Abstract
Context: The positive association of elevated fibroblast growth factor-23 (FGF23) with PTH levels in the setting of secondary hyperparathyroidism is paradoxical to the purported effects of FGF23 to suppress PTH secretion.
Objective: We used dynamic calcium-mediated suppression of PTH levels in hemodialysis (HD) patients to determine the relationship between FGF23 levels and parathyroid gland function.
Design: HD patients with elevated PTH were washed out of vitamin D analogs and/or calcimimetics and then exposed them to a high-calcium dialysate bath designed to suppress PTH.
Setting: The study was conducted at an outpatient HD unit of an academic medical center.
Participants: Eighteen maintenance HD patients with elevated PTH levels participated in the study.
Main Outcome Measures: Ionized calcium (iCa), PTH, and FGF23 levels were measured during HD. The slope of the relationship between iCa and PTH (a marker of parathyroid gland mass) and the iCa level required for a 50% reduction in PTH were determined, and the association of these with FGF23 levels was determined.
Results: Increased baseline log FGF23 levels were associated with putative alterations in gland mass as estimated by significantly shallower slopes of the iCa/PTH suppression curves (P = 0.0004), but there was no association between FGF23 and calcium sensing as measured by ionized Ca associated with a 50% suppression of PTH (P = 0.38). FGF23 levels decreased significantly during HD, but this change was not correlated with decrements in either renal phosphate or PTH.
Conclusions: High FGF23 levels may be a marker for parathyroid gland hyperplasia in HD patients. Acute reductions in neither PTH nor renal phosphate during dialysis correlated with PTH suppression.
Degree of calcium-mediated parathyroid hormone suppression in dialysis patients with secondary hyperparathyroidism is significantly associated with increased FGF23 levels.
Hyperphosphatemia and decreased 1,25 dihydroxyvitamin D3 [1,25(OH)2D] levels lead to progressive hyperparathyroidism in end-stage renal disease (ESRD) patients on hemodialysis (HD), a condition that is associated with mortality in this population (1). Secondary hyperparathyroidism (SHPT), characterized by increased PTH secretion and progressive parathyroid gland (PTG) hyperplasia, and fibroblast growth factor-23 (FGF23), a recently identified phosphaturic and vitamin D counterregulatory hormone secreted by osteocytes in bone, increase concurrently in chronic kidney disease (2,3,4). Circulating levels of FGF23 in ESRD patients with SHPT are typically substantially elevated relative to individuals with intact renal function (5,6,7,8).
The significance of elevated levels of FGF23 in chronic kidney disease is currently being elucidated. FGF23 targets FGF receptor-Klotho complexes in the kidney (9,10) to suppress 1α-hydroxylase activity and to decrease renal phosphate (P) reabsorption, thereby contributing to maintenance of P homeostasis and contributing to the development of SHPT via reductions in 1,25(OH)2D (3,4,11,12). FGF receptor-Klotho complexes are also present in the parathyroid-secreting cells, but the effects of FGF23 on PTG function are unclear.
Recent findings indicate that elevations of FGF23 are associated with mortality in incident dialysis patients (13) through mechanisms that remain to be determined. FGF23 also targets FGF-Klotho receptor complexes in the PTG. Animal studies indicate that FGF23 directly targets the PTG to suppress PTH (14,15). However, in clinical studies, FGF23 levels have been reported to be associated with elevations of PTH levels in some (5,6,16) but not all (8,17,18) studies, contributing to uncertainty in the relationship between FGF23 and the PTG. This progression of hyperparathyroidism in the setting of increased FGF23 levels has recently been explained by down-regulation of FGF receptors leading to end-organ resistance to the suppressive effects of FGF23 (19,20). A possible role of FGF23 to stimulate parathyroid cell proliferation leading to hyperplasia has not been examined.
