, having a body mass index (BMI) of 25–50 (BMI is calculated as weight (kg)/[height (m)]2), and reporting at least 10 urinary incontinent episodes (including both stress and urge incontinent episodes) on a 7-day voiding diary at baseline. Participants were also required to be able to walk two blocks in 10 minutes without assistance, to successfully monitor their dietary intake and activity for 1 week, and to agree not to initiate new treatments for incontinence or weight reduction during the trial. Prior medical therapy for incontinence or obesity did not affect eligibility. Participants who had urinary incontinence of neurological or functional origin, prior incontinence or urethral surgery, significant medical conditions of the genitourinary tract or current urinary tract infections, or more than four urinary tract infections in the past year were excluded. To maximize the safety of the intervention, we excluded participants with noninsulin-dependent diabetes mellitus requiring medical therapies that increase the risk of hypoglycemia, uncontrolled hypertension, coronary heart disease, or who were currently pregnant. Details of the study design, inclusion and exclusion criteria, and results have been previously reported.14
Eligible participants were allocated randomly in a 2:1 ratio to an 18-month behavioral weight loss program (weight loss; n=226) or to a structured education program (control; n=112). Assessments were completed at 6, 12, and 18 months by staff members who were unaware of the participant's treatment assignment. The study was approved by the Institutional Review Board at each site and written consent was obtained from all participants before enrollment.
Participants randomized to standard education were offered seven 1-hour group education sessions over the 18 months. These sessions provided general information about weight loss, physical activity, and healthy eating habits.
Participants assigned to the weight loss program received an intervention modeled after those used in the Diabetes Prevention Program15 and Look AHEAD,19,20 that was designed to produce an average loss of 7–9% of initial body weight by reducing calories and increasing physical activity. All participants were asked to attend weekly group meetings for months 1–6 and every other week for months 7–18. Participants were prescribed a calorie and fat restricted diet of 1,200–1,800 kcal/day, depending on initial weight, with less than 30% of calories from fat. Physical activity goals were gradually increased until participants were doing 200 minutes per week of activities similar in intensity to brisk walking. Behavior modification techniques, including self-monitoring of diet and exercise, were stressed throughout the program.14 After completion of the first 6 months of the program, each weight loss group was cluster-randomized to a maintenance program that focused on either refining skills or increasing motivation to improve longer-term weight loss maintenance. Women randomized to the two maintenance groups were similar to each other and to women in the control group on baseline characteristics. No differences in weight loss or urinary incontinence were observed between the two maintenance interventions over the 12-month maintenance period (West DS, Gorin AA, Subak LL, Foster G, Bragg C, Hecht J, et al. A motivation-focused weight loss maintenance program is an effective alternative to a skill-based approach. Int J Obes. in press).
At the beginning of intervention, participants in the weight loss and control groups were given a self-help incontinence behavioral treatment booklet with instructions for improving bladder control.21 No other information was provided pertaining to incontinence at the sessions or during follow-up visits.
Weight was measured with participants wearing street clothes, without shoes, using a calibrated digital scale (Tanita BWB 800, Tanita Corporation of America, Inc., Arlington Heights, IL) and recorded to the nearest 0.1 kg. Height was measured at baseline to the nearest centimeter using a calibrated, wall-mounted stadiometer and a horizontal measuring block. Physical activity was assessed with the Paffenbarger Activity Questionnaire.22
Urinary incontinence was assessed with a 7-day voiding diary.23 Participants were trained to record the time of each void and each incontinent episode and to identify each episode as stress (involuntary loss of urine associated with coughing, sneezing, straining, or exercise), urge (loss of urine associated with a strong need or urge to void), or other. These voiding diaries were reviewed by blinded assessment staff to reconcile any questions, inconsistencies, or omissions. As a secondary outcome, we assessed the quantity of urine lost involuntarily using a standardized pad test.24 Participants collected and returned in sealed plastic bags preweighed urinary incontinence pads used during a 24-hour period, and the posttest weight of each pad was recorded in grams. At each 6-month assessment, participants were also asked to rate their overall satisfaction with the changes in their incontinence (5-point scale).25 Subjective ratings of satisfaction have been shown to be valid and to correlate with changes in quality of life and urinary incontinence symptoms.25
Demographic characteristics and medical, behavioral, and incontinence histories were ascertained using self-reported questionnaires.
