Abstract
Context: The aim of this analysis was to evaluate glucagon and c-peptide concentrations in two scenarios: euglycemic hyperinsulinemia and hyperglycemic hyperinsulinemia. We postulated that worsening obesity and insulin resistance will be reflected as an up-regulated (less suppressible) islet secretion profile.
Methods: Eighty-two [34 obese with normal glucose tolerance (NGT), 30 obese with impaired glucose tolerance (IGT), and 18 nonobese with NGT] subjects underwent a euglycemic-hyperinsulinemic clamp (EHC) and a hyperglycemic clamp. C-peptide and glucagon were evaluated at basal and steady-state (SS) conditions.
Results: Basal glucagon was significantly elevated in obese insulin-resistant and obese IGT subjects as was basal c-peptide. SS glucagon and c-peptide levels during the EHC were lower in the lean and obese insulin-sensitive subjects compared with the obese insulin-resistant subjects with NGT or IGT. Fasting glucagon was the only significant determinant (β = 0.66, P < 0.001) of SS glucagon during the EHC (R2 = 0.57). In a longitudinal follow-up of a subsample, those who converted from normal to IGT significantly increased their fasting glucagon concentration in comparison with those who remained with NGT.
Conclusions: Islet up-regulation manifesting as basal elevated glucagon and c-peptide secretion that determines the suppressive effects of hyperinsulinemia appears early in the course of deteriorating glucose tolerance.
Alpha cell up-regulation manifesting as basal elevated glucagon appears early in the course of deteriorating glucose tolerance in obese youth.
Type 2 diabetes mellitus (T2DM) diagnosed in the pediatric age group is becoming increasingly common (1). The rise in the prevalence of the disease in childhood is tightly linked to the increasing rates of obesity, and they are therefore called the twin epidemics (2). The underlying pathophysiology of the development of altered glucose metabolism in childhood has been shown to closely mimic that observed in adults, yet the tempo of progression of the disease from impaired glucose tolerance (IGT) to overt T2DM seems to be accelerated (3). Early defects characteristic of obese children with IGT/T2DM are severe peripheral insulin resistance of the muscle as well as the liver (4,5). Moreover, early defects in first phase insulin secretion have been demonstrated in adolescents with IGT, whereas a double defect in first- and second-phase insulin secretion has been shown in adolescents with T2DM (4,6).
Glucose metabolism is governed by not only insulin but also glucagon. The mechanism of regulation of glucagon secretion from islet α-cells is still not clear. There is continuing controversy whether α-cells can directly sense and respond to fluctuations in plasma glucose (7) or whether their response is mediated via the autonomic nervous system response to glucose sensing elsewhere (8). The role of paracrine and endocrine effects of hormones secreted from neighboring cells, specifically insulin secretion by β-cells, in glucagon regulation is more established. Thus, both glucose and insulin have been shown to suppress glucagon secretion (9). Insulin itself has a paracrine/endocrine effect causing suppression of its own secretion mediated via phosphatidylinositol 3-kinase (10) and possibly via modulation of mitochondrial function (11). The ability of exogenous insulin or of hyperglycemia to suppress glucagon as well as c-peptide secretion in vivo in children with varying degrees of obesity and insulin sensitivity has not been studied.
The aim of this analysis was to evaluate basal glucagon and c-peptide concentrations as well as in two scenarios: euglycemic hyperinsulinemia and hyperglycemic hyperinsulinemia. Because basal pulsatile glucagon and c-peptide secretion has been shown to coincide (12,13), we hypothesized that the responses of these two would be highly correlated. We chose to investigate these issues in lean, obese insulin-sensitive and obese insulin-resistant adolescents and postulated that worsening obesity and insulin resistance will be reflected as an up-regulated and less suppressible islet secretion profile. We also evaluated changes of these parameters in a smaller number of normal glucose tolerant (NGT) subjects who repeated the euglycemic clamp and compared those whose glucose tolerance deteriorated with those who remained NGT.