In most studies of SHPT in HD patients, associations between levels of FGF23 and other markers of mineral metabolic status have been inferred principally through cross-sectional observation or by use of regression modeling to statistically isolate the association of FGF23 levels with levels of various markers of mineral metabolism. However, experimental systems exist, which enable direct investigation of the FGF23-PTG relationship. PTG function, size, and responsiveness to Ca can be inferred using a paradigm of dynamic Ca-mediated PTH suppression that has been used by previous investigators (21). PTH, which has both suppressible and nonsuppressible components, can be diminished in the circulation by establishing a state of transient relative hypercalcemia. The degree of PTH suppression in response to hypercalcemia, expressed as the slope of the ionized Ca-PTH relationship, has been correlated with PTG size (itself a marker of SHPT severity) as measured by ultrasound, whereas the level of ionized Ca associated with a 50% suppression of PTH (iCa50%PTH) is a marker of PTG sensitivity to hypercalcemia. Whether FGF23 levels are associated with the degree of PTH suppressibility or with PTG sensitivity to Ca has never been directly tested.
To gain insights into the potential clinical and regulatory relationships FGF23 might share with the PTG physiology, we designed a prospective interventional study of HD patients with SHPT. We first dialyzed chronic HD patients with established hyperparathyroidism on a high-Ca bath designed to raise serum ionized calcium (iCa) for several hours. After this, we later dialyzed patients on a normal-Ca bath. Our intent was to determine the following: 1) whether FGF23 levels are associated levels of P and PTH in prevalent HD patients, 2) whether FGF23 level is associated with the degree of dynamic Ca-mediated PTH suppression, and 3) how FGF23 levels change during the HD procedure itself.
Participants and Methods
Participants
Clinically stable adult maintenance HD patients with presumed SHPT were solicited for study participation. Patients were eligible if they were at least 18 yr of age, had been on HD for at least 3 months, and had a clinically diagnosed history of SHPT documented in the medical chart. Patients were excluded if they had been hospitalized within the previous 1 month, had acute ongoing medical issues (e.g. active infection), or had a history of parathyroidectomy. Laboratory criteria for inclusion were an intact PTH level of 300 pg/ml or greater while on treatment for at least 2 months with either activated vitamin D compounds (calcitriol analogs) and/or calcimimetics as well as a corrected Ca of 8.4 mg/dl or greater.
The study was conducted in accordance with the principles of the Declaration of Helsinki, and approval was obtained from the University of Kansas Medical Center’s Institutional Review Board. Informed consent was obtained from all participants.
Experimental design
The experimental design had two distinct phases. All participants underwent a 4-wk medication washout period during which activated vitamin D compounds and/or calcimimetics were withheld. All participants then underwent phase 1, in which they dialyzed on a 3.5-mEq/liter calcium bath, starting at the beginning of a dialysis session, for 150 min. To closely monitor the participants in the setting of the intervention, a physician was at the chairside to observe the patient’s clinical status at all times and to measure iCa levels at 0, 45, 90, and 150 min with a point-of-service device and observe the results in real time. Serum for P, intact PTH, and FGF23 were drawn at these same time points. At 150 min, the participant was changed to a 2.5-mEq/liter Ca bath (standard for our unit) and monitored by a physician for the remainder of the HD session. Medications for SHPT were then resumed, and the participants underwent regular dialysis with a 2.5-mEq/liter Ca dialysate bath for 3 months. After this, participants then underwent another 4-wk medication washout period in which they took no active vitamin D analogs or calcimimetics. They were next exposed to phase 2, in which they dialyzed on a typical 2.5-mEq/liter Ca bath; samples for Ca, P, PTH, and FGF23 were drawn at 0 and 150 min. Because measuring iCa in real time presents a modest technical challenge and because iCa would not be expected to rise dramatically on a 2.5-mEq Ca bath, only total Ca was measured during phase 2.
Measurement of circulating factors
Ionized Ca was measured at the chairside using the immunoradiometric assay Trupoint blood analysis system (International Technidyne Corp., Edison, NJ) to provide information on iCa levels in real time. Samples for P and intact PTH were collected in serum separator tubes, allowed to clot at room temperature, centrifuged, frozen at −70 C, and shipped on dry ice to ARUP Laboratories (Salt Lake City, UT) for measurement. Samples for FGF23 were assayed at the study site in triplicate using the full-length intact human FGF23 ELISA (Kainos, Tokyo, Japan).
Statistical analysis
Descriptive statistics were presented for baseline characteristics. Changes from baseline to 150 min in the analytes were evaluated with the Wilcoxon signed rank test. Because the distribution of FGF23 levels were, as expected, highly skewed, a natural log transformation of these measures was used. Similarly, the difference in log FGF23 was computed by subtracting log FGF23 at 150 min from log FGF23 at 0 min. Bivariate associations were examined with Spearman correlations.