To examine whether there was an association between the magnitude of weight loss and improvements in urinary incontinence, analyses were performed with participants grouped according to their weight loss from baseline to 6, 12, or 18 months. Log-rank tests were used to compare the proportion of participants with improvement in incontinence across four weight loss group categories. Generalized estimating equation negative binomial models were used to compare changes in mean incontinence frequency between women who gained weight and those who lost less than 5%, 5% to less than 10%, or 10% or greater of their initial body weight adjusting for potential confounders including treatment group, clinic, age, race, alcohol use, smoking status, number of live births, pelvic floor exercises, and self-reported kilocalories of physical activity.26 Similarly, we compared the proportion of women achieving at least a 70% reduction in self-reported weekly incontinence frequency across weight loss categories using adjusted generalized estimating equation logistic models to account for clustering within treatment groups. A priori, we selected a reduction of at least 70% in incontinent episode frequency because this amount of change has been reported as a threshold for improvement in patient satisfaction.25 The sample size provided 80% power in two-sided tests with a type I error of 5% to detect a 55% reduction in incontinence frequency among women who lost 0–5%, 5–10%, or more than 10% compared with those who gained weight.
For the generalized estimating equation analyses, we used multiple imputation of missing outcomes under the conservative assumption of nonignorable missingness. Specifically, we imputed missing weights assuming that dropouts in both groups returned on average to their baseline weight.14 We then imputed missing incontinence frequencies for both groups as if the participant had been assigned to the control group in which average weight losses were minimal but some reduction in incontinence frequency was observed. Summary effect estimates, standard errors, P values, and confidence intervals were computed using standard methods for multiply imputed data, which account for the additional uncertainty of imputed variables.27
All statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). A P value of <.05 was considered statistically significant.
RESULTS
The 338 randomized women had mean (±standard deviation) age of 53±11 years, baseline BMI of 36±6, weight of 92 (±18) kg, and 24 (±18) urinary episodes per week (10 stress and 14 urge episodes/week). Seventy-seven percent of the women were white, 19% were African American, and 4% reported other ethnic or racial groups. The 6-, 12-, and 18-month assessments, including weight and the 7-day voiding diary, were completed by 304 (90%), 294 (87%), and 287 (85%) of all participants. There were no significant differences between those who attended these assessments and those who did not on baseline demographic characteristics, BMI, or urinary incontinence frequency.
Participants were grouped into their category of weight loss at 6, 12, or 18 months without regard to randomized treatment assignment. The categories were gained weight, lost 0 to less than 5%, lost 5 to less than 10%, or lost 10% or greater of baseline body weight. Participants in the gained weight category gained on average 2.1 (±1.9) kg, 2.8 (±3.1) kg, and 3.5 (±3.2) kg at 6, 12, and 18 months, respectively. Table 1 shows the number and proportion of women in each of these categories at each time period. As expected, women in the weight loss intervention were overrepresented in the higher weight loss categories at 6, 12, and 18 months (all P<.001). Women in the various weight loss categories did not differ significantly from each other on baseline characteristics, including BMI and urinary incontinence measures (Table 2).
Table 1.
Women in Each Weight Loss Category by Visit
| Weight Gain | 0% to Less Than 5% Weight Loss | 5% to Less Than 10% Weight Loss | 10% or More Weight Loss | Total | |
|---|---|---|---|---|---|
| 6-mo visit | |||||
| Total | 47 (15) | 84 (28) | 89 (29) | 84 (28) | 304 (100) |
| Intervention | 15 (7) | 49 (23) | 68 (32) | 82 (38) | 214 (100) |
| Control | 32 (36) | 35 (39) | 21 (23) | 2 (2) | 90 (100) |
| 12-mo visit | |||||
| Total | 64 (22) | 72 (24) | 73 (25) | 85 (29) | 294 (100) |
| Intervention | 28 (14) | 43 (21) | 58 (28) | 78 (38) | 207 (100) |
| Control | 36 (41) | 29 (33) | 15 (17) | 7 (8) | 87 (100) |
| 18-mo visit | |||||
| Total | 76 (26) | 79 (28) | 61 (21) | 71 (25) | 287 (100) |
| Intervention | 38 (19) | 53 (27) | 45 (23) | 61 (31) | 197 (100) |
| Control | 38 (42) | 26 (29) | 16 (18) | 10 (11) | 90 (100) |
Data are n (%).
Present n (row percent) for women with urinary incontinence measures available at each time point.
Table 2.