Subjects and Methods
The obese participants of this study are part of a cohort of children and adolescents taking part in a longitudinal study aimed at defining the pathophysiology of prediabetes in obese youth. Inclusion criteria for obese participants were a body mass index (BMI) greater than the 95th percentile for age and gender, being on no medications that may affect glucose metabolism, being otherwise healthy, and a self-report of not participating in organized physical activity. Due to the Human Investigation Committee instructions, all lean participants were either siblings of obese participants or over the age of 18 yr; thus, euglycemic or hyperglycemic clamps were not performed in population-derived lean children. The inclusion criteria of lean participants were a BMI lower than the 85th percentile for age and gender, being on no medications that may affect glucose metabolism, and being otherwise healthy (with no restrictions on physical activity). Participants who were enrolled in this study had a detailed medical history, complete physical examination including assessment of Tanner stage of development, a dual-energy x-ray absorptiometry scan for determination of body composition and an oral glucose tolerance test to determine carbohydrate tolerance as previously described (14).
We analyzed data from 102 participants who underwent both a euglycemic-hyperinsulinemic clamp and a hyperglycemic clamp. To investigate the full spectrum of body habitus and glucose tolerance, we studied lean subjects with NGT, obese subjects with NGT, and obese subjects with IGT. Some of the obese subjects (14 with IGT and 32 with NGT) have been partially described in previous publications (4,15). The nature and purpose of the study were explained to the parent/guardian and child before written consent from the parent and written assent from the child were obtained. The study protocol was approved by the Human Investigation Committee of the Yale University School of Medicine.
Procedures
Hyperinsulinemic-euglycemic clamp
All participants underwent a euglycemic hyperinsulinemic clamp as previously described (4). In brief, after an overnight fast, two iv catheters, one for blood drawing and one for infusion of glucose, insulin, were inserted in the antecubital vein of each arm after local infiltration with lidocaine. Insulin sensitivity was measured by a hyperinsulinemic-euglycemic clamp by infusing insulin as a primed continuous infusion 80 mU/m2 · min for 120 min (16). Arterialized blood samples were collected every 10 min during the last 30 min of the insulin infusion period for determination of glucose and hormones. Insulin sensitivity was calculated as the glucose infusion rate divided by the insulin concentration during steady state (M over I).
Hyperglycemic clamp
To quantify insulin secretion, blood glucose was rapidly raised to 200 mg/dl by infusing 20% dextrose at variable rates, and plasma glucose was kept at that level for 120 min (16). First-phase insulin and c-peptide secretion were calculated as the mean of five samples drawn at 2, 4, 6, 8, and 10 min of the clamp.
Biochemical analysis
Plasma glucose levels were measured by the glucose oxidase method with a glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin, c-peptide, and glucagon concentrations were measured using double-antibody RIA kits (Linco Diagnostic Systems Laboratories, Webster, TX).
Statistical analysis
Parameters are presented as means ± sds. Parameters that were not normally distributed were ln transformed for the sake of analysis yet are presented in their absolute values. Group comparisons between parameters of interest during the clamps were performed using repeated-measures ANOVA. Adjustment for multiple comparisons was performed using a post hoc Bonferonni correction. Regression models of steady-state glucagon and c-peptide during the euglycemic clamps were performed using the general linear model procedure. Comparisons of longitudinal changes in parameters of interest were performed using nonparametric paired sample testing. P < 0.05 was considered statistically significant. The analysis was performed using SPSS 15 for Windows (SPSS, Chicago, IL).
Results
Fifty obese subjects with NGT were divided based on the 33rd and the 66th percentile of their clamp-derived insulin sensitivity to obese sensitive (below the 33rd percentage) and obese resistant (above the 66th percentage), and thus, 34 remained in the analysis. Of note, the obese insulin sensitive subjects had mild fasting hyperinsulinemia and should thus be considered as relatively insulin sensitive. Thus, a total of 82 subjects participated in this analysis. As expected, the lean participants were slightly yet significantly older than the rest of the participants (Table 1). Obviously the lean group had lower weight, BMI, percent body fat, and fat mass (P < 0.001 compared with all obese participants), whereas the three obese groups were comparable in those parameters. Lean body mass was comparable among the four groups. Insulin sensitivity, expressed as glucose infusion rate at steady state of the euglycemic-hyperinsulinemic clamp divided by lean body mass, was comparable between the lean and the obese-sensitive group and significantly greater than the obese resistant and IGT group.
