Abstract
Fish oil improves several features of metabolic syndrome such as dyslipidemia, insulin resistance and hepatic steatosis. Fish oil may mediate some of its beneficial effects by modulating the storage and/or secretory functions of adipose tissue. The storage of triglycerides in adipose tissue is regulated by the availability of free fatty acids as well as the degree of lipolysis in adipose tissue. Fish oil has been shown to reduce lipolysis in several studies indicating improved triglyceride storage. Importantly, adipose tissue secretes a variety of adipokines and fish oil feeding is associated with remarkable changes in the plasma levels of two key adipokines, adiponectin and leptin. Much attention has been focused on the contribution of adiponectin in fish oil mediated improvements in metabolic syndrome. However, emerging evidence also indicates a role of leptin in modulating the components of the metabolic syndrome upon fish oil feeding. In addition to improving the storage and secretory functions of adipose tissue, fish oil, and the n-3 fatty acids found in fish oil, has been shown to reduce inflammation in adipose tissue. These effects may be in part a result of activation of peroxisome proliferator-activated receptor γ or inhibition of toll-like receptor 4. Thus, there is compelling evidence that fish oil mediates its beneficial effects on metabolic syndrome by improving adipose tissue storage and secretory functions and by reducing inflammation.
Keywords: Fish oil, adipose tissue, adiponectin, leptin, AMPK, PPARγ, TLR4
I. Introduction
Obesity has led to alarming increases in the incidence of many chronic diseases, including type 2 diabetes and cardiovascular disease (CVD). Because overnutrition leads to obesity, manipulation of dietary nutrient content is a logical means of alleviating this problem. Fish oil consumption is associated with various health benefits. Along with lowering of plasma triglycerides (TG), fish oil also improves insulin sensitivity, reduces blood pressure, inflammation, thrombosis and arrhythmia, contributing to its role in lowering risk of CVD and diabetes (reviewed in [1, 2]). Hepatocentric hypolipidemic effects of fish oil have been extensively evaluated (for review, please see [3]) and undoubtedly play a major part in the reduction of risk for chronic disease that is associated with its consumption. However, potential adipocentric beneficial effects of fish oil have not been fully elucidated. Fish oil supplementation is a simple therapy for reducing the risk of both diabetes and CVD, and understanding the mechanisms through which fish oil improves metabolic phenotypes will allow for greater benefits from this nutraceutical. Evidence points to the role of adipose tissue (AT) in fish oil mediated improvements on features of metabolic syndrome (MetS) such as insulin resistance (IR) and dyslipidemia. The overall effects of n-3 fatty acids on AT biology and metabolism have been recently reviewed [4]. Thus, the current article is focused on the mechanistic details as to how peroxisome proliferator-activated receptor γ (PPARγ) and toll-like receptor 4 (TLR4) signaling modulate AT storage and/or secretory functions and inflammatory response, thereby modulating MetS.
II. Adipose Tissue
AT plays an important role in regulating lipid homeostasis by storing excess energy in the form of TG. Thus the lipid storage function of AT is critical in buffering the daily influx of dietary fatty acids entering the circulation. AT controls plasma levels of free fatty acids (FFA) by suppressing the release of non-esterified fatty acids into the circulation. Impairment of AT storage function, as seen in obesity and lipodystrophic conditions, is associated with increased lipolysis. This results in excessive release of FFAs into the circulation, which leads to ectopic lipid deposition thereby contributing to the development of IR. It has been well-established that AT is not just an inert storage organ but AT secretes many bioactive substances called adipokines. Adiponectin and leptin are two important adipokines secreted by AT that have an undeniable influence in modulating glucose and lipid metabolism via their autocrine and paracrine effects. In addition to the above mentioned adipokines, AT in obesity releases several inflammatory mediators such as monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6), and tumor necrosis factor α (TNFα). Mounting evidence suggests that macrophages accumulate in AT in obesity and secrete these inflammatory mediators thereby activating inflammatory pathways in obese AT [5, 6]. It is now widely accepted that AT inflammation modulates the storage and/or secretory functions of AT. Because of its crucial role in energy homeostasis, AT is now considered to be a potential target for therapeutic agents and dietary factors that are known to improve features of MetS.
III. Incorporation of n-3 fatty acids into AT
One mechanism by which dietary fish oil may exert its favorable effects in AT is by incorporation of the n-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), into lipid fractions of the adipocytes. Luo et al. [7] demonstrated that feeding Sprague-Dawley rats with a fish oil diet for 6 weeks increased the incorporation of n-3 fatty acids into the membrane phospholipid fraction of adipocytes. This was associated with increased insulin sensitivity in the adipocytes, as insulin-stimulated glucose uptake was positively correlated with the degree of unsaturation of membrane phospholipid fatty acids [7]. Fickova et al. [8] also demonstrated the incorporation of n-3 fatty acids into adipocyte membrane phospholipids after feeding of male Wistar rats with a fish oil diet for only one week. This was associated with a significantly lower concentration of serum TG, cholesterol and insulin [8]. Fish oil feeding has also been shown to increase incorporation of n-3 fatty acids into the TG fraction of AT in rats [9]. Several other human studies have also shown that intake of dietary n-3 fatty acids results in accumulation of these lipids in AT [10–12]. Thus, dietary fish oils clearly make their way into the membrane phospholipids and TG lipid droplets of adipocytes, where they have the potential to modulate signaling events, thereby altering the metabolic activity of AT.
IV. Dietary fish oil and AT storage function
A. AT mass
Because AT functions as a reservoir for storage of excess fatty acids into TGs, the fatty acid flux in AT determines the degree of adiposity or AT mass. In some animal models and in human subjects, fish oil supplementation reduced adiposity, thus exerting an anti-obesity effect. The reduced AT mass upon fish oil feeding was shown to be associated with improvements in several risk factors of MetS. For example, fish oil reduced IR and ameliorated dyslipidemia and hepatic steatosis (Table 1). Because the n-3 fatty acids promote hepatic fatty acid oxidation and reduce fatty acid synthesis in liver (reviewed in [3]), the reduced adiposity in these studies may be due to a decreased availability of FFAs for storage.
Table 1.
Effect of dietary fish oil or n-3 fatty acids on AT mass and the associated metabolic parameters in mice:
| Change in AT mass with fish oil or n-3 fatty acids | |||
|---|---|---|---|
| Metabolic Parameter | Decreased AT mass | Increased AT mass | No change in AT mass |
| Systemic IR | ↓ [7, 13–19] | ↓ [20] | ↓ [21] |
| Dyslipidemia | ↓ [7, 8, 12, 13, 16, 17, 19, 22–25] | ↓ [26] | ↓ [21] |
| Hepatic steatosis | ↓ [13, 19, 25] | ↓ [20, 26] | ↓ [21] |
| AT inflammation and/or IR | ↓ [7, 13, 14, 22, 27] | ↓ [20, 26] | ↓ [21, 28] |
| Atherosclerosis | --- | ↓ [26] | --- |
| Plasma adiponectin | ↑ [12, 19, 23, 24] | ↑ [20, 26] | ↔ [28] |
| Plasma leptin | ↓ [12, 13, 17, 18, 29]; ↑ [19, 22, 30] | ↑[20] | ↑[28] |
↑, increase; ↓, decrease; ↔, no change
Despite the fact that fish oil was shown to exert an anti-obesity effect in a number of mouse studies, it is becoming clear that this effect of fish oil may depend on the genetic background. For example, Ide reported that dietary fish oil increased AT mass in ICR mice fed a high sucrose diet [20]. Interestingly, in this study, the increased fat mass was associated with improved insulin sensitivity and reduced hepatic steatosis. In addition, our own study demonstrated that fish oil feeding increased AT mass in low density lipoprotein receptor deficient (LDLR−/−) mice [26] and the increased fat mass was associated with improvements in dyslipidemia and hepatic steatosis. Moreover, our study showed that an increase in AT mass, as a result of improved TG storage, is also associated with reduction in atherosclerotic lesion formation, indicating a role for AT in modulating the development of cardiovascular disease. It should also be noted that in some studies, fish oil did not alter the degree of adiposity but still improved the pattern of adipokines secreted from AT and exerted beneficial effects on features of MetS [21, 28]. Taken together, dietary fish oil exerts an overall beneficial effect in overcoming the features of MetS independent of its effects on AT mass (Table 1).