A previous investigation using a nearly identical experimental paradigm (21) generated slopes from subject-specific ordinary least squares regression lines to characterize the association between change in iCa and change in log-transformed percent change of PTH. Analogously, using linear mixed models (22), we generated iCa/PTH suppression curves to derive slope parameters to quantify the association between PTH suppression in response to increases in iCa. Specifically, we used a random coefficients model (23), predicting the natural log of PTH at each time point (0, 45, 90, and 150 min) as a function of iCa at the corresponding time point and the (time specific) iCa-by-baseline log FGF23 measure interaction. By treating the intercept and iCa terms as random, this model allowed for each subject to have unique slope and intercept parameters, analogous to the ordinary least squares approach by Indridason et al. (21). Using the Kenward-Rogers adjustment to the degrees of freedom (23) the t test of the iCa-by-baseline log FGF23 interaction provided a direct test of the baseline level of FGF23 on the response of PTH to the iCa challenge. The iCa/PTH suppression curve slopes of each individual participant derived from this model were then plotted against baseline log FGF23 level to visually present this relationship from the linear mixed model. Finally, for the exploratory analysis, the relationship between iCa50%PTH and log FGF23 was examined. For this analysis, participants who did not experience significant PTH suppression (n = 4) were eliminated. The Spearman correlation coefficient for the relationship between iCa50%PTH and baseline log FGF23 was estimated.
Analyses were conducted with SAS 9.2 software (SAS Institute Inc., Cary, NC). A type I error rate of 5% was used to determine statistical significance with no corrections for multiple comparisons due to the exploratory nature of this study.
Results
All 18 participants underwent phase 1 (the high Ca bath exposure), whereas 13 of these participants underwent subsequent phase 2 (the normal Ca bath exposure). The five participants who did not undergo the phase 2 exposure did not do so because of death (n = 1), acute or ongoing illness (n = 2), poor control of SHPT precluding washout of treatment (n = 1), and transplantation (n = 1). Table 1 illustrates baseline participant characteristics. Mean age was 54.3 yr, and median duration of dialysis was 38 months. Median PTH level was 645 pg/ml and median FGF23 level was 4705 pg/ml.
Table 1.
Participant baseline characteristics (n = 18)
| Variable | Value |
|---|---|
| Age (yr) | 54.3 ± 13.5 |
| Sex, male, n (%) | 12 (67) |
| Race | |
| White, n (%) | 6 (33) |
| Black, n (%) | 7 (39) |
| Hispanic, n (%) | 5 (28) |
| Duration of dialysis (months) | 38 (16, 72) |
| iCa (mmol/liter) | 1.16 ± 1.0 |
| P (mg/dl) | 6.3 ± 2.2 |
| Intact PTH (pg/ml) | 644 (438,772) |
| FGF23 (pg/ml) | 4705 (795, 15,649) |
Data shown as mean ± sd except for variables that demonstrated skew (duration of dialysis, intact PTH, and FGF23), for which median and 25th and 75th percentile levels are shown.
We first investigated factors associated with baseline log FGF23 levels. Baseline log FGF23 was positively correlated with baseline P at the start of both experimental phases (P < 0.02 at each time point). Baseline log FGF23 showed positive correlations with baseline PTH level; this correlation was significant at phase 1 (the high calcium bath exposure; P = 0.011) and nearly significant during phase 2 (the normal calcium bath exposure; P = 0.07), during which there were fewer participants.
Next, we confirmed that during phase 1, exposure to the high-Ca bath resulted in the expected Ca-mediated PTH suppression (ρ = −0.59, P < 0.01). Results of changes in several analytes during both phases are shown in Table 2. Mean iCa increased from 1.16 ± 0.10 mmol/liter to a peak of 1.47 ± 0.19 mmol/liter at 90 min, an elevation that was sustained at 150 min (1.42 ± 0.16 mmol/liter at the final time point). The Wilcoxon rank sum test indicated an increase in the median iCa at 150 min (P < 0.0001). Concurrently, PTH fell significantly, from a median of 645 pg/ml to 131 pg/ml (P = 0.006). Total Ca was unchanged during phase 2 (data not shown). PTH was unchanged in phase 2 (P = 0.50). In both phases, P decreased significantly. Likewise, relative log FGF23 levels decreased significantly, regardless of the Ca bath concentration (P < 0.0001 in phase 1 and P = 0.0002 in phase 2). Overall, changes in raw (untransformed) FGF23 levels were modest. For example, during phase 1, FGF23 levels fell from a median of 4705 [interquartile range (IQR) 795-15649] pg/ml to 3690 (IQR 639–10644) pg/ml, a drop of 21.6% in the median value.