Table 1.
Anthropometric and biochemical characteristics of study participants
| Lean (n = 18) | Obese NGT sensitive (n = 17) | Obese NGT resistant (n = 17) | Obese IGT (n = 30) | P value | |
|---|---|---|---|---|---|
| Sex (male/female) | 10/8 | 11/6 | 8/9 | 12/18 | 0.39 |
| Ethnicity (C/AA/H) | 11/4/3 | 7/4/6 | 4/7/6 | 10/9/11 | 0.36 |
| Age (yr) | 19.0 ± 2.1 | 13.6 ± 3.2 | 14.0 ± 2.6 | 13.9 ± 3.1 | <0.001a |
| Height (cm) | 170 ± 8 | 165 ± 15 | 164 ± 11 | 162 ± 11 | 0.14 |
| Weight (kg) | 64 ± 10 | 88 ± 27 | 102 ± 18 | 94 ± 23 | <0.001a |
| BMI (kg/m2) | 22.0 ± 2.2 | 31.9 ± 6.2 | 37.6 ± 5.3 | 35.3 ± 5.7 | <0.001b |
| Body fat (%) | 20 ± 8 | 37 ± 6 | 40 ± 6 | 39 ± 7 | <0.001a |
| Lean body mass (kg) | 48 ± 11 | 52 ± 16 | 57 ± 12 | 54 ± 13 | 0.22 |
| Fat mass | 15 ± 4 | 33 ± 11 | 39 ± 9 | 37 ± 10 | <0.001a |
| Puberty (pre/pub) | 0/18 | 4/13 | 3/14 | 6/24 | 0.20 |
| Insulin sensitivity (M/I) mg/kg/(lbmμU/ml) | 0.094 ± 0.034 | 0.085 ± 0.017 | 0.025 ± 0.007 | 0.030 ± 0.01 | <0.001c |
C, Caucasian; AA, African-American; H, Hispanic.
P < 0.001 for lean vs. all other groups.
P < 0.001 for lean vs. all other groups, P = 0.006 and P = 0.05 for obese sensitive vs. obese resistant and IGT, respectively.
P < 0.001 for lean vs. all other groups, P < 0.001 for obese sensitive vs. obese resistant and IGT.
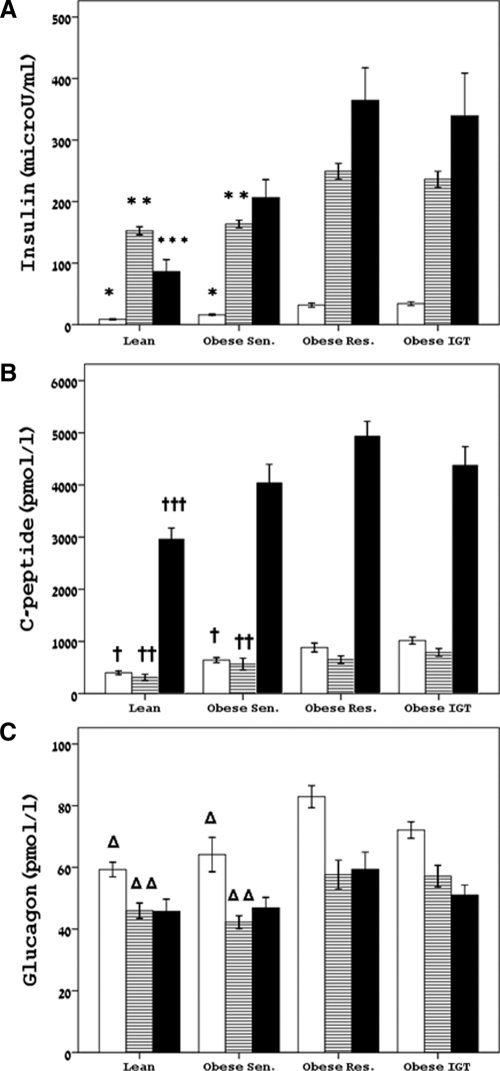
Fasting glucose was normal and similar in all four groups. Figure 1 shows the insulin (Fig. 1A), c-peptide (Fig. 1B), and glucagon (Fig. 1C) levels during fasting, steady-state euglycemia-hyperinsulinemia, and during steady-state hyperglycemia. Fasting insulin was significantly lower in the lean and obese-sensitive groups in comparison with their obese-resistant and IGT counterparts. Fasting c-peptide was significantly lower in the lean group in comparison with all the obese groups, whereas the obese-sensitive subjects had significantly lower levels than the IGT group. Fasting glucagon was significantly lower in the lean and obese insulin-sensitive subjects in comparison with the obese insulin-resistant and IGT group.