B. AT lipolysis
As mentioned, obese AT releases excess FFAs into circulation via enhanced lipolysis. Thus, the rate of lipolysis in AT is inversely correlated to storage of TG in AT in obesity. A large body of evidence suggests that fish oil and n-3 fatty acids reduce lipolysis in AT. For example, Soria et al. reported that isolated adipocytes from rats fed a sucrose-rich diet exhibited increased basal and stimulated lipolysis whereas normalization of lipolysis was observed in adipocytes derived from rats fed a diet rich in fish oil [16]. In addition, Rossi et al. showed that basal lipolysis in isolated epididymal AT was significantly reduced by fish oil [31]. Rustan et al. showed that both basal and stimulated lipolysis are increased in adipocytes derived from epididymal and perirenal AT upon feeding rats with a lard diet and this effect was reduced by a diet rich in n-3 fatty acids [32]. Other studies also showed that fish oil reduced AT lipolysis [33, 34].
Potential mechanism(s) by which fish oil improves TG storage in AT is via modulation of lipoprotein lipase (LPL), hormone sensitive lipase (HSL), and fatty acid synthase (FAS) in AT. While the first two are involved in the release of fatty acids, FAS facilitates the storage of fatty acids as TGs in AT. Haug et al. showed that fish oil reduced both LPL and HSL activity in AT [35]. Moreover, the mRNA expression levels of LPL and HSL were significantly reduced in AT of rats fed an n-3 fatty acid rich diet [18]. Lombardo et al. showed that dietary n-3 fatty acids reduced LPL activity in AT in rats concomitant with improvements in the risk factors of MetS [36]. On the other hand, fish oil increased LPL activity in AT in a separate study, which was associated with reduced AT mass. However, the activity of FAS, a lipogenic enzyme, was also increased upon fish oil feeding, leading the authors to conclude that fish oil increases lipid mobilization but does not decrease lipid storage [37]. Because fish oil reduced AT lipolysis and modulated the expression and/or activities of enzymes involved in TG storage in AT, these various reports indicate that fish oil improves TG storage in AT.
V. Dietary fish oil and adipokines
As mentioned, AT is an active endocrine organ secreting several adipokines with potent systemic physiologic and pathophysiologic effects. In particular, adiponectin and leptin regulate energy homeostasis and metabolism. Fish oil has repeatedly been shown to increase plasma levels of adiponectin, an anti-inflammatory and insulin sensitizing adipokine, in rodents as well as in human subjects (see Table 1). On the other hand, the impact of dietary fish oil on plasma leptin levels varies depending on the study. In addition to these two adipokines, fish oil has also been shown to modulate the mRNA expression of other adipocyte-derived adipokines such as visfatin and apelin; however, the circulating levels of these adipokines were not altered significantly with fish oil feeding [38]. With regard to resistin another adipokine considered to modulate IR, EPA has been shown to decrease its mRNA expression in 3T3 L1 adipocytes [39]. However, another study showed that resistin mRNA levels were not altered in the AT of ob/ob mice that received a diet enriched in n-3 fatty acids [21]. Because fish oil has been shown to greatly modulate plasma levels of adiponectin and leptin, this article focuses on the role of these two adipokines in mediating the beneficial effects of fish oil in regulating glucose and lipid homeostasis.
A. Adiponectin
Adiponectin is unlike other adipocytokines in that its production is decreased with obesity and its concentrations in the circulation are inversely correlated with IR. Various research groups have found an increase in circulating adiponectin with fish oil supplementation both in animal models [19, 26, 40] as well as in human subjects [12, 23]. Because a number of studies have shown an increase in plasma adiponectin levels by dietary fish oil, it is accepted that fish oil mediates some of its beneficial effects via modulating adiponectin levels.
With regard to improving features of MetS, adiponectin is a pleiotropic adipokine with several beneficial effects (reviewed in [41]). Because adiponectin plays a critical role in improving insulin sensitivity, it is reasonable to speculate that fish oil exerts its insulin sensitizing effects via adiponectin. In fact, high sucrose diet-induced IR was ameliorated by fish oil consumption in a study completed by Rossi et al. [19], and this was accompanied by a significant elevation in plasma adiponectin concentrations. Adiponectin can also promote hepatic fatty acid oxidation, thus reducing lipid accumulation in liver [42]. Yano et al. have shown that mice deficient for both leptin and adiponectin exhibit an increase in hepatic TGs relative to mice that are only leptin deficient [43]. Thus, the beneficial effects of fish oil on hepatic steatosis may be mediated via adiponectin. It should also be noted that adiponectin regulates the activity of 5′-adenosine monophosphate activated protein kinase (AMPK), which is an important modulator of both glucose and lipid metabolism (reviewed in [44]). Thus, dietary fish oil, via increasing circulating adiponectin levels, can improve features of MetS such as IR, dyslipidemia, and hepatic steatosis (Table 1).
B. Leptin
Similar to adiponectin, leptin has been shown to regulate several aspects of lipid metabolism, such as increasing hepatic fatty acid oxidation and plasma TG clearance, as well as reducing TG secretion into plasma [45, 46]. However, unlike adiponectin, the plasma level of which is consistently increased by fish oil, the effect of fish oil in modulating plasma leptin levels is unclear. Plasma leptin levels are almost always positively correlated with the degree of adiposity. Consistent with this observation, fish oil has been shown to decrease plasma leptin in association with reduced adiposity [19, 22, 30], and to increase plasma leptin in association with increased adiposity ([20, 47] and our unpublished data). Interestingly, other evidence suggests that fish oil feeding increases plasma leptin levels despite reducing the degree of adiposity [19, 22, 30]. It should also be noted that even without altering AT mass, fish oil can increase plasma leptin (Table 1) [28]. Importantly, it has been reported that EPA can increase the mRNA and protein levels of leptin in 3T3-L1 cells in vitro, suggesting that the n-3 fatty acids present in fish oil can increase the expression of leptin in adipocytes. These studies suggest that fish oil has the potential to increase plasma leptin levels independent of AT mass.