Baseline Characteristics According to Magnitude of Weight Loss at 6 Months
| 6 Months (n=304) | Weight Gain | 0% to Less Than 5% Weight Loss | 5% to Less Than 10% Weight Loss | 10% or Greater Weight Loss | P |
|---|---|---|---|---|---|
| Total | 47 (15%) | 84 (28) | 89 (29%) | 8 (32) | |
| Intervention | 15 (7) | 49 (23) | 68 (32) | 82 (38) | <.001 |
| Control | 32 (36) | 35 (39) | 21 (23) | 2 (2) | |
| Age (y) | 51.4 (±9.4) | 52.1 (±10.1) | 54.7 (±10.5) | 53.5 (±10.4) | .18 |
| Race | .02 | ||||
| White | 40 (17) | 57 (24) | 69 (29) | 74 (31) | |
| African American | 7 (13) | 21 (39) | 18 (33) | 8 (15) | |
| Other | 0 (0) | 6 (60) | 2 (20) | 2 (20) | |
| Education high school or less | 9 (24) | 7 (19) | 15 (41) | 6 (16) | .07 |
| Weight (kg) | 96.6 (±16.6) | 94.7 (±17.2) | 98.3 (17.2) | 95.9 (±17) | .56 |
| Body mass index (kg/m2) | 36.4 (±5.4) | 35.9 (±5.8) | 36.9 (5.8) | 35.8 (±5.6) | .86 |
| Total incontinent episodes per wk | 22.2 (±19) | 22.3 (±14.8) | 24.6 (19.2) | 25.1 (±18.9) | .51 |
| Stress incontinent episodes per wk | 8.8 (±10.2) | 7.7 (±9.6) | 9.4 (9.2) | 11 (±13.3) | .13 |
| Urge incontinent episodes per wk | 12.5 (±17.4) | 13.6 (±12.8) | 14 (14.1) | 12.8 (±13.6) | .67 |
Data are n (%) or mean±standard deviation unless otherwise specified.
Among women who gained weight, lost 0 to less than 5%, lost 5% to less than 10%, and lost at least 10% at 6 months, 18.5%, 12.4%, 5.5%, and 9.5%, respectively, dropped out by 18 months. Participants who dropped out of the study had a greater number of incontinence episodes at baseline (P=.03) and were less likely to be married (P=.02).
We tested the interaction between randomization group and magnitude of weight loss and found that the effect of specific magnitudes of weight loss on urinary incontinence did not differ for women in the weight loss compared with the control group (Pvalues range from .12 to .93). Thus, the weight loss and control group were combined for all subsequent analyses.
Table 3 shows the adjusted changes in urinary incontinence in each of the weight loss categories. As shown, there was a strong association between magnitude of weight loss and absolute percent reductions in weekly frequency of total, stress, and urge urinary incontinence at 6, 12, and 18 months such that greater weight losses were associated with greater reductions in incontinent episodes frequency. There were no significant interactions between baseline BMI (BMI of less than 35 compared with more than 35) or baseline frequency of urinary incontinence and the weight change categories for any of the measures of urinary incontinence; thus, the relationship between weight change category and improvements in urinary incontinence was not modified by initial BMI or initial severity of incontinence. Similarly, women in the various weight loss categories did not differ in their reported use of the strategies described in the incontinence booklet.
Table 3.