Figure 1.
Concentrations of fasting and steady state-levels of insulin (A), c-peptide (B), and glucagon (C) during fasting, euglycemic hyperinsulinemia, and hyperglycemic hyperinsulinemia. White bars, fasting state; dashed bars, SS euglycemic-hyperinsulinemic clamp; black bars, SS hyperglycemic clamp. *, P < 0.001 for lean vs. obese resistant (Res.) and IGT, respectively, P < 0.001 and P = 0.002 for obese sensitive (Sen.) vs. obese resistant and IGT. **, P < 0.001 for lean vs. obese resistant and IGT, respectively, P < 0.001 and P = 0.001 for obese sensitive vs. obese resistant and IGT. ***, P = 0.01 for lean vs. obese resistant and IGT, respectively; †, <0.001 for lean vs. obese resistant and IGT, respectively, P = 0.001 for obese sensitive vs. IGT; ††, P < 0.001 for lean vs. all others, P = 0.04 for obese sensitive vs. obese resistant and obese IGT; †††, P = 0.001 and P = 0.02 for lean vs. obese resistant and IGT, respectively; Δ, P < 0.001 for lean vs. obese resistant and P = 0.002 vs. IGT, P = 0.002 and P = 0.04 for obese sensitive vs. obese resistant and IGT, respectively; Δ Δ, P = 0.03 and P = 0.02 for lean vs. obese resistant and IGT, respectively, P = 0.007 and P = 0.02 for obese sensitive vs. obese resistant and IGT.
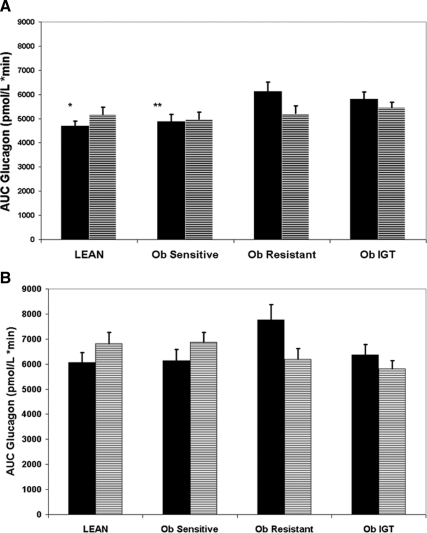
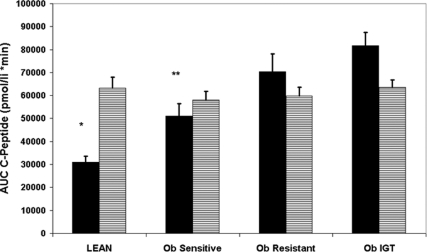
The steady-state (SS) glucose levels during the euglycemic-hyperinsulinemic clamp were comparable between the four groups (91 ± 6, 95 ± 7, 93 ± 5, and 94 ± 8 mg/dl for the lean, obese sensitive, obese resistant, and IGT, respectively). Despite infusing a body surface area adjusted insulin dose, SS insulin levels were different among the four groups. Specifically, the lean subjects had lower SS insulin concentrations compared with the obese-resistant and IGT (P < 0.001 for both), as did the obese sensitive (P < 0.001 vs. obese resistant and IGT, respectively). C-peptide levels were lower in the lean (P < 0.001 vs. obese resistant and IGT, respectively) and lower in the sensitive obese group in comparison with the obese with IGT (P = 0.002). SS glucagon during the euglycemic-hyperinsulinemic clamp was lower in the lean group compared with the obese-resistant and IGT groups (P = 0.02 and P = 0.03, respectively) as well as in the obese sensitive group (P = 0.04 for both). The area under the curve (AUC) for glucagon was significantly lower in the lean and obese sensitive subjects (Fig. 2A) in comparison with the obese-resistant and IGT participants indicating greater absolute suppression of glucagon levels during the euglycemic clamp. AUC of c-peptide during the euglycemic hyperinsulinemic clamp was significantly lower in the lean and obese insulin-sensitive subjects in comparison with the obese-resistant and IGT groups (Fig. 3), indicating greater absolute suppression during the clamp.
