Abstract
Context: The extent to which 25-hydroxyvitamin D [25(OH)D] and IGF-I influence bone mineral content (BMC) accrual from early to mid-puberty is unclear.
Objective, Setting, and Participants: This study sought to determine relationships among 25(OH)D, IGF-I, and BMC in community-dwelling prepubertal females (n = 76; aged 4–8 yr at baseline) over a period of up to 9 yr.
Design: The hypothesis that changes in IGF-I vs. 25(OH)D are more strongly associated with BMC accrual was formulated after data collection. 25(OH)D and IGF-I were log-transformed and further adjusted using two-way ANOVA for differences in season and race. Linear mixed modeling (including a random subject-specific intercept and a random subject-specific slope on age) was employed to analyze the proportion of variance the transformed 25(OH)D and IGF-I variables explained for the bone outcomes.
Results: IGF-I was more strongly associated with BMC accrual than 25(OH)D at the total body (R2 = 0.874 vs. 0.809), proximal femur (R2 = 0.847 vs. 0.771), radius (R2 = 0.812 vs. 0.759), and lumbar spine (R2 = 0.759 vs. 0.698). The rate of BMC accrual was positively associated with changes in IGF-I but negatively associated with 25(OH)D. When IGF-I and 25(OH)D were included in the same regression equation, 25(OH)D did not have a significant predictive effect on BMC accrual above and beyond that of IGF-I.
Conclusions: These prospective data in early adolescent females indicate that both 25(OH)D and IGF-I have a significant impact on bone mineral accrual; however, the positive association of IGF-I and BMC accrual is greater than the negative association of 25(OH)D and BMC accrual.
Both 25(OH)D and IGF-I impact bone mineral accrual; however, the positive association of IGF-I is greater than the negative association of 25(OH)D on BMC accrual.
Whereas considerable knowledge exists regarding vitamin D status and bone in older adults (e.g. poor vitamin D status in the elderly is associated with low bone mass, and vitamin D supplementation has been shown to increase calcium absorption, suppress intact PTH secretion, slow bone loss, reduce the number of skeletal fractures, and favorably alter functional outcomes of bone metabolism) (1,2,3), much less is known about vitamin D and bone in growing children. Low 25-hydroxyvitamin D [25(OH)D] concentrations in children may have significant health implications with respect to bone metabolism, because approximately 90% of adult bone mineral content (BMC) is achieved by the end of adolescence with approximately 28% of BMC accrued during the 2-yr period surrounding peak BMC velocity (4). Currently, 25(OH)D concentrations reflecting optimal vitamin D status in children are unknown, and the influences of vitamin D status on BMC accrual during growth have yet to be determined. Contributing to these uncertainties is that few vitamin D supplementation trials have been conducted in pediatric populations. Supplementation with vitamin D in adolescents has been shown to significantly increase circulating 1,25-dihydroxycholecalciferol [1,25(OH)2D] (5,6,7) and decrease intact PTH (6) but only with larger doses of vitamin D and in those with lower initial serum 25(OH)D. Two vitamin D intervention studies in adolescents have reported modest improvements in femoral BMC with the equivalent of 2000 IU/d (5) and 200–400 IU/d (8) and in vertebral BMC with 400 IU/d (8), although in the latter study, positive results were shown only in the compliance-based analyses.