Table 2.
Changes in mineral metabolic analytes during the two different calcium bath exposures
| Variable | Phase 1 (high Ca bath, 3.5 mEq/liter) (n = 18)
|
Phase 2 (normal Ca bath, 2.5 mEq/liter) (n = 13)
|
||||
|---|---|---|---|---|---|---|
| 0 min | 150 min | P value | 0 min | 150 min | P value | |
| iCa (mmol/liter)a | 1.16 ± 0.10 | 1.42 ± 0.16 | <0.0001 | Not measured | Not measured | — |
| PTH (pg/ml)b | 645 (438, 772) | 131 (90, 392) | 0.006 | 581 (443, 928) | 586 (449, 929) | 0.50 |
| P (mg/dl) | 6.3 ± 2.2 | 3.2 ± 1.1 | <0.0001 | 6.8 ± 1.6 | 3.0 ± 0.8 | 0.0002 |
| log FGF23 | 3.59 ± 0.77 | 3.45 ± 0.78 | <0.0001 | 3.96 ± 0.63 | 3.81 ± 0.65 | 0.0002 |
P values were generated by the Wilcoxon signed rank test.
Ca measured as ionized in the high-calcium bath phase only.
PTH levels reported as median and 25th and 75th percentiles.
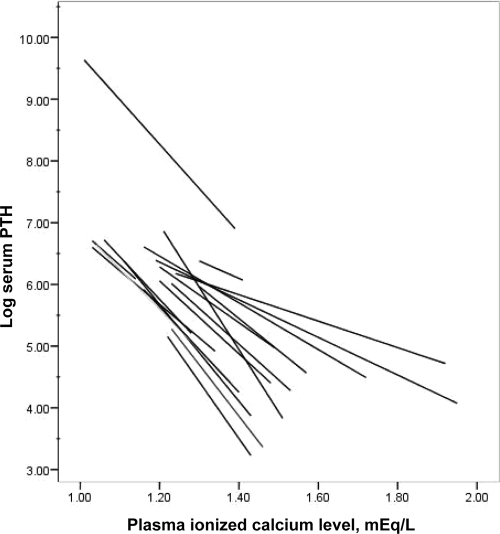
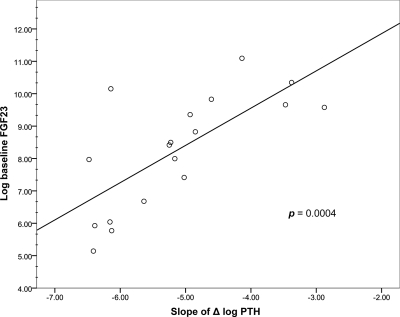
We then examined the association between baseline FGF23 levels and both the iCa/PTH suppression curve slope and the iCa50%PTH. The effect of raising iCa on PTH suppression on each individual subject during phase 1 is shown in Fig. 1. Some participants exhibited far more robust suppression than others. The association of the slope of the iCa/PTH suppression curve with baseline log FGF23 levels is shown in Fig. 2. The plot indicates that slope was significantly associated with baseline log FGF23 levels (P = 0.0004) and that the iCa/PTH suppression relationship was attenuated by larger values of baseline log FGF23 (β = 0.28). These results demonstrated that, in general, individuals with higher baseline FGF23 levels had significantly less Ca-mediated PTH suppression relative to individuals with lower baseline FGF23 levels.
Figure 1.
Modeled change in log-transformed PTH level as a function of plasma ionized Ca for each participant during the high-Ca dialysate bath exposure. Individual lines represent ordinary least squares regression lines for each participant over his or her range of iCa values.
Figure 2.
Plot of baseline log FGF23 levels with the slope of the Ca-mediated PTH suppression curve generated during the high-Ca bath exposure. Baseline log FGF23 levels were significantly associated with slope of the Ca-mediated PTH suppression curve (P = 0.0004 from the linear mixed model).