Given that leptin significantly affects lipid metabolism, these studies indicate that leptin may play a role, at least in part, in mediating the lipid lowering effects of fish oil in plasma and liver. This notion is supported by the observation that an EPA supplemented diet did not reduce plasma TG levels in leptin deficient ob/ob mice [23]. In fact, we recently showed that fish oil exerts a potent hypolipidemic effect in lean LDLR−/− mice but not in obese leptin deficient ob/ob;LDLR−/− mice [47]. Leptin, like adiponectin, regulates the activity of AMPK, modulating both glucose and lipid metabolism. Of note, fish oil feeding has been shown to increase hepatic AMPK activity and to promote lipid oxidation [48]. Our recent study also showed that fish oil increases hepatic AMPK phosphorylation, which was associated with reduced hepatic steatosis and dyslipidemia in lean LDLR−/− mice. Interestingly, the lipid-lowering effects of fish oil are impaired in leptin deficient ob/ob;LDLR−/− mice concomitant with an impairment in hepatic AMPK phosphorylation [47]. Thus, in addition to adiponectin, leptin also plays a role in mediating the beneficial effects of dietary fish oil against features of metS.
VI. Dietary fish oil and inflammation and oxidative stress
A. Inflammation
Fish oil has been shown to lower inflammatory response in various in vitro models as well as in human subjects (reviewed in [49]). Recent studies suggest that fish oil may exert an anti-inflammatory effect in AT. It is well-established that macrophages accumulate in AT in obesity and participate in inflammatory pathways that are activated in obese AT. Thus, it is possible that fish oil, being a potent anti-inflammatory agent, modulates macrophage infiltration into AT and the subsequent induction of inflammatory responses in AT. In fact, Todoric et al. demonstrated that an n-3 fatty acid-enriched diet abolished macrophage infiltration into AT and also reduced inflammatory markers in AT of db/db mice [50]. In addition, we demonstrated that dietary fish oil reduced macrophage infiltration and inflammation in AT in LDLR−/− mice [26]. EPA treatment has also been shown to reduce the expression of IL-6 in AT in obese rats [27]. Muurling et al. have shown that a fish oil diet reduced the protein level of TNFα in the AT of apoE*3-Leiden transgenic mice fed a high fat diet which did not reduce IR but inhibited hepatic VLDL-TG production [51]. Thus, these studies indicate that fish oil can reduce inflammatory events in AT.
B. Oxidative Stress
While the antioxidant effects on other cell types and tissues have been demonstrated [52, 53] little is known regarding the antioxidant potential of n-3 fatty acids or fish oil in AT. Our previous study has shown that fish oil feeding is associated with a significant reduction in F2-isoprostane levels in AT [26]. Because measurement of F2- isoprostanes is considered the “gold standard” for assessment of systemic oxidative stress, our study indicates that fish oil contributes to improved AT function via reducing oxidative stress in AT.
VII. Potential mechanisms by which fish oil improves AT functions
A. PPARγ
PPARs play an integral role in reducing IR, dyslipidemia, and inflammation (reviewed in [54]). Because EPA and DHA are endogenous ligands for PPARs, it is possible that PPARs mediate the insulin sensitizing, lipid lowering, and anti-inflammatory properties of fish oil. Available evidence indicates that not only the n-3 fatty acids but also their metabolites are potent PPARγ agonists [55]. Increasing evidence suggests that the interaction between fish oil and PPARs plays a critical role in regulating glucose and lipid metabolism in liver (reviewed in [21, 56, 57] and [21]). However, little is known regarding the contributions of PPARs to the beneficial effects of fish oil in AT. Although PPARγ is expressed in liver, muscle, colon, and AT, its expression is much greater in AT than other tissues. PPARγ is necessary for adipocyte differentiation and thus for lipid storage [58]. In addition, expression of genes involved in adipogenesis and lipid storage are upregulated by stimulation of PPARγ [59]. In AT, PPARγ enhances lipid uptake, increases fatty acid synthesis, increases TG formation to promote fat storage, and prevents TG lipolysis. As mentioned, fish oil has differential effects on adiposity depending on different animal models. Fish oil has been shown to reduce adiposity in a number of studies and it is not clear whether PPARγ was altered in these conditions. On the other hand, fish oil increases adiposity in LDLR−/− mice and in ICR mice and the potential role of PPARγ in modulating adiposity in these models is unclear. However, based on the overall beneficial effects of fish oil in improving insulin sensitivity and lipid metabolism in these models, it is reasonable to speculate that PPARγ may have a role in mediating the improved storage function of AT upon fish oil feeding.
With regard to the secretion of adipokines, PPARγ agonists have been shown to increase circulating levels of adiponectin [60, 61]. Moreover, the administration of fish oil to PPARγ null mice did not have an effect on circulating adiponectin, indicating that the effects are PPARγ-dependent [40]. In vitro experiments in adipocytes have shown that EPA and DHA significantly increase synthesis and secretion of the high molecular weight form of adiponectin, and that this effect is PPARγ dependent [62]. Thus, these studies suggest that fish oil increases circulating adiponectin, and this effect is mediated via PPARγ.
It should also be noted that the increase in circulating adiponectin with PPARγ stimulation may depend more on post-transcriptional or post-translational modifications than on alterations in mRNA synthesis, as some researchers have failed to find significant changes in adiponectin synthesis with PPARγ agonist treatment despite large increases in circulating adiponectin [63, 64]. Banga et al. [62] treated adipocytes with the PPARγ agonist pioglitazone, EPA or DHA; and found that all 3 treatments increased adiponectin protein content without affecting adiponectin mRNA. Addition of a PPARγ inhibitor prevented this increase in protein synthesis. The authors speculated that adipocytes contain a protein, or some other constituent, that inhibits adiponectin translation, and stimulation of PPARγ leads to reduction in the inhibitory effects of this molecule. More research is necessary, but it appears promising that adiponectin secretion is stimulated post-transcriptionally by fish oil and other PPARγ agonists.
In addition, adiponectin undergoes post-transcriptional hydroxylation and glycosylation, which may alter how it is secreted [65, 66]. Exciting research indicates that the secretion of adiponectin oligomers may be regulated by the endoplasmic reticulum (ER) chaperones ERp44 and Ero1-Lα [67, 68]. It was found that a large portion of adiponectin is bound to ERp44 in the AT, inhibiting its release; whereas elevated Ero1-Lα allows for release of adiponectin from ERp44 and greater secretion [69]. The mechanisms through which fish oil and other PPARγ agonists (TZDs) increase adiponectin secretion are still unknown. However, promising work by Wang et al. (66) and Qiang et al. (67) indicates that TZDs may affect expression of ERo1-Lα. In these studies, 3T3-L1 adipocytes and AT from ob/ob mice displayed greater expression of Ero1-Lα after TZD treatment, providing a potential mechanism for increased release of adiponectin and greater circulating HMW adiponectin [67, 68]. More research is needed to assess the effects of fish oil and other PPARγ agonists on thiol binding and other post-translational modifications that may elevate circulating adiponectin.
Although much is known regarding the involvement of PPARγ in regulating plasma adiponectin, its role in regulating plasma leptin levels is not clear. Some studies have shown that PPARγ decreases plasma leptin [70, 71]. However, AT-specific PPARγ knock out mice have been reported to exhibit decreased levels of plasma leptin and adiponectin [72]. Moreover, PPARγ agonists improve insulin sensitivity with increased serum leptin levels [73, 74]. As mentioned, fish oil and n-3 fatty acids have been shown to increase plasma leptin in a number of studies [20, 22, 28, 30, 47]. These studies suggest that PPARγ may play a role in regulating plasma leptin levels and that modulation of PPARγ and leptin signaling by n-3 fatty acids is one mechanism by which these fatty acids mediate their lipid lowering and insulin sensitizing effects.