Adjusted Percent Change in Weekly Urinary Incontinence Episode Frequency and Urine Loss According to Magnitude of Weight Loss*
| Weight Gain† |
0% to Less Than 5% Weight Loss |
5% to Less Than 10% Weight Loss |
More Than 10% Weight Loss |
|||||
|---|---|---|---|---|---|---|---|---|
| Percent Change UI (95% CI) | Percent Change UI (95% CI) | P‡ | Percent Change UI (95% CI) | P‡ | Percent Change UI (95% CI) | P‡ | P§ | |
| Total incontinence episodes per wk from voiding diary | ||||||||
| 6 mo | −11 (−29, 12) | −28 (−45, −7) | .11 | −53 (−65, −38) | ≤.001 | −52 (−64, −37) | <.001 | <.001 |
| 12 mo | −31 (−48, −10) | −46 (−62, −25) | .16 | −63 (−75, −46) | .002 | −64 (−73, −52) | <.001 | <.001 |
| 18 mo | −40 (−52, −25) | −50 (−62, −33) | .27 | −63 (−74, −47) | .02 | −66 (−75, −55) | <.001 | <.001 |
| Stress incontinence episodes per wk from voiding diary | ||||||||
| 6 mo | −42 (−59, −17) | −48 (−65, −24) | .60 | −68 (−80, −49) | .003 | −74 (−82, −62) | <.001 | <.001 |
| 12 mo | −51 (−65, −29) | −63 (−79, −37) | .28 | −74 (−84, −59) | .02 | −74 (−83, −59) | .02 | .009 |
| 18 mo | −50 (−65, −29) | −63 (−77, −39) | .28 | −70 (−83, −49) | .05 | −77 (−86, −61) | .002 | .001 |
| Urge incontinence episodes per wk from voiding diary | ||||||||
| 6 mo | 3 (−23, 38) | −21 (−41, 6) | .10 | −43 (−58, −23) | ≤.001 | −30 (−49, −5) | .009 | .003 |
| 12 mo | −27 (−48, 4) | −38 (−57, −8) | .43 | −58 (−72, −35) | .02 | −56 (−71, −35) | .01 | .005 |
| 18 mo | −33 (−51, −8) | −48 (−66, −19) | .20 | −56 (−71, −33) | .06 | −62 (−73, −46) | .001 | .002 |
| 24-h involuntary urine loss from pad test | ||||||||
| 6 mo | −38 (−8, −53) | −23 (0, −41) | .45 | −50 (−35, − 60) | .23 | −55 (−41, −66) | .1 | .01 |
| 12 mo | −46 (−27, −61) | −47 (−30, −60) | .97 | −63 (−50, −72) | .08 | −66 (−55, −74) | .05 | .02 |
| 18 mo | −40 (−20, −56) | −59 (−45, −69) | .06 | −56 (−40, −68) | .18 | −62 (−47, −73) | .06 | .10 |
Adjusted for treatment group, clinic, age, race, alcohol use, smoking status, number of live births, pelvic floor exercises, and kilocalories burned.
Reference group.
P value for comparison with weight gain as the reference group.
Test for trend based on orthogonal contrasts in parameters of the generalized estimating equation negative binomial model.
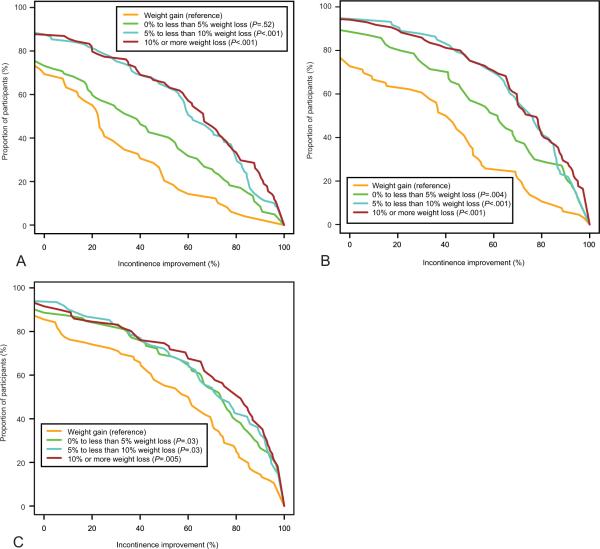
Figure 1 shows the cumulative percentage of women who experienced 0% to 100% reductions in total urinary incontinence episodes at 6, 12, and 18 months, respectively, within each of the weight change categories. At 6 months, the proportion of women who achieved any given degree of improvement in urinary incontinence was lowest in the gained weight category followed by those who lost 0% to less than 5% and highest in those who lost 5% to less than 10% or 10% or greater. There was no further improvement for those who lost 10% or greater compared with those who lost 5 to less than 10% (P=.47). An identical pattern was seen at 12 months (P=.78); however, at 18 months, the gained weight category differed from all three of the weight loss categories with no difference between those who lost 0% to less than 5%, 5% to less than 10%, or 10% or greater (pairwise comparison P values from log-rank test ranged from .47 to .85).
Fig. 1.
Cumulative proportion of participants in each weight change category who experienced 0–100% reductions in total urinary incontinent episodes at 6 months (A), 12 months (B), and 18 months (C).
As noted, we selected a 70% reduction in total, stress, or urge incontinent episodes per week as a clinically significant threshold. Table 4 shows the number and percentage of women in each weight loss category who achieved or failed to achieve this threshold. Using those who gained weight as the reference, we found (Table 5) that the adjusted odds of achieving at least a 70% reduction in total, stress, or urge incontinent episodes per week was significantly increased by greater weight losses (with the exception of stress incontinence at 6 months). Again, we found a strong association between weight losses of 5% to less than 10% of body weight and urinary incontinence improvement without further benefit among women with 10% or greater weight loss (P>.20 for all outcomes at 6, 12, and 18 months). Women who lost 5% to less than 10% of their body weight had 3.7 times the odds of achieving a 70% or greater reduction in total incontinent episodes compared with women who gained weight at 12 months and 2.4 times the odds at 18 months.