In an exploratory analysis, we investigated whether baseline log FGF23 level was associated with the level of iCa required for an iCa50%PTH. Four of 18 individuals experienced a trivial suppression of PTH in absolute terms and were therefore not included in the analysis; three of these four individuals had markedly high FGF23 levels (>11,500 pg/ml), which were well above the median FGF23 value, as well as PTH levels above the median. As these individuals ostensibly represented patients with refractory hyperparathyroidism, we performed the analysis on the 14 individuals who did demonstrate physiologically relevant PTH suppression during the experimental procedure. Baseline log FGF23 was not significantly correlated with iCa50%PTH (r = 0.2571, P = 0.37).
Finally, the association of acute changes in log FGF23 levels with changes in P and PTH during HD was examined. Changes in log FGF23 during HD were not significantly associated with changes in P during either exposure (P = 0.41 in the phase 1 and P = 0.48 in phase 2). Neither of the slopes of the two relationships was significantly different from zero (data not shown). Changes in log FGF23 during HD were not robustly significantly associated with changes in PTH during either phase (P = 0.06 in phase 1 and P = 0.13 in phase 2). An overall summary of the key correlations tested during the study is shown in Table 3. In addition to those noted above, we tested for the association of PTH level with change in PTH during both phases of the study; no significant associations were found. Likewise, there was no significant association between the baseline log FGF23 level and the change in PTH at either time.
Table 3.
Summary of associations of levels of mineral metabolic parameters under various experimental conditions
| Spearman correlation | Ca bath conditiona | ρ | P value |
|---|---|---|---|
| ΔiCa with ΔPTH | High | −0.59 | <0.01 |
| Baseline log FGF23 with baseline P | High | 0.70 | <0.002 |
| Normal | 0.66 | 0.014 | |
| Baseline log FGF23 with baseline PTH | High | 0.58 | 0.011 |
| Normal | 0.52 | 0.07 | |
| Baseline log FGF23 with ΔPTH | High | 0.13 | 0.59 |
| Normal | −0.34 | 0.25 | |
| Baseline PTH with ΔPTH | High | −0.28 | 0.26 |
| Normal | −0.03 | 0.91 | |
| ΔLog FGF23 with ΔP | High | 0.21 | 0.41 |
| Normal | 0.22 | 0.48 | |
| ΔLog FGF23 with ΔPTH | High | 0.45 | 0.06 |
| Normal | 0.44 | 0.13 |
n = 18 for the high-Ca dialysate exposure; n = 13 for the normal-Ca dialysate exposure. Δ, Change.
Discussion
The present investigation is the first study to use dynamic Ca-mediated PTH suppression to investigate how FGF23 levels correlate with indices of PTG function. Our most important finding is that baseline FGF23 levels are significantly associated with the slope of the Ca-mediated PTH suppression curve [a quantity reported to be an indirect marker of PTG size (21)]. We did not see an association between baseline FGF23 levels and iCa50%PTH, which measures the sensitivity to calcium-mediated PTH suppression. We also did not find an association between changes in PTH levels and changes in FGF23 levels during calcium-mediated PTH suppression, suggesting that, at least under the present experimental conditions, acute changes in PTH do not regulate circulating levels of FGF23.
The regulatory relationship between FGF23 and PTH appears to be complex. The direct correlation between PTH and FGF23 could represent one of several possibilities: an effect of PTH to directly stimulate FGF23, an action of FGF23 to stimulate PTG hyperplasia leading to long-term increases in PTH secretion, or the result of common factors in ESRD leading to concordant increases in both hormones via distinct mechanisms. The possibility that PTH stimulates FGF23 is supported by studies demonstrating a reduction in FGF23 levels after parathyroidectomy (16). However, no data in humans have demonstrated a direct regulatory effect of PTH on FGF23 synthesis, and in vitro studies show that PTH does not stimulate FGF23 promoter activity (10). It is unlikely in ESRD that the effect of PTH on FGF23 is indirectly mediated by 1,25(OH)2D because 1,25(OH)2D, which can stimulate FGF23, is decreased in ESRD. It also seems unlikely that PTH-induced increased bone turnover leads to increased FGF23 because recent studies in animals indicated that decreased, rather than increased, bone remodeling is associated with increased FGF23 levels in rodent models (24). Moreover, PTH administration sufficient to increased bone remodeling leads to reduction in FGF23 levels in animal models.