Figure 2.
The AUC of glucagon during the euglycemic-hyperinsulinemic (A) and the hyperglycemic clamp (B). Black bars, Unadjusted; dashed bars, adjusted for group, sex, age, ethnicity, pubertal status, and fasting glucagon. *, P = 0.004 and P = 0.01 vs. obese resistant and IGT, respectively; **, P = 0.01 and P = 0.03 vs. obese resistant and IGT, respectively.
Figure 3.
The AUC of c-peptide during the euglycemic-hyperinsulinemic clamp. Black bars, Unadjusted; dashed bars, adjusted for group, sex, age, ethnicity, pubertal status, and fasting c-peptide. *, P < 0.001 vs. obese resistant and IGT; **, P = 0.003 and P < 0.001 vs. obese resistant and IGT.
Using a multivariate regression model, we evaluated SS glucagon during the euglycemic clamp as the dependent variable and included group, age, gender, and ethnicity in the model. The only significant factor in the model, which explained 15% of the variability was the subject group [with the lean being significantly different than the obese resistant and IGT (P < 0.05 for both) as for the obese sensitive (P = 0.01 for both)]. Upon adding fasting glucagon to the model (which now explained 57% of the variability), the effect of the group was lost, and only fasting glucagon was the significant determinant of SS glucagon (β = 0.66, P < 0.001). Similarly, we modeled SS c-peptide during the euglycemic clamp as the dependent variable and included group, age, gender, and ethnicity in the model. Only the group was a significant determinant in the model, which explained 20% of the variability, with the lean being significantly different from the others (P < 0.01 vs. all groups). Upon adding fasting c-peptide to the model, (now explaining 61% of the variability), the impact of the group disappeared and only the fasting c-peptide level emerged as a significant determinant of SS c-peptide during the clamp (β = 0.84, P < 0.001).
SS glucose levels during the hyperglycemic clamp were comparable among the groups (200 ± 7, 205 ± 6, 205 ± 4, and 201 ± 6 mg/dl for the lean, obese sensitive, obese resistant, and IGT, respectively). As shown in Fig. 1, insulin levels during the hyperglycemic clamp were greater in the obese-resistant and the IGT group in comparison with the lean and obese-sensitive groups. C-peptide was lower in the lean subjects in comparison with the other three groups (P = 0.02 vs. the obese sensitive and P < 0.001 vs. obese resistant and IGT). SS glucagon levels were similar in all four groups as was the glucagon AUC during the hyperglycemic clamp (Fig. 2B). Interestingly, fasting glucagon was significantly associated with first phase insulin and c-peptide secretion (r = 0.46 and r = 0.48, P = 0.001 for both) in subjects with normal glucose tolerance, whereas no such associations were evident in subjects with impaired glucose tolerance.