Table 4.
Observed Number and Percent of Women With 70% Reduction in Number of Incontinent Episodes per Week Reported in Voiding Diary
| Weight Gain |
0% to Less Than 5% Weight Loss |
5% to Less Than 10% Weight Loss |
10% or Greater Weight Loss |
|||||
|---|---|---|---|---|---|---|---|---|
| 70% or Greater | Less Than 70% | 70% or Greater | Less Than 70% | 70% or Greater | Less Than 70% | 70% or Greater | Less Than 70% | |
| Total UI (mo) | ||||||||
| 6 | 6 (13) | 41 (87) | 22 (26) | 62 (74) | 39 (44) | 50 (56) | 37 (44) | 47 (56) |
| 12 | 12 (19) | 52 (81) | 28 (39) | 44 (61) | 42 (58) | 31 (42) | 49 (58) | 36 (42) |
| 18 | 28 (37) | 48 (63) | 43 (54) | 36 (46) | 33 (54) | 28 (46) | 43 (61) | 28 (39) |
| Stress UI (mo) | ||||||||
| 6 | 13 (30) | 31 (70) | 23 (35) | 43 (65) | 43 (54) | 36 (46) | 39 (54) | 33 (46) |
| 12 | 15 (27) | 40 (73) | 31 (51) | 30 (49) | 39 (64) | 22 (36) | 52 (71) | 21 (29) |
| 18 | 33 (49) | 34 (51) | 44 (67) | 22 (33) | 33 (62) | 20 (38) | 43 (72) | 17 (28) |
| Urge UI (mo) | ||||||||
| 6 | 6 (14) | 37 (86) | 26 (33) | 53 (67) | 38 (46) | 45 (54) | 37 (46) | 43 (54) |
| 12 | 19 (31) | 43 (69) | 32 (48) | 35 (52) | 40 (58) | 29 (42) | 48 (59) | 33 (41) |
| 18 | 24 (35) | 44 (65) | 40 (51) | 38 (49) | 31 (54) | 26 (46) | 42 (63) | 25 (37) |
UI, urinary incontinence.
Data are n (%).
Table 5.
Adjusted Odds of at Least 70% Reduction in Number of Incontinent Episodes per Week Reported in Voiding Diary by Type of Incontinence and Magnitude of Weight Loss Compared With Weight Gain (Reference Group)*
| 0% to Less Than 5% Weight Loss |
5% to Less Than 10% Weight Loss |
More Than 10% Weight Loss |
|||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | P† | |
| Total UI (mo) | |||||||
| 6 | 1.7 (0.7–4.6) | 0.26 | 3.7 (1.6–8.2) | <.001 | 3.8 (1.5–9.6) | <.001 | <.001 |
| 12 | 1.6 (0.8–3.3) | 0.19 | 3.7 (1.7–8.3) | <.001 | 4.1 (2.1–7.9) | <.001 | <.001 |
| 18 | 1.9 (1.1–3.3) | 0.02 | 2.4 (1.1–5.1) | .02 | 3.3 (1.7–6.4) | <.001 | <.001 |
| Stress UI (mo) | |||||||
| 6 | 0.9 (0.4–2.0) | 0.76 | 1.8 (0.9–3.7) | .11 | 1.6 (0.6–3.9) | .33 | .13 |
| 12 | 1.7 (0.9–3.4) | 0.12 | 2.4 (1.1–5.3) | .02 | 3.4 (1.4–8.1) | .01 | .001 |
| 18 | 1.8 (0.9–3.4) | 0.09 | 1.7 (0.8–3.4) | .17 | 2.3 (1.0–5.1) | .04 | .05 |
| Urge UI (mo) | |||||||
| 6 | 2.2 (0.8–6.6) | 0.15 | 4.1 (1.5–10.7) | <.001 | 4.5 (1.4–14.1) | .01 | <.001 |
| 12 | 1.6 (0.8–3.2) | 0.19 | 2.4 (1.2–5.0) | .02 | 3.2 (1.6–6.2) | <.001 | <.001 |
| 18 | 2.3 (1.5–3.6) | <0.001 | 2.9 (1.5–5.5) | <.001 | 4.0 (2.1–7.9) | <.001 | <.001 |
OR, odds ratio; CI, confidence interval; UI, urinary incontinence.