A possible explanation for the observed PTH-FGF23 correlation could be a direct effect of FGF23 on PTG function. Although the PTG is known to be a target of FGF23, with Klotho and an isoform of the FGF receptor [FGF receptor 1(IIIc)] reconstituting the FGF23 receptor in the chief cells of the PTG (25), studies of FGF23 in animal models demonstrate that, at least in the short term, FGF23 directly down-regulates PTH production (15). This latter finding suggests that FGF23 opposes excessive PTH synthesis and that high FGF23 levels would be inversely correlated with PTH levels. This finding, however, does not preclude the possibility that elevations of FGF23 stimulate PTG hyperplasia, an effect of FGF23 not yet established but nevertheless consistent with FGF function in other tissues (26,27,28). Our data suggest that high FGF23 levels could contribute to a worsening of SHPT chronically because the present association between the iCa-PTH slope [a quantity associated with gland size as measured by ultrasound (21)] and FGF23 implicates the latter in PTG hyperplasia. Further evidence for this hypothesis comes from observations by other investigators that FGF23 levels predict future refractoriness to SHPT treatment with calcitriol analogs (6), suggesting that FGF23 levels may be directly a marker for the status of the PTG. Nevertheless, we must note that the association between FGF23 and the degree of PTG dysfunction could merely result from common factors associated with ESRD that exert independent effects on FGF23 secretion and PTG function. As such, definitive explanation of our findings remains uncertain, and future studies, such as those investigating whether FGF23 levels directly correlate with chief cell proliferation and PTG size as well as whether reduction of FGF23 alters the natural history of SHPT progression, must be performed to establish causal relationships.
Our other major finding is that relative FGF23 levels decrease during the HD procedure. Such acute changes in FGF23 could be theorized to be influenced by, or associated with, changes in PTH, Ca, P, or other factors. Because FGF23 levels decreased in similar fashion in both experimental phases (i.e. in both the high and normal Ca bath exposures) and because PTH and Ca behaved differently in the two conditions (i.e. PTH decreasing in the high Ca bath exposure as iCa rose, whereas PTH and Ca remained stable in the normal Ca bath exposure), our experimental system could not detect evidence of either PTH or Ca sharing a direct regulatory relationship with FGF23 in the acute setting. It is conceivable that the acute drop in P in both conditions (as a result of dialytic clearance of P during HD) could be responsible for the changes observed in FGF23 levels, but the mechanism of how P might regulate FGF23 levels is uncertain and the magnitude of change in FGF23 due to alterations in P levels is generally modest (7,29); we found no evidence of a statistically significant association between the decrease in P and the decrease in FGF23 in our subjects. Another possible explanation for the observed behavior of FGFG23 in response to HD would be dialytic clearance of FGF23. However, the large size of the FGF23 molecule (∼32 kDa) makes permeability across the dialysis membrane unlikely, and in the only other study that examined FGF23 changes during the HD procedure itself, FGF23 was undetectable in the dialysis effluent (18). These investigators also found, paradoxically, that FGF23 increased during HD. Although we cannot definitely determine why FGF23 decreased during dialysis (a finding confirmed by the identical behavior of FGF23 during the two distinct phases of our study), we do not believe the answer lies in the acute changes of Ca or PTH or in the dialytic clearance of FGF23 itself.