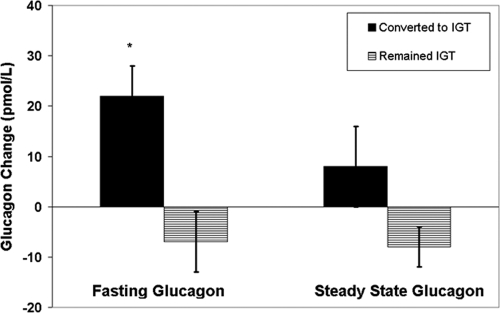
We evaluated 16 participants who were NGT at baseline and repeated the euglycemic hyperinsulinemic clamp after about 3 yr. During the follow-up, eight (two males, six females) converted to IGT (follow-up of 2.7 ± 1.2 yr) and eight (three males, five females) remained NGT (follow-up of 3.1 ± 0.8 yr). Insulin sensitivity showed a significant deterioration of −1.93 ± 2.0 mg/kg lbm per minute in the group that converted to IGT while remaining relatively stable in the group that remained NGT (a change of 0.42 ± 1.76 mg/kg lbm per minute, P = 0.05 vs. converters). At baseline, fasting glucagon was comparable (61 ± 15 vs. 62 ± 19 in converters and nonconverters, respectively) as was fasting c-peptide (862 ± 381 vs. 750 ± 169 pmol/liter in converters vs. nonconverters, respectively). As shown in Fig. 4, fasting glucagon increased in the converters in comparison with those who remained NGT (+22 ± 16 vs. −7 ± 16 pmol/liter, P = 0.008). Upon adjustment for time, pubertal status, and change in insulin sensitivity, the difference in fasting glucagon remained significant (P = 0.04) between the groups. Fasting c-peptide showed a similar trend for increasing in those who converted to IGT in comparison with those who remained NGT (+281 ± 300 vs. −21 ± 244, P = 0.06). SS concentrations of c-peptide and glucagon during euglycemic hyperinsulinemic clamp were comparable between the groups.
Figure 4.
Changes in fasting glucagon between baseline and follow-up studies. Black bars, Converted to IGT; dashed bars, remained IGT. *, P = 0.008.
Discussion
In this analysis we demonstrate elevated fasting glucagon levels in obese insulin-resistant adolescents with normal tolerance and with IGT. Moreover, we show reduced absolute suppression of glucagon in the face of hyperinsulinemic euglycemia along the transition from insulin sensitivity to insulin resistance in obese adolescents. The main determinant of this reduced suppression is the fasting glucagon level. Only when exposed to two suppressive stimuli, i.e. hyperinsulinemia and hyperglycemia, glucagon was similarly suppressed in all groups. We also show reduced suppression of c-peptide secretion during exogenous hyperinsulinemia in obese insulin-resistant adolescents that is also determined solely by the baseline level of c-peptide. Deterioration of glucose tolerance in these subjects is associated with increases in fasting glucagon.
The regulation of glucagon secretion by α-cells is dependent on ambient glucose, paracrine and endocrine hormonal stimuli, and mainly parasympathetic activation (17). Our analysis demonstrates that across the spectrum of insulin sensitivity in obese adolescents, the glucagon level tends to be elevated in the fasting state in association with decreasing insulin sensitivity. Moreover, suppression of glucagon secretion in the face of euglycemic hyperinsulinemia seems to be dependent on baseline glucagon secretion. Importantly, the elevated fasting glucagon levels observed in obese insulin-resistant adolescents with normal glucose tolerance or IGT seem inappropriate in the face of the elevated fasting insulin levels observed in those groups. Because insulin has been shown to regulate glucagon secretion (18), our findings may suggest that the α-cells manifest insulin resistance to the suppressive effect of insulin on glucagon secretion (19). Only upon providing two suppressive stimuli, namely hyperglycemia and hyperinsulinemia (at greater concentrations than those in the euglycemic clamp), glucagon secretion equalized between the groups. This factor may suggest that the glucose-sensing component of glucagon suppression, whether within the islet or elsewhere is intact, yet the accompanying hyperinsulinemia inherent to our study design (which was greater than that observed during the euglycemic clamp) prevents us from teasing out this effect.
Relative glucagon hypersecretion has been demonstrated in adults with T2DM in the fasting state and in response to a large carbohydrate meal, iv glucose, and arginine administration (20,21), yet early hypersecretion in obese insulin resistant adolescents with alterations in glucose metabolism have not been shown before. Because glucagon has a major effect on both fasting and postprandial hyperglycemia (22), its early up-regulation in obese insulin-resistant adolescents may contribute to their deteriorating glucose tolerance. In lean and obese subjects with normal glucose metabolism, there was a significant correlation between fasting glucagon and first-phase insulin/c-peptide secretion, whereas no such correlation was present in obese subjects with IGT. Together with the relation between fasting glucagon and peripheral insulin resistance, this suggests that basal hyperglucagonemia precedes the development of β-cell failure and is associated with a compensatory insulin secretion in subjects with NGT as their insulin sensitivity is decreasing. When β-cell failure occurs, insulin secretion is reduced, whereas glucagon hypersecretion remains, which explains the lack of association between fasting glucagon and insulin secretion in subjects with IGT.