PPARγ also plays a role in regulating the inflammatory response in various ways. In monocytes, PPARγ reduces inducible nitric oxide synthase (iNOS), matrix metalloproteinase-9 (MMP-9) [75], TNFα, interleukin-1β (IL-1β), and IL-6 [76]. This reduction in pro-inflammatory cytokine expression is associated with a decrease in the transcriptional activity of signal transducer and activator of transcription-1 (STAT1), nuclear factor κB (NFκB) and activator protein-1 (AP-1) [75]. This provides a potential mechanism through which a PPARγ agonist like fish oil could prevent the transcription of pro-inflammatory cytokines. It should also be pointed out that PPARs are now recognized as important determinants of macrophage polarization. Obesity is associated with a shift in macrophage phenotype in AT from the alternatively activated (anti-inflammatory) M2 phenotype to a pro-inflammatory M1 phenotype [77], and PPARγ has been reported to promote macrophage polarization towards an anti-inflammatory M2 phenotype [78, 79]. Thus, based on the crucial role of PPARγ in regulating AT functions, future studies are required to determine whether EPA and DHA, as ligands of PPARγ, may facilitate fish oil-mediated improvement of AT functions.
B. Alterations in Pattern Recognition Receptors
The anti-inflammatory effects of fish oil may be attributable in part to their ability to blunt signaling of the pattern recognition receptors such as TLR4 and the family of nucleotide-binding oligomerization domain-containing proteins (Nods) that play a role in host defense. Lipopolysaccharide (LPS) is the main ligand for TLR4; however, saturated fatty acids have also been shown to stimulate the TLR4 signaling pathway. On the other hand, n-3 polyunsaturated fatty acids inhibit TLR4 signaling. For example, Lee et al. found that DHA decreased NFκB activation by TLR4 in a dose-dependent manner in macrophages [80]. Mice deficient in TLR4 are protected against inflammation and IR resulting from diet-induced obesity [81, 82]; pointing to inhibition of TLR4 as a potential mechanism through which fish oil n-3 fatty acids reduce inflammation.
The mechanisms by which n-3 fatty acids modulate TLR4 signaling are not completely clear. However, evidence suggests a role for n-3 fatty acids in preventing the translocation of TLR4 into lipid rafts, an initial event involved in TLR4 signaling. Wong et al. reported that TLR4 is recruited to lipid rafts on the membrane of macrophages upon treatment with its ligand LPS or lauric acid [83]. This recruitment appears necessary for TLR4 signaling, as lipid raft disruption with nystatin inhibits NFκB activation by LPS and lauric acid. DHA prevents this translocation, interfering with the interaction between TLR4 and MyD88, thus inhibiting the signaling pathway through which this receptor stimulates secretion of pro-inflammatory cytokines.
There are two potential mechanisms put forth for how DHA may prevent TLR4 localization to lipid rafts; reduction of reactive oxygen species (ROS) generation via suppression of NADPH oxidase and disruption of lipid raft formation as a result of DHA incorporation into plasma membranes. As for the role of NADPH oxidase in modulating TLR4 signaling, it has been shown that induction of the ROS H2O2 in HEK 293 cells is dependent on stimulation of NADPH oxidase 4, which interacts directly with TLR4 [84]. Nakahira et al. found that ROS production by NADPH oxidase was necessary for LPS-stimulated TLR4 translocation to lipid rafts [85]. Wong et al. showed that LPS and lauric acid stimulate NADPH oxidase whereas DHA inhibits this enzyme [83].
With regard to the role of fatty acids in modulating lipid raft formation, saturated fatty acids aggregate to form membrane regions that are resistant to detergents, helping to stabilize lipid rafts. In fact, the majority of polar lipids present in lipid rafts are saturated fatty acids. Interestingly, Stulnig et al. reported that treatment of Jurkat T cells with EPA caused significant incorporation of n-3 PUFAs into the lipid rafts [86]. Thus, modulation of lipid raft formation and/or composition may destabilize the lipid rafts, serving as a mechanism for fish oil-mediated inhibition of TLR4 signaling and prevention of downstream production of pro-inflammatory cytokines. As mentioned, fish oil n-3 fatty acids reduce the AT levels of TNFα [26, 51]. TNFα has been shown to stimulate hormone sensitive lipase (HSL) (reviewed in [87]) and promote lipolysis in adipocytes [88]. Thus, a reduction in AT lipolysis by fish oil [28, 31, 33, 34] may be attributed, at least in part, to a decrease in secretion of TNFα with TLR4 inhibition. Therefore, by reducing lipolysis, fish oil limits the release of FFAs from AT in obesity thus ameliorating features of metS and inhibition of TLR4 may be important for these effects of fish oil.
Similar to TLRs, Nod proteins stimulate pro-inflammatory pathways in response to bacterial products. Zhao et al. [89] treated colonic epithelial cells with the saturated fatty acid lauric acid, and reported a dose-dependent activation of NFκB and induction of IL-8. However, the pro-inflammatory effects of lauric acid were not present in Nod1 or Nod2 dominant negative cells. Mirroring TLR4 data presented above, n-3 fatty acids inhibited the stimulation of NFκB and production of IL-8 by lauric acid. Additionally, while lauric acid promoted Nod2 signaling in HEK293T cells, the pathway was inhibited by DHA [89]. Further research is necessary to determine if inhibition of Nod proteins contributes to the anti-inflammatory effects of fish oil in AT.
VIII. Fish oil n-3 fatty acids and different AT depots
It is not clear whether the effects of fish oil n-3 fatty acids in modulating adiposity and AT gene expression are depot-specific. Belzung et al. have shown that fish oil-derived n-3 fatty acids selectively limit the hypertrophy of abdominal fat depots [90]. In this study, they showed that the AT mass was significantly increased in rats that received a high fat diet supplemented with different concentrations of n-3 fatty acids compared to control rats that received standard laboratory diet, in all four major fat depots such as subcutaneous (SC), mesenteric (MES), retroperitoneal (RP), and epididymal (EPI). Interestingly, the AT mass in RP and EPI fat depots was significantly lower in rats that received a high amount of n-3 fatty acids in their diet than rats fed a low or a medium amount of n-3 fatty acids. On the other hand, the presence of high amount of n-3 fatty acids did not change the AT mass in SC and MES fat depots. Although this study suggests the depot-specific effects of n-3 fatty acids on adiposity, further evidence is needed to support this notion.
Not only AT mass but also the gene expression profile appears to vary with different fat depots. N-3 fatty acids upregulate the mRNA expression of fatty acid synthase (FAS), HSL, lipoprotein lipase (LPL), CCAAT/enhancer binding protein alpha, and leptin in RP but not in SC AT [18]. However, with regard to the expression of inflammatory markers, Todoric et al. have shown that a fish oil diet reduced the expression of inflammatory genes in both gonadal and SC fat depots suggesting that the preventive effects of n-3 fatty acids on AT inflammation may be common to different fat depots [50]. Thus, the depot-specific effect of n-3 fatty acids on gene expression in AT is still unclear. Moreover, it is not known whether the effects of fish oil in different AT depots were regulated by PPARγ and TLR4.
In addition to modulating the functions of white AT, fish oil has also been shown to alter the expression of genes involved in thermogenesis in brown AT (BAT). Raclot et al. have shown that fish oil feeding is associated with incorporation of n-3 fatty acids into the TG fraction of BAT [91]. A fish oil diet has been shown to increase the mRNA expression of uncoupling protein-1 (UCP-1) in the BAT in rats. On the other hand, Oudart et al. have shown that n-3 fatty acids increase thermogenesis in BAT without altering the uncoupling protein (UCP) content in rats [92]. Finally, fish oil feeding has been shown to increase UCP-2 mRNA and decrease UCP-3 mRNA in BAT in mice [93]. These studies suggest that fish oil may also modulate lipid metabolism via altering UCP expression in BAT.