Several important limitations to our study must be noted. First, the sample size was small, and statistical power to discern true associations was limited. We also cannot be certain that our participants universally had a phenotype of SHPT and high bone turnover disease. Indeed, it seems likely that we included some patients with tertiary hyperparathyroidism because several of them did not experience robust suppression of PTH in response to hypercalcemia. Whereas even more stringent inclusion criteria would have been useful to more definitively identify patients with SHPT, capturing individuals with a more realistic chance of PTH suppression in response to hypercalcemic stimulus would likely have strengthened our conclusions because this would have enriched our study sample with likely responders. We did not have information on baseline 25 hydroxyvitamin D [25(OH)D] levels, but a recent study in which cholecalciferol was used to robustly increase 25(OH)D levels failed to significantly decrease FGF23 levels (30), making it less likely that any finding attributed to FGF23 levels was in reality due to 25(OH)D status. Additionally, because our focus was specifically calcium-mediated PTH suppression, we did not generate a true calcium set point, a quantity that would have required exposing the participants to a hypocalcemic stimulus (thereby complementing our hypercalcemic challenge). Such an intervention would be unlikely to change the present conclusions, given the findings of previous work that used calcium-mediated PTH suppression (21), but it is important avenue for future study that is required for a full understanding of the role of that FGF23 plays in PTG physiology. Lastly, our data do not provide definitive information on whether and how FGF23 and PTH might share regulatory relationships. This area of investigation is currently in flux, with recent studies suggesting that PTH suppresses FGF23 (27), and we acknowledge that much work remains to be done. However, our limitations are probably counterbalanced by the biologically provocative exposure to transient hypercalcemia (which reliably produced the stimulus we intended); by the study design, which exploited a limited number of subjects to act as their own controls and to be tested for numerous important outcomes and relationships; and by the use of the highly sensitive Kainos full-length FGF23 assay, which demonstrated levels of FGF23 similar to those widely reported in the literature.
In summary, FGF23 levels are associated with the degree of Ca-mediated PTH suppression, a marker of PTG size and function. This finding suggests that FGF23 levels may be a marker of PTG physiology. FGF23 levels decrease during dialysis, but the decrease does not appear to be associated with the changes in PTH or decrements of P during the HD procedure. More studies are needed to determine the regulatory relationship FGF23 shares with PTH, how FGF23 impacts PTG function, and whether FGF23 directly targets the PTG to induce long-term gland hyperplasia.
Acknowledgments
We thank Connie Wang, M.D., for technical assistance with the manuscript. J.B.W. helped design the study, assisted with conductance of the experiment, and wrote the manuscript; P.S. helped design the study and conducted the experiment; J.D.M. and R.K. performed the biostatistical analysis and assisted in manuscript preparation; R.M. measured FGF23 levels; H.G. assisted with conductance of the experiment; and L.D.Q. helped design the study, cowrote the manuscript, and oversaw all aspects of the study.
Footnotes
This work was supported by National Institutes of Health (National Institute of Arthritis and Musculoskeletal and Skin Diseases) Grants RO1AR045955 and R56AR045955 (to L.D.Q.).
Disclosure Summary: None of the authors have potential conflicts of interest to declare.
First Published Online October 13, 2010
Abbreviations: ESRD, End-stage renal disease; FGF23, fibroblast growth factor-23; iCa, ionized Ca; iCa50%PTH, ionized Ca associated with a 50% suppression of PTH; 1,25(OH)2D, 1,25 dihydroxyvitamin D3; P, renal phosphate; PTG, parathyroid gland; SHPT, secondary hyperparathyroidism.
References
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK 1998 Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 31:607–617 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Yoshioka M, Itoh N 2000 Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun 277:494–498 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Konishi M, Miyake A, Inui K, Itoh N 2002 Fibroblast growth factor (FGF)-23 inhibits renal phosphate reabsorption by activation of the mitogen-activated protein kinase pathway. J Biol Chem 277:28265–28270 [DOI] [PubMed] [Google Scholar]
- Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T 2004 FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435 [DOI] [PubMed] [Google Scholar]
- Imanishi Y, Inaba M, Nakatsuka K, Nagasue K, Okuno S, Yoshihara A, Miura M, Miyauchi A, Kobayashi K, Miki T, Shoji T, Ishimura E, Nishizawa Y 2004 FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int 65:1943–1946 [DOI] [PubMed] [Google Scholar]