It is plausible that during childhood, other hormones, notably IGF-I, may play a more prominent role in bone mineral accrual than vitamin D. IGF-I is available to bone through exocrine delivery via the circulation, paracrine and autocrine synthesis, and liberation from stores in the bone matrix (9,10). IGF-I is involved in the regulation, development, and homeostatic maintenance of bone and cartilage. In vitro and in vivo studies provide substantive evidence to indicate IGF-I involvement in osteoblast and osteoclast cell proliferation, but its precise role has yet to be elucidated (11,12,13). Studies comparing IGF-I knockout (KO) mice with controls show IGF-I is involved in bone mineral accretion. Mohan et al. (14) reported no significant increase in bone mineral density (BMD) in IGF-I KO mice during the pubertal period, whereas the control mice exhibited a 40% increase in BMD. Additionally, clinical studies in humans ascertaining mutations in IGF-I or IGF-I receptor result in intrauterine and postnatal growth retardation (15,16,17,18). The pubertal period of adolescence is marked by a rapid increase in serum IGF-I, which later declines with increasing age and mirrors the slope of bone accrual and age-related bone loss (19,20,21). The majority of cross-sectional studies show a positive association between IGF-I and bone mineral accrual in healthy children; however, the extent to which the hormone exerts an influence on bone mineralization is uncertain (22,23,24,25,26). These inconsistencies may be due to between-study variability in the maturational stage of participants, the form of IGF-I measured (e.g. serum IGF-I, IGF-binding protein (1–6), and IGF-I to IGF binding protein-3 ratio), the bone variable observed (e.g. areal BMD, BMC, cortical thickness, and bone biomarkers), and the bone site measured (e.g. lumbar spine, total body, and metacarpal). Furthermore, there is a lack of prospective research on the IGF-I/bone accrual relationship during the pubertal years.
Although IGF-I and 25(OH)D have been independently studied with respect to their impact on bone, studies investigating the influences of each of these hormones on bone mineral accrual are limited. Cell culture studies and animal model studies support an interaction between IGF-I and 1,25(OH)2D [i.e. 1,25(OH)2D promotes the action of IGF-I by increasing IGF-I receptor expression, and in turn, IGF-I stimulates the hydroxylation of the circulating 25(OH)D to the active 1,25(OH)2D form in the kidneys through the stimulation of the 1α-hydroxylase enzyme] (27,28). To our knowledge, IGF-I and vitamin D have never been prospectively examined together with regard to their relationship to bone mineral accrual during childhood. The purpose of this study was to determine relationships between BMC accrual and plasma concentrations of both IGF-I and 25(OH)D in pre- and early-pubertal females, aged 4–15 yr. We hypothesized that changes in plasma concentrations of IGF-I are more strongly associated with BMC accrual than changes in plasma concentrations of 25(OH)D in adolescent females.
Subjects and Methods
Participants
The study participants were females, aged 4–8 yr at baseline, who were recruited to participate in a prospective study investigating the influence of artistic gymnastics on bone (29). The present study followed subjects only in the nonintervention group (n = 96) for a period of up to 9 yr (median of 5 yr). Although testing occurred for all subjects annually, not all subjects were tested every year during the 9-yr time span. Subjects were healthy and were not taking medications known to affect bone metabolism. The participants’ ethnicity and race were classified through parent identification using the National Institutes of Health Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research (30). Ethnic/racial groups with small sample sizes (n < 7) were excluded, leaving 83 participants (49 White and 34 Black) of those originally recruited. Sixty-five (34 White, 31 Black) subjects provided annual serum samples over 4 yr, 12 (11 White, one Black) over 5 yr, six (four White, two Black) over 6 yr, and one (White) over 7 yr, and one (White) subject provided a serum sample over 9 yr. The University of Georgia Institutional Review Board for Human Subjects approved the study protocol. Each participant and her guardian completed informed assent and consent forms, respectively.
Data collection
Data collection took place in the Bone and Body Composition Laboratory at The University of Georgia between October 1997 and October 2008. Enrollment was staggered throughout the fall (September to November), winter (December to February), spring (March to May), and summer (June to August). Annual testing of each participant occurred within the same season as the baseline testing session. Blood was collected between 0730 and 1000 h after a 12-h fast. Participants returned to the laboratory within 1 wk of their blood draw to undergo bone scans and anthropometric measures and to complete questionnaires assessing demographic, dietary, and physical activity information.
Sexual maturation
A physician conducted annual sexual maturation assessments using criteria for stages of breast development (stages 1–5) as described by Tanner (31). Computed results of one-way random-effects model, single-measure intraclass correlation coefficients (ICC) (R value) indicated perfect agreement (R = 1.0) from physician-assessed staging of breast development in females aged 6–10 yr (n = 10), evaluated twice in a 2-wk period.