The elevated fasting insulin and c-peptide levels observed in the obese insulin-resistant participants seem to be a normal appropriate compensatory response. This may be achieved by reducing insulin clearance or increasing basal insulin secretion. On the other hand, exogenous insulin has been shown to significantly attenuate β-cells and cause reduced insulin secretion, even in the face of hyperglycemia (23); thus, in the face of euglycemic hyperinsulinemia, one should expect suppression of c-peptide secretion. Our findings indicate that SS c-peptide levels achieved during the euglycemic hyperinsulinemic clamp were different between the groups and that the major determinant of this concentration was the fasting c-peptide. Thus, a comparable relative degree of suppression of endogenous c-peptide was observed between the groups, in the face of different insulin concentrations, yet the SS concentration, reflecting possibly a basal level of secretion, was the most important determinant of the overall β-cell suppression. Because the clearance rate of c-peptide is slower than that of glucagon and insulin, longer metabolic studies may have unraveled this phenomenon in a clearer fashion. These findings suggest that there may a β-cell insulin resistance, even at early stages of altered glucose metabolism that may be partly due to defects in insulin signaling pathways within the β-cell.
A limitation of this study is that the δ-cell, another important factor within the pancreatic islet that secretes somatostatin, was not evaluated. The δ-cell is influenced in opposite directions by glucagon, which stimulates somatostatin secretion, and insulin, which suppresses it (24). On the other hand, somatostatin serves as an insulin break and suppresses β-cells. Whether early fasting hyperglucagonemia as described in this study leads to increased local somatostatin levels, which can eventually suppress insulin secretion, is still not known and deserves further investigation.
To overcome the cross-sectional nature of this analysis, which prevents from indicating whether islet up-regulation, specifically of the α-cell, precedes the development of altered glucose tolerance, we analyzed the longitudinal data of our subjects. Our findings indicate that increased basal glucagon appears early in the development of impaired glucose metabolism and potentially precedes it. Our findings unravel islet up-regulation that manifests as basal elevated glucagon and c-peptide secretion that is relatively resistant to the suppressive effects of exogenous hyperinsulinemia. Despite the similar magnitude of suppression in all groups, the elevated basal secretion of glucagon and c-peptide in severely insulin-resistant obese youth is the determinant of a the absolute elevated glucagon and c-peptide concentrations observed during SS conditions of the clamp. These findings indicate that early defects in islet regulation may be present before overt altered glucose metabolism and represent potential novel therapeutic targets. Specifically, targeting the α-cell to suppress glucagon secretion or potentially antagonizing the systemic and local paracrine effects of glucagon early in the development of altered glucose metabolism seem to be promising therapeutic targets.
Acknowledgments
We thank the patients who participated in these studies as well as their families.
Footnotes
R.W. was supported by the Stephen Morse Diabetes Research Fund.
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 15, 2010
Abbreviations: AUC, Area under the curve; BMI, body mass index; IGT, impaired glucose tolerance; NGT, normal glucose tolerance; SS, steady state; T2DM, type 2 diabetes mellitus.