- Kazama JJ, Sato F, Omori K, Hama H, Yamamoto S, Maruyama H, Narita I, Gejyo F, Yamashita T, Fukumoto S, Fukagawa M 2005 Pretreatment serum FGF-23 levels predict the efficacy of calcitriol therapy in dialysis patients. Kidney Int 67:1120–1125 [DOI] [PubMed] [Google Scholar]
- Koiwa F, Kazama JJ, Tokumoto A, Onoda N, Kato H, Okada T, Nii-Kono T, Fukagawa M, Shigematsu T 2005 Sevelamer hydrochloride and calcium bicarbonate reduce serum fibroblast growth factor 23 levels in dialysis patients. Ther Apher Dial 9:336–339 [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Kazama JJ, Nii-Kono T, Omori K, Yamashita T, Fukumoto S, Gejyo F, Shigematsu T, Fukagawa M 2005 Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int 67:1171–1178 [DOI] [PubMed] [Google Scholar]
- Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD 2003 Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem 278:37419–37426 [DOI] [PubMed] [Google Scholar]
- Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD 2006 Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 17:1305–1315 [DOI] [PubMed] [Google Scholar]
- Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, Miyamoto K, Fukushima N 2003 Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1α,25-dihydroxyvitamin D3 production. J Biol Chem 278:2206–2211 [DOI] [PubMed] [Google Scholar]
- Perwad F, Zhang MY, Tenenhouse HS, Portale AA 2007 Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1α-hydroxylase expression in vitro. Am J Physiol Renal Physiol 293:F1577–F1583 [DOI] [PubMed] [Google Scholar]
- Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M 2008 Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359:584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajisnik T, Björklund P, Marsell R, Ljunggren O, Akerström G, Jonsson KB, Westin G, Larsson TE 2007 Fibroblast growth factor-23 regulates parathyroid hormone and 1α-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol 195:125–131 [DOI] [PubMed] [Google Scholar]
- Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J 2007 The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117:4003–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Tominaga Y, Ueki T, Goto N, Matsuoka S, Katayama A, Haba T, Uchida K, Nakanishi S, Kazama JJ, Gejyo F, Yamashita T, Fukagawa M 2004 Total parathyroidectomy reduces elevated circulating fibroblast growth factor 23 in advanced secondary hyperparathyroidism. Am J Kidney Dis 44:481–487 [PubMed] [Google Scholar]
- Nishi H, Nii-Kono T, Nakanishi S, Yamazaki Y, Yamashita T, Fukumoto S, Ikeda K, Fujimori A, Fukagawa M 2005 Intravenous calcitriol therapy increases serum concentrations of fibroblast growth factor-23 in dialysis patients with secondary hyperparathyroidism. Nephron Clin Pract 101:c94–c99 [DOI] [PubMed] [Google Scholar]
- Urena Torres P, Friedlander G, de Vernejoul MC, Silve C, Prié D 2008 Bone mass does not correlate with the serum fibroblast growth factor 23 in hemodialysis patients. Kidney Int 73:102–107 [DOI] [PubMed] [Google Scholar]
- Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, Mendoza FJ, Munoz-Castaneda JR, Shalhoub V, Almaden Y, Rodriguez M 2010 FGF23 fails to inhibit uremic parathyroid glands. J Am Soc Nephrol 21:1125–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T 2010 Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int 77:211–218 [DOI] [PubMed] [Google Scholar]
- Indridason OS, Heath 3rd H, Khosla S, Yohay DA, Quarles LD 1996 Non-suppressible parathyroid hormone secretion is related to gland size in uremic secondary hyperparathyroidism. Kidney Int 50:1663–1671 [DOI] [PubMed] [Google Scholar]
- McCullough CE, Searle SR 2001 Generalized, linear, and mixed models. Chap 6. New York: John Wiley, Sons [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O 2006 SAS for mixed models. 2nd ed. Chap 8. Cary, NC: SAS Institute, Inc. [Google Scholar]
- Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P 2003 FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest 112:683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T 2006 Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–774 [Google Scholar]
- Nabel EG, Yang ZY, Plautz G, Forough R, Zhan X, Haudenschild CC, Maciag T, Nabel GJ 1993 Recombinant fibroblast growth factor-1 promotes intimal hyperplasia and angiogenesis in arteries in vivo. Nature 362:844–846 [DOI] [PubMed] [Google Scholar]
- Samadfam R, Richard C, Nguyen-Yamamoto L, Bolivar I, Goltzman D 2009 Bone formation regulates circulating concentrations of fibroblast growth factor 23. Endocrinology 150:4835–4845 [DOI] [PubMed] [Google Scholar]
- Izikki M, Guignabert C, Fadel E, Humbert M, Tu L, Zadigue P, Dartevelle P, Simonneau G, Adnot S, Maitre B, Raffestin B, Eddahibi S 2009 Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents. J Clin Invest 119:512–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett SM, Gunawardene SC, Bringhurst FR, Jüppner H, Lee H, Finkelstein JS 2006 Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 21:1187–1196 [DOI] [PubMed] [Google Scholar]
- Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD 2010 Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol 21:353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]