Anthropometric measures
Participants wore light clothing and no shoes for the height and weight measurements, taken using the Anthropometric Standardization Reference protocol (32). Using a wall-mounted stadiometer (Novel Products, Rockton, IL), height was measured to the nearest 0.1 cm. Weight was measured to the nearest 0.25 kg using a calibrated double-beam balance scale (Fairbanks Scales, Kansas City, MO). One-way random-effects model, single-measure ICCs were calculated in females aged 6–10 yr (n = 10) measured twice in a 2-wk period by the same individual. The height and weight ICC and test-retest coefficient of variation (CV) (percentage) values were 0.99 and 0.4% and 0.99 and 1.4%, for height and weight, respectively. Body mass index (BMI) (kilograms per square meter) values were plotted on BMI-for-age percentile charts (33).
BMC and body composition
Participants’ bone area (square centimeters) and BMC (grams) were assessed at the total body, lumbar spine, nondominant proximal femur, and nondominant distal radius by dual-energy x-ray absorptiometry (DXA; QDR-1000W, Hologic, Inc., Bedford, MA). DXA calibration was performed daily using a calcium hydroxyapatite and epoxy lumbar spine phantom embedded in a Lucite cube (Hologic, model DPA/QDR-1). The laboratory CV calculated from 365 scans over 5 yr was 0.27%. In our laboratory, test-retest measurements with DXA in 6- to 10-yr-old females (n = 10) showed the following CVs for areal bone mineral density: total body, 1.2%; lumbar spine, 1.3%; proximal femur, 1.6%; and forearm, 2.1%. The lumbar spine was analyzed using DXA low-density spine software version 4.74 (Hologic). The fat-free soft tissue (FFST) mass (grams) and percent body fat were determined by DXA and analyzed using pediatric whole-body analysis version 5.73 (Hologic). An external three-step soft tissue wedge composed of aluminum and lucite, calibrated against stearic acid (100% fat) and water (8.6% fat), was run in tandem with each whole-body scan for quality control. The percent body fat single-measure ICC and CV in participants aged 5–8 yr (n = 10) scanned twice in a 1-wk period were R = 0.99 and 2.0%, respectively.
Biochemical assays
Fasting blood samples were collected and stored at −70 C until analysis of plasma 25(OH)D and IGF-I. 25(OH)D samples were assayed using a RIA (DiaSorin Laboratories, Stillwater, MN) and were reported by Willis et al. (34). The inter- and intraassay CVs were 7.3–10.5 and 5.9–7.0%, respectively. IGF-I concentrations were determined using recombinant human IGF-I quantitative sandwich immunoassay technique (ELISA; R&D Systems, Minneapolis, MN). The plasma samples were pretreated with 1) an acidic dissociation solution and 2) a buffered protein with blue dye and preservatives and lyophilized to release IGF-I from binding proteins. The inter- and intraassay CVs were 7.5–8.3and 3.5–4.3%, respectively. Both 25(OH)D and IGF-I samples were run in duplicate using a block design, such that all samples from the same subject were assayed at one time, and an equal number of Black and White subjects were analyzed using the same kit.
Dietary intake
Dietary intakes were estimated using 3-d diet records, which have shown reliability and validity in estimating energy and nutrient intakes in children (35,36,37). The records included type of food, preparation method, and time of eating. A laboratory technician, using photographs of serving sizes and a 24-h recall with food models, helped train the participants’ guardians in accurately recording food intake. Energy (kilocalories), calcium (milligrams), and vitamin D (micrograms) intake was assessed using Food Processor II software (version 7.5; ESHA Research, Salem, OR). The ICC for average measure of dietary intake of 3 d (n = 10) completed twice within a 2-wk period was determined using a one-way random-effects model and calculated for energy, calcium, and vitamin D (R = 0.47, 0.71, and 0.94, respectively).
Physical activity
Trained researchers administered a modified version of a physical activity questionnaire developed by Slemenda et al. (38) to each participant and her guardian. Physical activity was subjectively quantified using a five-point Likert scale comparing the participant’s usual activity level to that of her peers scaling from 1 being much less active to 5 being much more active.