References
- Rosenbloom AL, Joe JR, Young RS, Winter WE 1999 Emerging epidemic of type 2 diabetes in youth. Diabetes Care 22:345–54 [DOI] [PubMed] [Google Scholar]
- Smyth S, Heron A 2006 Diabetes and obesity: the twin epidemics. Nat Med 12:75–80 [DOI] [PubMed] [Google Scholar]
- Weiss R, Taksali SE, Tamborlane WV, Burgert TS, Savoye M, Caprio S 2005 Predictors of changes in glucose tolerance status in obese youth. Diabetes Care 28:902–909 [DOI] [PubMed] [Google Scholar]
- Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, Boselli L, Barbetta G, Allen K, Rife F, Savoye M, Dziura J, Sherwin R, Shulman GI, Caprio S 2003 Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 362:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali AM, De Oliveira AM, Kim H, Chen S, Reyes-Mugica M, Escalera S, Dziura J, Taksali SE, Kursawe R, Shaw M, Savoye M, Pierpont B, Constable RT, Caprio S 2009 Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology 49:1896–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor N, Bacha F, Saad R, Janosky J, Arslanian S 2005 Youth type 2 diabetes: insulin resistance, beta-cell failure, or both? Diabetes Care 28:638–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich JE 1983 Glucose in the control of glucagons secretion. In: Lefebvre PJ, ed. Handbook of experimental pharmacology. Berlin: Springer-Verlag; 3–18 [Google Scholar]
- Ertl AC, Mann S, Richardson A, Briscoe VJ, Blair HB, Tate DB, Davis SN 2008 Effects of oral carbohydrate on autonomic nervous system counterregulatory responses during hyperinsulinemic hypoglycemia and euglycemia. Am J Physiol Endocrinol Metab 295:E618–E625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich JE, Charles MA, Grodsky GM 1976 Regulation of pancreatic insulin and glucagon secretion. Ann Rev Physiol 38:353–388 [DOI] [PubMed] [Google Scholar]
- Eto K, Yamashita T, Tsubamoto Y, Terauchi Y, Hirose K, Kubota N, Yamashita S, Taka J, Satoh S, Sekihara H, Tobe K, Iino M, Noda M, Kimura S, Kadowaki T 2002 Phosphatidylinositol 3-kinase suppresses glucose-stimulated insulin secretion by affecting post-cytosolic [Ca(2+)] elevation signals. Diabetes 51:87–97 [DOI] [PubMed] [Google Scholar]
- Liu S, Okada T, Assmann A, Soto J, Liew CW, Bugger H, Shirihai OS, Abel ED, Kulkarni RN 2009 Insulin signaling regulates mitochondrial function in pancreatic β-cells. PLoS One 4:e7983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang DA, Matthews DR, Peto J, Turner RC 1979 Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N Engl J Med 301:1023–1027 [DOI] [PubMed] [Google Scholar]
- Goodner CJ, Walike BC, Koerker DJ, Ensinck JW, Brown AC, Chideckel EW, Palmer J, Kalnasy L 1977 Insulin, glucagon, and glucose exhibit synchronous, sustained oscillations in fasting monkeys. Science 195:177–179 [DOI] [PubMed] [Google Scholar]
- Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, Savoye M, Rieger V, Taksali S, Barbetta G, Sherwin RS, Caprio S 2002 Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 346:802–810 [DOI] [PubMed] [Google Scholar]
- Weiss R, Taksali SE, Dufour S, Yeckel CW, Papademetris X, Cline G, Tamborlane WV, Dziura J, Shulman GI, Caprio S 2005 The “obese insulin-sensitive” adolescent: importance of adiponectin and lipid partitioning. J Clin Endocrinol Metab 90:3731–3737 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R 1979 Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- Gromada J, Franklin I, Wollheim CB 2007 α-Cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28:84–116 [DOI] [PubMed] [Google Scholar]
- Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB 2005 β-Cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54:1808–1815 [DOI] [PubMed] [Google Scholar]
- Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y, Wheeler MB, Braun M, Wang Q 2006 Intraislet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab 3:47–58 [DOI] [PubMed] [Google Scholar]
- Unger RH, Aguilar-Parada E, Müller WA, Eisentraut AM 1970 Studies of pancreatic α-cell function in normal and diabetic subjects. J Clin Invest 49:837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller WA, Faloona GR, Aguilar-Parada E, Unger RH 1970 Abnormal α-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med 283:109–115 [DOI] [PubMed] [Google Scholar]
- Dunning BE, Gerich JE 2007 The role of α-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 28:253–283 [DOI] [PubMed] [Google Scholar]
- Hee-Park S, Lim B, Baek WK, Bae JH, Song DK 2007 Negative and positive feedback regulation of insulin in glucose-stimulated Ca2+ response in pancreatic β cells. Diabetes Res Clin Pract 77(Suppl 1):S143–S149 [DOI] [PubMed] [Google Scholar]
- Kawai K, Unger RH 1983 Opposing actions of glucagon and insulin on splanchnic d cell function. J Clin Invest 71:721–725 [DOI] [PMC free article] [PubMed] [Google Scholar]