IX. Fish oil in human research and clinical application
While the hypolipidemic effects of fish oil are well-established in humans (reviewed in [3]), the effects on insulin sensitivity are inconclusive. For example, treatment for 2 months with 3g/day of n-3 polyunsaturated fatty acids reduced adiposity and some atherogenic factors but did not improve insulin sensitivity in women with type 2 diabetes [94]. This discrepancy may be due to several factors, including differences in metabolism between animals and humans, inadequate doses of n-3 fatty acids, or health status and dietary intake of the subjects studied. Kopecky et al. speculated that, given the significantly greater relative metabolic rate of mice compared to humans, plasma and tissue saturation with n-3 fatty acids may be a better indicator of dose adequacy than direct extrapolation of intake [95]. The dose to achieve saturation of plasma phospholipids is physiological, ~2 g/day of DHA according to Arterburn et al. [96], but the effectiveness of a fish oil dose can be affected by various confounding dietary factors. The typical Western diet, abundant in sucrose and trans, saturated and n-6 polyunsaturated fatty acids, provide a potential for inflammation that may offset the insulin-sensitizing effects of a supplemental dose of n-3 fatty acids. Putative DHA metabolites have been shown to be ligands for PPARγ and increase its transcriptional activity [55], promoting an anti-inflammatory environment. However, stimulation of pro-inflammatory pathways by saturated fatty acids or formation of pro-inflammatory prostaglandins and leukotrienes by n-6 fatty acids may counteract fish oil’s beneficial anti-inflammatory effects. Careful attention to saturated and trans fat intake and the n-3/n-6 ratio of PUFA intake is necessary for determination of the efficacy of fish oil supplementation in humans.
X. Conclusions
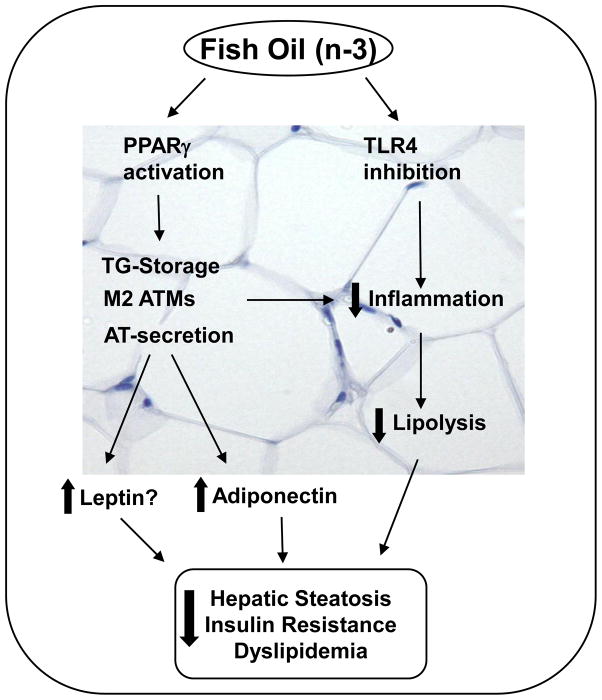
Fish oil is known to improve several features of MetS associated with CVD. These various reports indicate that improved AT storage and secretory functions as well as a reduction in AT-specific inflammation have a central role in mediating the beneficial effects of fish oil against the risk factors of MetS (Figure 1). Although adiponectin is recognized as an important player in mediating the beneficial effects of fish oil on the risk factors of MetS, increasing evidence suggests that leptin may also contribute to improvements in glucose and lipid homeostasis by fish oil. Future studies are warranted to understand the mechanisms, in particular the role of PPARγ, in modulating leptin signaling upon fish oil feeding. Moreover, the role of TLR4 and Nod inhibition in mediating the beneficial effects of fish oil in AT remains to be established.
Figure 1. Potential mechanisms by which fish oil improves metabolic syndrome.
Fish oil and n-3 fatty acids improve the storage and secretory functions of AT and reduce AT-specific inflammation via PPARγ and/or TLR4 signaling. Adiponectin, a pleiotropic adipokine, mediates the lipid lowering and insulin sensitizing effects of fish oil. However, leptin may also be involved in fish oil mediated improvements in metabolic syndrome. In addition to regulating adipokine signaling fish oil also reduces local inflammation in AT. The anti-inflammatory effect of fish oil in AT may be mediated by PPARγ which promotes ATM polarization towards an M2 phenotype thereby reducing AT-specific inflammation. Next, n-3 fatty acids may also exert their anti-inflammatory effects through inhibiting TLR4 signaling. The improved storage/secretory functions and/or reduced AT-specific inflammation reduces lipolysis in AT thereby reducing the release of free fatty acids which, in turn, leads to reduced hepatic steatosis, dyslipidemia and insulin resistance. AT-adipose tissue; ATM-Adipose tissue macrophage
Acknowledgments
V. Saraswathi is supported by an American Heart Association Scientist Development Grant (0930335N) and by a Pilot and Feasibility Grant from the Vanderbilt Digestive Diseases Research Center (DK 058404). M.J. Puglisi is supported by the Vanderbilt Molecular Endocrinology Training Program (NIH T32DK07563), and A.H. Hasty is supported by a grant from the National Institutes of Health (HL089466) and by a Career Development Award from the American Diabetes Association (1-07-CD-10).