Statistical analyses
Statistical analyses were performed using Statistical Analysis Software version 9.1 (SAS, Cary, NC). Descriptive statistics are expressed as mean ± sd, and a P value ≤0.05 was considered statistically significant. Linear mixed-effects models were used to analyze the effects of plasma IGF-I and 25(OH)D on bone mineral accrual and to determine which of these variables is most strongly associated with bone mineral accrual. A common set of three models was fit to BMC at each scan site: total body, lumbar spine, nondominant proximal femur, and nondominant forearm. Generally speaking, these three models were designed to describe the extent to which variability in patterns of change in BMC with age was explained by 1) plasma IGF-I, 2) 25(OH)D, and 3) both plasma IGF-I and 25(OH)D. The longitudinal nature of the study design requires the models to account for possible within-subject correlation among repeated measures of BMC taken over time. In addition, patterns of change in BMC are, a priori, not likely to be, and, empirically, were not observed to be, linear in age. To account for these data features, all models included both linear and quadratic effects of age at the time of measurement (current age) as well as subject-specific random intercepts and (linear) age effects. In addition, the models all included an effect of race and, because the sample was heterogeneous with respect to age at entry of the study, separate baseline and longitudinal effects of age were accounted for by also including linear and quadratic effects of the subject’s age at the start of the study. Thus, all models took the following form:
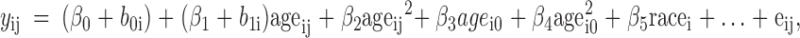 |
1 |
where yij represents the BMC at the jth measurement occasion for subject i, ageij and agei0 are the ith subject’s age at the jth measurement occasion and baseline, respectively; b0i and b1i are correlated, bivariate normal random effects; racei is an indicator for whether subject i is White; eij is a mean 0, constant variance, normal error term; and the β terms are regression coefficients. Note that age and baseline age were centered around the mean age (8.52 yr) averaged over all subjects at all testing sessions. A similar approach to modeling observational longitudinal data involving a sample of subjects heterogeneous in age has been published by Fitzmaurice et al. (39).
Models 1–3 differed in the specification of the “…” portion of equation 1.1 by including additional effects of plasma IGF-I, 25(OH)D, or both. Because of the right-skewed nature of these variables, both IGF-I and 25(OH)D were (natural) log-transformed (loge). In addition, the variable loge[25(OH)D] was adjusted for seasonal and race differences by centering it at each girl’s season of measurement by race baseline mean. For simplicity of notation, let Xij = loge(IGF-I)ij and Zij = loge[25(OH)D]ij. Although the explanatory effect of Xij in a longitudinal model like equation 1.1 could be assessed by the inclusion of an additional regression effect β6Xij, such an approach ignores possible interactions between age and Xij (that is, it ignores the possibility that, for example, the trend in BMC with age depends upon IGF-I). To avoid such assumptions, our models took the form of equation 1.1 with either 1) an additional main effect of Xij plus interaction effects between Xij and all of the age variables already in the model, 2) an additional main effect of Zij plus interaction effects between Zij and all of the age variables already in the model, and 3) both 1) and 2) combined. Specifically, model 1 takes the following form:
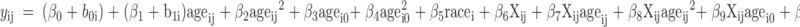 |
2 |
Model 2 takes the following form:
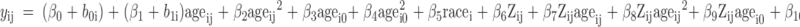 |
3 |
And model 3 takes the following form:
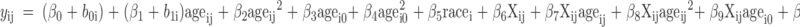 |
4 |
R2 values (40) from these models were compared to address the question of which variable’s combined main and interaction effects explain more of the variability in BMC growth.
Absolute values for IGF-I and 25(OH)D vs. Z-scores or δ-values were used in the models. For each bone site, based on the fitted models 1 and 2, predictive curves of BMC accrual were plotted for a hypothetical subject with a fixed value of logeIGF-I (model 1) or a fixed value of loge25(OH)D (model 2). Fixed values were set equal to the sample deciles of the explanatory variable [logeIGF-I or loge25(OH)D] to generate a family of curves to give a graphical display of the relative explanatory power of the two variables. To compare the explanatory power of logeIGF-I vs. loge25(OH)D on bone mineral accrual after accounting for FFST, the three models were refit, with the inclusion of linear effects of both FFST and baseline FFST. Again R2 values were compared across these lean mass-adjusted models. The addition of FFST to the models allows an assessment of which partial association is larger: the one between BMC accrual and changes in logeIGF-I after controlling for FFST or the partial association between BMC accrual and changes in loge25(OH)D after controlling for FFST.