Adjusted for treatment group, use of incontinence behavioral treatment booklet, clinic, age, alcohol use, smoking status, race, number of live births, and amount of calories burned.
Test for trend from orthogonal contrasts in statistical model.
Satisfaction with urinary incontinence outcomes was also strongly associated with amount of weight loss At 6 months, the percent of participants who reported being moderately or very satisfied with overall changes in leakage was 37%, 64%, 75%, and 80% for participants who gained weight and those losing 0% to less than 5%, 5% to less than 10%, or 10% or greater, respectively (P<.001). The percentages at 12 months were 49%, 62%, 78%, and 81% (P<.001), respectively, and 54%, 63%, 74%, and 79% at 18 months (P=.01).
DISCUSSION
The goal of this report was to determine the magnitude of weight loss that is associated with significant improvements in urinary incontinence. We have shown that significant improvements in the frequency of urinary incontinent episodes occur with weight losses of 5% to less than 10% of body weight relative to women who gained weight both for overall reductions in weekly incontinent episodes and for stress and urge incontinent episodes separately. In addition, the proportion of women achieving a 70% reduction in total and urge incontinence frequency was also significantly related to weight change category with somewhat weaker effects for stress incontinent episodes. Women who lost 5–10% of their body weight were two to four times more likely to achieve at least a 70% reduction in total and urge incontinent episode frequency compared with women who gained weight at 6, 12, and 18 months. It is important to note that the benefits of 5% to less than 10% weight losses were observed even in those women with higher baseline body weight or greater frequency of incontinence. Moreover, larger weight losses (10% or greater) did not appear to result in greater improvements, although we cannot strongly rule out such effects. Thus, losing 5–10% of initial body weight appears to be sufficient for clinically meaningful improvement in incontinence in overweight and obese women.
As seen in Table 3, the absolute changes in stress episodes were far greater than the absolute changes in urge episodes at each time point and in each category of weight loss. As a result of the large improvement in stress incontinent episodes in the weight gain group (which is used as the reference group), the relative changes in incontinent episodes and the odds of achieving a 70% reduction with greater weight loss appear smaller for stress incontinent episodes than for urge incontinent episodes.
Whereas weight losses of 5% to less than 10% were sufficient to produce improvements in frequency of incontinent episodes, changes on the pad test as a measure of 24-hour involuntary urine loss were seen only for those who lost 10 kg or greater relative to those who gained weight. Thus, a larger magnitude of weight loss may be needed to produce significant improvements on this measure of incontinence.
The women's subjective reports of satisfaction with their overall changes in urinary leakage were also strongly associated with weight loss. Approximately 75% of women who lost 5% to less than 10% of their body weight reported being moderately or very satisfied with the improvement in their leakage. Although satisfaction with urinary incontinence improvement does not always reflect the actual changes in urinary incontinence episodes,28 these subjective assessments suggest a high benefit: risk ratio for a lifestyle approach to treating urinary incontinence.
It is of note that some of the women who gained weight reported improvements in their incontinence and approximately 40–50% of those who gained weight were moderately or very satisfied with these improvements. These findings may reflect the beneficial effects of joining a clinical trial with other women confronting similar issues, the provision of a booklet on behavioral approaches to incontinence, or fluctuations in the severity of incontinence over time.
These results are encouraging because weight losses of 5–10% can be achieved and largely maintained with current behavioral weight loss programs. For example, in the Diabetes Prevention Program,15 49% of women lost at least 7% of their initial weight at 6 months and 37% were still 7% below baseline after an average of 2.8 years of intervention. Similarly, in a large cohort of overweight individuals with diabetes, 55.2% lost over 7% of their body weight at 1 year.29
Whereas other treatments for incontinence, including both behavioral and pharmacologic approaches, are targeted to urinary incontinence alone, weight loss has a wide spectrum of benefits for overweight and obese women. Several studies show that modest weight losses of 5–10% of body weight are associated with improvements in a range of health outcomes, including reducing the risk of developing noninsulin-dependent diabetes mellitus and controlling and preventing hypertension.15–18 A sustained decrease in urinary incontinence can now be added to the extensive list of health improvements associated with modest weight loss.