Abbreviations
- AP-1
activator protein-1
- AMPK
5′-adenosine monophosphate activated protein kinase
- AT
adipose tissue
- CVD
cardiovascular disease
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- EPI
epididymal
- FAS
fatty acid synthase
- FFA
free fatty acid
- HSL
hormone sensitive lipase
- iNOS
inducible nitric oxide synthase
- IR
insulin resistance
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- LDLR−/−
low density lipoprotein receptor deficient
- LPL
lipoprotein lipase
- MCP-1
macrophage chemoattractant protein-1
- MES
mesenteric
- MMP-9
matrix metalloproteinase-9
- MetS
metabolic syndrome
- NF-κB
nuclear factor κB,
- PPARγ
peroxisome proliferator-activated receptor γ
- RP
retroperitoneal
- SC
subcutaneous
- STAT-1
signal transducer and activator of transcription-1
- TG
triglyceride
- TLR4
toll-like receptor 4
- UCP
uncoupling protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 2.Connor WE. Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000;71:171S–5S. doi: 10.1093/ajcn/71.1.171S. [DOI] [PubMed] [Google Scholar]
- 3.Bays HE, Tighe AP, Sadovsky R, Davidson MH. Prescription omega-3 fatty acids and their lipid effects: physiologic mechanisms of action and clinical implications. Expert Rev Cardiovasc Ther. 2008;6:391–409. doi: 10.1586/14779072.6.3.391. [DOI] [PubMed] [Google Scholar]
- 4.Flachs P, Rossmeisl M, Bryhn M, Kopecky J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin Sci (Lond) 2009;116:1–16. doi: 10.1042/CS20070456. [DOI] [PubMed] [Google Scholar]
- 5.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Barnes G, Yang Q, Tan G, Yang D, Chou C, Sole J, Nichols A, Ross J, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo J, Rizkalla SW, Boillot J, Alamowitch C, Chaib H, Bruzzo F, Desplanque N, Dalix AM, Durand G, Slama G. Dietary (n-3) polyunsaturated fatty acids improve adipocyte insulin action and glucose metabolism in insulin-resistant rats: relation to membrane fatty acids. J Nutr. 1996;126:1951–8. doi: 10.1093/jn/126.8.1951. [DOI] [PubMed] [Google Scholar]
- 8.Fickova M, Hubert P, Cremel G, Leray C. Dietary (n-3) and (n-6) polyunsaturated fatty acids rapidly modify fatty acid composition and insulin effects in rat adipocytes. J Nutr. 1998;128:512–9. doi: 10.1093/jn/128.3.512. [DOI] [PubMed] [Google Scholar]
- 9.Herzberg GR, Skinner C. Differential accumulation and release of long-chain n-3 fatty acids from liver, muscle, and adipose tissue triacylglycerols. Can J Physiol Pharmacol. 1997;75:945–51. doi: 10.1139/cjpp-75-8-945. [DOI] [PubMed] [Google Scholar]
- 10.London SJ, Sacks FM, Caesar J, Stampfer MJ, Siguel E, Willett WC. Fatty acid composition of subcutaneous adipose tissue and diet in postmenopausal US women. Am J Clin Nutr. 1991;54:340–5. doi: 10.1093/ajcn/54.2.340. [DOI] [PubMed] [Google Scholar]
- 11.Tjonneland A, Overvad K, Thorling E, Ewertz M. Adipose tissue fatty acids as biomarkers of dietary exposure in Danish men and women. Am J Clin Nutr. 1993;57:629–33. doi: 10.1093/ajcn/57.5.629. [DOI] [PubMed] [Google Scholar]
- 12.Krebs JD, Browning LM, McLean NK, Rothwell JL, Mishra GD, Moore CS, Jebb SA. Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int J Obes (Lond) 2006;30:1535–44. doi: 10.1038/sj.ijo.0803309. [DOI] [PubMed] [Google Scholar]
- 13.Ruzickova J, Rossmeisl M, Prazak T, Flachs P, Sponarova J, Veck M, Tvrzicka E, Bryhn M, Kopecky J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids. 2004;39:1177–85. doi: 10.1007/s11745-004-1345-9. [DOI] [PubMed] [Google Scholar]
- 14.Samane S, Christon R, Dombrowski L, Turcotte S, Charrouf Z, Lavigne C, Levy E, Bachelard H, Amarouch H, et al. Fish oil and argan oil intake differently modulate insulin resistance and glucose intolerance in a rat model of dietary-induced obesity. Metabolism. 2009;58:909–19. doi: 10.1016/j.metabol.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Jang IS, Hwang DY, Chae KR, Lee JE, Kim YK, Kang TS, Hwang JH, Lim CH, Huh YB, Cho JS. Role of dietary fat type in the development of adiposity from dietary obesity-susceptible Sprague-Dawley rats. Br J Nutr. 2003;89:429–38. doi: 10.1079/BJN2002801. [DOI] [PubMed] [Google Scholar]
- 16.Soria A, Chicco A, Eugenia D’Alessandro M, Rossi A, Lombardo YB. Dietary fish oil reverse epididymal tissue adiposity, cell hypertrophy and insulin resistance in dyslipemic sucrose fed rat model small star, filled. J Nutr Biochem. 2002;13:209–18. doi: 10.1016/s0955-2863(01)00214-5. [DOI] [PubMed] [Google Scholar]
- 17.Hun CS, Hasegawa K, Kawabata T, Kato M, Shimokawa T, Kagawa Y. Increased uncoupling protein2 mRNA in white adipose tissue, and decrease in leptin, visceral fat, blood glucose, and cholesterol in KK-Ay mice fed with eicosapentaenoic and docosahexaenoic acids in addition to linolenic acid. Biochem Biophys Res Commun. 1999;259:85–90. doi: 10.1006/bbrc.1999.0733. [DOI] [PubMed] [Google Scholar]
- 18.Raclot T, Groscolas R, Langin D, Ferre P. Site-specific regulation of gene expression by n-3 polyunsaturated fatty acids in rat white adipose tissues. J Lipid Res. 1997;38:1963–72. [PubMed] [Google Scholar]
- 19.Rossi AS, Lombardo YB, Lacorte JM, Chicco AG, Rouault C, Slama G, Rizkalla SW. Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrose-fed, insulin-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R486–R94. doi: 10.1152/ajpregu.00846.2004. [DOI] [PubMed] [Google Scholar]
- 20.Ide T. Interaction of fish oil and conjugated linoleic acid in affecting hepatic activity of lipogenic enzymes and gene expression in liver and adipose tissue. Diabetes. 2005;54:412–23. doi: 10.2337/diabetes.54.2.412. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Periz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, Titos E, Martinez-Clemente M, Lopez-Parra M, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–57. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peyron-Caso E, Taverna M, Guerre-Millo M, Veronese A, Pacher N, Slama G, Rizkalla SW. Dietary (n-3) polyunsaturated fatty acids up-regulate plasma leptin in insulin-resistant rats. J Nutr. 2002;132:2235–40. doi: 10.1093/jn/132.8.2235. [DOI] [PubMed] [Google Scholar]
- 23.Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, Tanaka M, Kawano H, Yano T, Aoe S, et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol. 2007;27:1918–25. doi: 10.1161/ATVBAHA.106.136853. [DOI] [PubMed] [Google Scholar]
- 24.Flachs P, Mohamed-Ali V, Horakova O, Rossmeisl M, Hosseinzadeh-Attar MJ, Hensler M, Ruzickova J, Kopecky J. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia. 2006;49:394–7. doi: 10.1007/s00125-005-0053-y. [DOI] [PubMed] [Google Scholar]
- 25.Nascimento FA, Barbosa-da-Silva S, Fernandes-Santos C, Mandarim-de-Lacerda CA, Aguila MB. Adipose tissue, liver and pancreas structural alterations in C57BL/6 mice fed high-fat-high-sucrose diet supplemented with fish oil (n-3 fatty acid rich oil) Exp Toxicol Pathol. 2009 doi: 10.1016/j.etp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Saraswathi V, Gao L, Morrow JD, Chait A, Niswender KD, Hasty AH. Fish oil increases cholesterol storage in white adipose tissue with concomitant decreases in inflammation, hepatic steatosis, and atherosclerosis in mice. J Nutr. 2007;137:1776–82. doi: 10.1093/jn/137.7.1776. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Echarri N, Perez-Matute P, Marcos-Gomez B, Baena MJ, Marti A, Martinez JA, Moreno-Aliaga MJ. Differential inflammatory status in rats susceptible or resistant to diet-induced obesity: effects of EPA ethyl ester treatment. Eur J Nutr. 2008;47:380–6. doi: 10.1007/s00394-008-0738-3. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Matute P, Perez-Echarri N, Martinez JA, Marti A, Moreno-Aliaga MJ. Eicosapentaenoic acid actions on adiposity and insulin resistance in control and high-fat-fed rats: role of apoptosis, adiponectin and tumour necrosis factor-alpha. Br J Nutr. 2007;97:389–98. doi: 10.1017/S0007114507207627. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Storlien LH, Huang XF. Effects of dietary fat types on body fatness, leptin, and ARC leptin receptor, NPY, and AgRP mRNA expression. Am J Physiol Endocrinol Metab. 2002;282:E1352–9. doi: 10.1152/ajpendo.00230.2001. [DOI] [PubMed] [Google Scholar]
- 30.Cha MC, Jones PJ. Dietary fat type and energy restriction interactively influence plasma leptin concentration in rats. J Lipid Res. 1998;39:1655–60. [PubMed] [Google Scholar]
- 31.Rossi AS, Lombardo YB, Chicco AG. Lipogenic enzyme activities and glucose uptake in fat tissue of dyslipemic, insulin-resistant rats: Effects of fish oil. Nutrition. 2009 doi: 10.1016/j.nut.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Rustan AC, Hustvedt BE, Drevon CA. Postprandial decrease in plasma unesterified fatty acids during n-3 fatty acid feeding is not caused by accumulation of fatty acids in adipose tissue. Biochim Biophys Acta. 1998;1390:245–57. doi: 10.1016/s0005-2760(97)00194-x. [DOI] [PubMed] [Google Scholar]
- 33.Ghafoorunissa, Ibrahim A, Rajkumar L, Acharya V. Dietary (n-3) long chain polyunsaturated fatty acids prevent sucrose-induced insulin resistance in rats. J Nutr. 2005;135:2634–8. doi: 10.1093/jn/135.11.2634. [DOI] [PubMed] [Google Scholar]
- 34.Gaiva MH, Couto RC, Oyama LM, Couto GE, Silveira VL, Riberio EB, Nascimento CM. Polyunsaturated fatty acid-rich diets: effect on adipose tissue metabolism in rats. Br J Nutr. 2001;86:371–7. doi: 10.1079/bjn2001392. [DOI] [PubMed] [Google Scholar]
- 35.Haug A, Hostmark AT. Lipoprotein lipases, lipoproteins and tissue lipids in rats fed fish oil or coconut oil. J Nutr. 1987;117:1011–7. doi: 10.1093/jn/117.6.1011. [DOI] [PubMed] [Google Scholar]
- 36.Lombardo YB, Hein G, Chicco A. Metabolic syndrome: effects of n-3 PUFAs on a model of dyslipidemia, insulin resistance and adiposity. Lipids. 2007;42:427–37. doi: 10.1007/s11745-007-3039-3. [DOI] [PubMed] [Google Scholar]
- 37.Peyron-Caso E, Quignard-Boulange A, Laromiguiere M, Feing-Kwong-Chan S, Veronese A, Ardouin B, Slama G, Rizkalla SW. Dietary fish oil increases lipid mobilization but does not decrease lipid storage-related enzyme activities in adipose tissue of insulin-resistant, sucrose-fed rats. J Nutr. 2003;133:2239–43. doi: 10.1093/jn/133.7.2239. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Echarri N, Perez-Matute P, Marcos-Gomez B, Martinez JA, Moreno-Aliaga MJ. Effects of eicosapentaenoic acid ethyl ester on visfatin and apelin in lean and overweight (cafeteria diet-fed) rats. Br J Nutr. 2009;101:1059–67. doi: 10.1017/S0007114508048307. [DOI] [PubMed] [Google Scholar]
- 39.Haugen F, Zahid N, Dalen KT, Hollung K, Nebb HI, Drevon CA. Resistin expression in 3T3-L1 adipocytes is reduced by arachidonic acid. J Lipid Res. 2005;46:143–53. doi: 10.1194/jlr.M400348-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Neschen S, Morino K, Rossbacher JC, Pongratz RL, Cline GW, Sono S, Gillum M, Shulman GI. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes. 2006;55:924–8. doi: 10.2337/diabetes.55.04.06.db05-0985. [DOI] [PubMed] [Google Scholar]
- 41.Lara-Castro C, Fu Y, Chung BH, Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr Opin Lipidol. 2007;18:263–70. doi: 10.1097/MOL.0b013e32814a645f. [DOI] [PubMed] [Google Scholar]
- 42.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 43.Yano W, Kubota N, Itoh S, Kubota T, Awazawa M, Moroi M, Sugi K, Takamoto I, Ogata H, et al. Molecular mechanism of moderate insulin resistance in adiponectin-knockout mice. Endocr J. 2008;55:515–22. doi: 10.1507/endocrj.k08e-093. [DOI] [PubMed] [Google Scholar]
- 44.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–83. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang W, Dedousis N, Bandi A, Lopaschuk GD, O’Doherty RM. Liver triglyceride secretion and lipid oxidative metabolism are rapidly altered by leptin in vivo. Endocrinology. 2006;147:1480–7. doi: 10.1210/en.2005-0731. [DOI] [PubMed] [Google Scholar]
- 46.Wein S, Ukropec J, Gasperikova D, Klimes I, Sebokova E. Concerted action of leptin in regulation of fatty acid oxidation in skeletal muscle and liver. Exp Clin Endocrinol Diabetes. 2007;115:244–51. doi: 10.1055/s-2007-956166. [DOI] [PubMed] [Google Scholar]
- 47.Saraswathi V, Morrow JD, Hasty AH. Dietary fish oil exerts hypolipidemic effects in lean and insulin sensitizing effects in obese LDLR−/− mice. J Nutr. 2009;139:2380–6. doi: 10.3945/jn.109.111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suchankova G, Tekle M, Saha AK, Ruderman NB, Clarke SD, Gettys TW. Dietary polyunsaturated fatty acids enhance hepatic AMP-activated protein kinase activity in rats. Biochem Biophys Res Commun. 2005;326:851–8. doi: 10.1016/j.bbrc.2004.11.114. [DOI] [PubMed] [Google Scholar]
- 49.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–19S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 50.Todoric J, Loffler M, Huber J, Bilban M, Reimers M, Kadl A, Zeyda M, Waldhausl W, Stulnig TM. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia. 2006;49:2109–19. doi: 10.1007/s00125-006-0300-x. [DOI] [PubMed] [Google Scholar]
- 51.Muurling M, Mensink RP, Pijl H, Romijn JA, Havekes LM, Voshol PJ. A fish oil diet does not reverse insulin resistance despite decreased adipose tissue TNF-alpha protein concentration in ApoE-3*Leiden mice. J Nutr. 2003;133:3350–5. doi: 10.1093/jn/133.11.3350. [DOI] [PubMed] [Google Scholar]
- 52.Wang HH, Hung TM, Wei J, Chiang AN. Fish oil increases antioxidant enzyme activities in macrophages and reduces atherosclerotic lesions in apoE-knockout mice. Cardiovasc Res. 2004;61:169–76. doi: 10.1016/j.cardiores.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi M, Tsuboyama-Kasaoka N, Nakatani T, Ishii M, Tsutsumi S, Aburatani H, Ezaki O. Fish oil feeding alters liver gene expressions to defend against PPARalpha activation and ROS production. Am J Physiol Gastrointest Liver Physiol. 2002;282:G338–48. doi: 10.1152/ajpgi.00376.2001. [DOI] [PubMed] [Google Scholar]
- 54.Robinson E, Grieve DJ. Significance of peroxisome proliferator-activated receptors in the cardiovascular system in health and disease. Pharmacol Ther. 2009;122:246–63. doi: 10.1016/j.pharmthera.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto K, Itoh T, Abe D, Shimizu M, Kanda T, Koyama T, Nishikawa M, Tamai T, Ooizumi H, Yamada S. Identification of putative metabolites of docosahexaenoic acid as potent PPARgamma agonists and antidiabetic agents. Bioorg Med Chem Lett. 2005;15:517–22. doi: 10.1016/j.bmcl.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 56.Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–6. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- 57.Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr Opin Lipidol. 2008;19:242–7. doi: 10.1097/MOL.0b013e3282ffaf6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–7. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 59.Auwerx J. PPARgamma, the ultimate thrifty gene. Diabetologia. 1999;42:1033–49. doi: 10.1007/s001250051268. [DOI] [PubMed] [Google Scholar]