Strengths of this study include the large, diverse, well-characterized cohort of overweight and obese women, the use of a standardized lifestyle intervention program, the validated outcome measures collected by blinded assessors, and the excellent follow-up through 18 months. Participants in this trial reported levels of incontinence (mean of 24 episodes per week) that appear similar to cohorts of women undergoing surgery for incontinence30 (mean of 22.4 episodes per week) and in trials of incontinence medication and a continence pessary.31,32 However, in interpreting our findings, it is important to note that we are reporting associations between categories of weight change and urinary incontinence outcomes; participants were not randomly assigned to different categories of weight loss. Thus, the groups may not have differed only in weight loss. Most notably, our lifestyle intervention included both weight loss and physical activity; thus, changes in physical activity as well as weight loss may contribute to the urinary incontinence improvements reported here. However, to adjust for these possible confounders, we have added key variables to our multivariable statistical models, including demographic characteristics, use of pelvic floor exercises, and physical activity, in examining our results.
In summary, modest weight losses of 5–10% were associated with statistically and clinically significant reductions in urinary incontinent episodes and with satisfaction with improvements in continence. The broad range of health benefits achieved with weight reduction strongly supports consideration of this approach as initial treatment for overweight women with incontinence.
Acknowledgments
Financial Disclosure Dr. Richter is a consultant for IDEO and Xanodyne Pharmaceuticals, Inc and has received grants on behalf of her institution from Pfizer, Astellas, the University of California-San Francisco/Pfizer, and the American Geriatrics Society. Dr. Burgio is a consultant with Pfizer, Astellas, and Johnson & Johnson and has received grant support from Pfizer. Dr. Subak has received grants on behalf of her institution from Pfizer. The other authors did not report any potential conflicts of interest.
Supported by grant nos. U01DK067860, U01 DK067861, and U01 DK067862 from the National Institute of Diabetes and Digestive and Kidney Diseases. Funding also was provided by the Office of Research on Women's Health.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
CLINICAL TRIAL REGISTRATION: ClinicalTrials.gov, www.clinicaltrials.gov, NCT00091988.
REFERENCES
- 1.Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: a population-based study. Arch Intern Med. 2005;165:537–42. doi: 10.1001/archinte.165.5.537. [DOI] [PubMed] [Google Scholar]
- 2.Thom DH, Brown JS. Reproductive and hormonal risk factors for urinary incontinence in later life: a review of the clinical and epidemiologic literature. J Am Geriatr Soc. 1998;46:1411–7. doi: 10.1111/j.1532-5415.1998.tb06009.x. [DOI] [PubMed] [Google Scholar]
- 3.Grimby A, Milsom I, Molander U, Wiklund I, Ekelund P. The influence of urinary incontinence on the quality of life of elderly women. Age Ageing. 1993;22:82–9. doi: 10.1093/ageing/22.2.82. [DOI] [PubMed] [Google Scholar]
- 4.Hunskaar S, Vinsnes A. The quality of life in women with urinary incontinence as measured by the sickness impact profile. J Am Geriatr Soc. 1991;39:378–82. doi: 10.1111/j.1532-5415.1991.tb02903.x. Erratum in J Am Geriatr Soc 1992;40:976–7. [DOI] [PubMed] [Google Scholar]
- 5.Fantl JA, Wyman JF, McClish DK, Harkins SW, Elswick RK, Taylor JR, et al. Efficacy of bladder training in older women with urinary incontinence. JAMA. 1991;265:609–13. [PubMed] [Google Scholar]
- 6.Burgio KL, Robinson JC, Engel BT. The role of biofeedback in Kegel exercise training for stress urinary incontinence. Am J Obstet Gynecol. 1986;154:58–64. doi: 10.1016/0002-9378(86)90393-5. [DOI] [PubMed] [Google Scholar]
- 7.Fantl J, Newman D, Colling J, Delancey JO, Keeys C, McDowell B. Clinical practice guideline. No. 2. Rockville (MD): 1996. Urinary incontinence in adults: acute chronic management. [Google Scholar]
- 8.Danforth KN, Townsend MK, Lifford K, Curhan GC, Resnick NM, Grodstein F. Risk factors for urinary incontinence among middle-aged women. Am J Obstet Gynecol. 2006;194:339–45. doi: 10.1016/j.ajog.2005.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannestad YS, Rortveit G, Daltveit AK, Hunskaar S. Are smoking and other lifestyle factors associated with female urinary incontinence? The Norwegian EPINCONT Study. BJOG. 2003;110:247–54. [PubMed] [Google Scholar]
- 10.Waetjen LE, Liao S, Johnson WO, Sampselle CM, Sternfield B, Harlow SD, et al. Factors associated with prevalent and incident urinary incontinence in a cohort of midlife women: a longitudinal analysis of data: study of women's health across the nation. Am J Epidemiol. 2007;165:309–18. doi: 10.1093/aje/kwk018. [DOI] [PubMed] [Google Scholar]
- 11.Bump RC, Sugerman HJ, Fantl JA, McClish DK. Obesity and lower urinary tract function in women: effect of surgically induced weight loss. Am J Obstet Gynecol. 1992;167:392–7. doi: 10.1016/s0002-9378(11)91418-5. discussion 397–9. [DOI] [PubMed] [Google Scholar]
- 12.Subak LL, Whitcomb E, Shen H, Saxton J, Vittinghoff E, Brown JS. Weight loss: a novel and effective treatment for urinary incontinence. J Urol. 2005;174:190–5. doi: 10.1097/01.ju.0000162056.30326.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgio KL, Richter HE, Clements RH, Redden DT, Goode PS. Changes in urinary and fecal incontinence symptoms with weight loss surgery in morbidly obese women. Obstet Gynecol. 2007;110:1034–40. doi: 10.1097/01.AOG.0000285483.22898.9c. [DOI] [PubMed] [Google Scholar]
- 14.Subak LL, Wing R, West DS, Franklin F, Vittinghoff E, Creasman JM, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360:481–90. doi: 10.1056/NEJMoa0806375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller ER, 3rd, Erlinger TP, Young DR, Jehn M, Charleston J, Rhodes D, et al. Results of the Diet, Exercise, and Weight Loss Intervention Trial (DEW-IT) Hypertension. 2002;40:612–8. doi: 10.1161/01.hyp.0000037217.96002.8e. [DOI] [PubMed] [Google Scholar]
- 17.Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 18.Tuomilehto J, Lindstrom J, Eriksson J, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 19.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 20.Look AHEAD Research Group. Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it [published erratum appears in Obesity (Silver Spring) 2007;15:1339] Obesity (Silver Spring) 2006;14:737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgio KL, Pearce LK, Lucco AJ. Staying dry: a practical guide to bladder control. The John Hopkins University Press; Baltimore (MD): 1989. [Google Scholar]
- 22.Paffenbarger RS, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–75. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 23.Nygaard I, Holcomb R. Reproducibility of the seven-day voiding diary in women with stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:15–7. doi: 10.1007/pl00004021. [DOI] [PubMed] [Google Scholar]
- 24.Abrams P, Blaivas JG, Stanton SL, Andersen JT. The standardisation of terminology of lower urinary tract function. The International Continence Society Committee on Standardisation of Terminology. Scand J Urol Nephrol Suppl. 1988;114:5–19. [PubMed] [Google Scholar]
- 25.Burgio KL, Goode PS, Richter HE, Locher JL, Roth DL. Global ratings of patient satisfaction and perceptions of improvement with treatment for urinary incontinence: validation of three global patient ratings. Neurourol Urodyn. 2006;25:411–7. doi: 10.1002/nau.20243. [DOI] [PubMed] [Google Scholar]
- 26.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 27.Little RJA, Rubin DB. Statistical analysis with missing data. 2nd ed. John Wiley & Sons; New York (NY): 2002. [Google Scholar]
- 28.Albo ME, Richter HE, Brubaker L, Norton P, Kraus SR, Zimmern PE, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143–55. doi: 10.1056/NEJMoa070416. [DOI] [PubMed] [Google Scholar]
- 29.Look AHEAD Research Group. Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richter HE, Burgio KL, Brubaker L, Moalli PA, Markland AD, Mallet V, et al. Factors associated with incontinence frequency in a surgical cohort of stress incontinent women. Am J Obstet Gynecol. 2005;193:2088–93. doi: 10.1016/j.ajog.2005.07.068. [DOI] [PubMed] [Google Scholar]
- 31.Burgio KL, Kraus SR, Menefee S, Borello-France D, Corton M, Johnson HW, et al. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Ann Intern Med. 2008;149:161–9. doi: 10.7326/0003-4819-149-3-200808050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richter HE, Burgio KL, Brubaker L, Nygaard IE, Ye W, Weidner A, et al. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609–17. doi: 10.1097/AOG.0b013e3181d055d4. [DOI] [PMC free article] [PubMed] [Google Scholar]