- 60.Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, Tanen M, Berg AH, O’Rahilly S, et al. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 61.Tao L, Wang Y, Gao E, Zhang H, Yuan Y, Lau WB, Chan L, Koch WJ, Ma XL. Adiponectin. An Indispensable Molecule in Rosiglitazone Cardioprotection Following Myocardial Infarction. Circ Res. 2009 doi: 10.1161/CIRCRESAHA.109.211797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banga A, Unal R, Tripathi P, Pokrovskaya I, Owens RJ, Kern PA, Ranganathan G. Adiponectin translation is increased by the PPARgamma agonists pioglitazone and omega-3 fatty acids. Am J Physiol Endocrinol Metab. 2009;296:E480–9. doi: 10.1152/ajpendo.90892.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bodles AM, Banga A, Rasouli N, Ono F, Kern PA, Owens RJ. Pioglitazone increases secretion of high-molecular-weight adiponectin from adipocytes. Am J Physiol Endocrinol Metab. 2006;291:E1100–5. doi: 10.1152/ajpendo.00187.2006. [DOI] [PubMed] [Google Scholar]
- 64.Rasouli N, Yao-Borengasser A, Miles LM, Elbein SC, Kern PA. Increased plasma adiponectin in response to pioglitazone does not result from increased gene expression. Am J Physiol Endocrinol Metab. 2006;290:E42–E6. doi: 10.1152/ajpendo.00240.2005. [DOI] [PubMed] [Google Scholar]
- 65.Sato C, Yasukawa Z, Honda N, Matsuda T, Kitajima K. Identification and adipocyte differentiation-dependent expression of the unique disialic acid residue in an adipose tissue-specific glycoprotein, adipo Q. J Biol Chem. 2001;276:28849–56. doi: 10.1074/jbc.M104148200. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Xu A, Knight C, Xu LY, Cooper GJ. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem. 2002;277:19521–9. doi: 10.1074/jbc.M200601200. [DOI] [PubMed] [Google Scholar]
- 67.Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, Scherer PE. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol. 2007;27:3716–31. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol Cell Biol. 2007;27:4698–707. doi: 10.1128/MCB.02279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolf G. New insights into thiol-mediated regulation of adiponectin secretion. Nutr Rev. 2008;66:642–5. doi: 10.1111/j.1753-4887.2008.00115.x. [DOI] [PubMed] [Google Scholar]
- 70.De Vos P, Lefebvre AM, Miller SG, Guerre-Millo M, Wong K, Saladin R, Hamann LG, Staels B, Briggs MR, Auwerx J. Thiazolidinediones repress ob gene expression in rodents via activation of peroxisome proliferator-activated receptor gamma. J Clin Invest. 1996;98:1004–9. doi: 10.1172/JCI118860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kallen CB, Lazar MA. Antidiabetic thiazolidinediones inhibit leptin (ob) gene expression in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1996;93:5793–6. doi: 10.1073/pnas.93.12.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–7. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim HJ, Kim SK, Shim WS, Lee JH, Hur KY, Kang ES, Ahn CW, Lim SK, Lee HC, Cha BS. Rosiglitazone improves insulin sensitivity with increased serum leptin levels in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2008;81:42–9. doi: 10.1016/j.diabres.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 74.Liu LF, Purushotham A, Wendel AA, Belury MA. Combined effects of rosiglitazone and conjugated linoleic acid on adiposity, insulin sensitivity, and hepatic steatosis in high-fat-fed mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1671–82. doi: 10.1152/ajpgi.00523.2006. [DOI] [PubMed] [Google Scholar]
- 75.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 76.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–6. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 77.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S, Muller M. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J Biol Chem. 2008;283:22620–7. doi: 10.1074/jbc.M710314200. [DOI] [PubMed] [Google Scholar]
- 79.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–51. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 81.Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, Knauf C, Peiretti F, Verdier M, Juhan-Vague I, et al. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia. 2007;50:1267–76. doi: 10.1007/s00125-007-0654-8. [DOI] [PubMed] [Google Scholar]
- 82.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–98. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 83.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–92. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–93. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 85.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Sasidhar M, Nabel EG, et al. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–89. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stulnig TM, Huber J, Leitinger N, Imre EM, Angelisova P, Nowotny P, Waldhausl W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J Biol Chem. 2001;276:37335–40. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- 87.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–56. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- 88.Laurencikiene J, van Harmelen V, Arvidsson Nordstrom E, Dicker A, Blomqvist L, Naslund E, Langin D, Arner P, Ryden M. NF-kappaB is important for TNF-alpha-induced lipolysis in human adipocytes. J Lipid Res. 2007;48:1069–77. doi: 10.1194/jlr.M600471-JLR200. [DOI] [PubMed] [Google Scholar]
- 89.Zhao L, Kwon MJ, Huang S, Lee JY, Fukase K, Inohara N, Hwang DH. Differential modulation of Nods signaling pathways by fatty acids in human colonic epithelial HCT116 cells. J Biol Chem. 2007;282:11618–28. doi: 10.1074/jbc.M608644200. [DOI] [PubMed] [Google Scholar]
- 90.Belzung F, Raclot T, Groscolas R. Fish oil n-3 fatty acids selectively limit the hypertrophy of abdominal fat depots in growing rats fed high-fat diets. Am J Physiol. 1993;264:R1111–8. doi: 10.1152/ajpregu.1993.264.6.R1111. [DOI] [PubMed] [Google Scholar]
- 91.Raclot T, Groscolas R, Leray C. Composition and structure of triacylglycerols in brown adipose tissue of rats fed fish oil. Lipids. 1994;29:759–64. doi: 10.1007/BF02536697. [DOI] [PubMed] [Google Scholar]
- 92.Oudart H, Groscolas R, Calgari C, Nibbelink M, Leray C, Le Maho Y, Malan A. Brown fat thermogenesis in rats fed high-fat diets enriched with n-3 polyunsaturated fatty acids. Int J Obes Relat Metab Disord. 1997;21:955–62. doi: 10.1038/sj.ijo.0800500. [DOI] [PubMed] [Google Scholar]
- 93.Tsuboyama-Kasaoka N, Takahashi M, Kim H, Ezaki O. Up-regulation of liver uncoupling protein-2 mRNA by either fish oil feeding or fibrate administration in mice. Biochem Biophys Res Commun. 1999;257:879–85. doi: 10.1006/bbrc.1999.0555. [DOI] [PubMed] [Google Scholar]
- 94.Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulange A, Vidal H, Slama G, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86:1670–9. doi: 10.1093/ajcn/86.5.1670. [DOI] [PubMed] [Google Scholar]
- 95.Kopecky J, Rossmeisl M, Flachs P, Kuda O, Brauner P, Jilkova Z, Stankova B, Tvrzicka E, Bryhn M. n-3 PUFA: bioavailability and modulation of adipose tissue function. Proc Nutr Soc. 2009;68:361–9. doi: 10.1017/S0029665109990231. [DOI] [PubMed] [Google Scholar]
- 96.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–76S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]