Finally, it was desired to measure the correlation between logeIGF-I and loge25(OH)D and to test whether this correlation was equal to 0. This task is complicated by the longitudinal nature of the data because the within-subject correlation and nonconstant mean through time for each variable invalidates a simple correlation analysis. Instead, inference on the correlation between these variables was performed by fitting a bivariate linear mixed-effect model to logeIGF-I and loge25(OH)D simultaneously, in which random subject-specific effects for each response variable were included to account for within-subject correlation, different linear trends through time were fit for each response variable, and contemporaneous correlation between the two response variables was allowed and estimated.
Results
Participant characteristics
Baseline characteristics of the participants are presented in Table 1. All but four girls were classified as breast stage 1 (i.e. prepubertal) at baseline. Over the 9 yr, nine subjects reached menarche and had advanced to breast stages 4/5. At the time of study exit, 25 stayed in prepubertal stage 1 and 39 advanced to early pubertal stage 2/3. The majority of participants (n = 44) at baseline had BMI-for-age percentiles that were between the 5th and 85th percentile, three participants had BMI-for-age percentiles that were less than the 5th percentile, 15 participants had BMI-for-age percentiles between the 85th and 95th percentiles, and 14 participants had BMI-for-age percentiles that were 95th or higher percentile. At baseline, 83% of the participants’ intake of vitamin D exceeded two thirds of the dietary reference intake recommendation for vitamin D, and 86% of the participants exceeded two thirds of the dietary reference intake recommendation for calcium. The mean physical activity level of the participants was between average and above average.
Table 1.
Baseline descriptive characteristics of participants
| Variable | Mean | sd | n |
|---|---|---|---|
| Age (yr) | 6.4 | 1.6 | 76 |
| Breast stagea | |||
| Stage 1 | NA | NA | 71 |
| Stage 2 | NA | NA | 3 |
| Stage 3 | NA | NA | 1 |
| Anthropometrics | |||
| Weight (kg) | 25.8 | 8.4 | 73 |
| Height (cm) | 120.0 | 11.8 | 73 |
| BMI | 17.5 | 3.2 | 73 |
| BMI-for-age percentile | 69.0 | 28.3 | 73 |
| % body fatb | 27.3 | 9.8 | 73 |
| FFST mass (kg)b | 16.8 | 4.3 | 73 |
| BMC (g)b | |||
| Total body | 859.7 | 262.5 | 73 |
| Lumbar spine | 17.8 | 4.8 | 73 |
| Proximal femur | 10.7 | 4.0 | 72 |
| Forearm | 2.4 | 0.8 | 73 |
| 25(OH)D (nmol/liter) | 88.3 | 24.5 | 74 |
| IGF-I (ng/ml) | 251.3 | 94.1 | 76 |
| Dietary Intake | |||
| Vitamin D (μg)c | 6.34 | 2.34 | 73 |
| Calcium (mg) | 861.0 | 355.0 | 73 |
| Physical activityd | 3.5 | 0.8 | 73 |
NA, Not applicable.
Sexual maturation stage (breast) as described by Tanner (31).
Body fat percentage, fat-free soft tissue mass, and bone mineral content measured by DXA.
Vitamin D (in micrograms) is presented using the geometric mean ± geometric sd [exp(arithmetic mean of ln-transformed data) ± exp(usual sd of ln-transformed data)].
Physical activity level assessed by parent questionnaire developed by Slemenda et al. (38): 1, inactive; 2, below average; 3, average; 4, above average; 5, very high.
Baseline plasma 25(OH)D and IGF-I are presented in Table 1. The majority of participants (n = 51) had 25(OH)D values above 80 nmol/liter, 20 participants had 25(OH)D values that were between 50 and 80 nmol/liter, and three participants had 25(OH)D values that were less than 50 nmol/liter. A large percentage of participants (∼75%) had 25(OH)D concentrations that fell below 80 nmol/liter at least once during the investigation. Baseline plasma IGF-I values ranged from 55–470 ng/ml.
Influence of plasma concentrations of 25(OH)D and IGF-I on BMC accrual
Table 2 depicts R2 values for change over time in total body, lumbar spine, proximal femur, and forearm BMC and changes in either IGF-I plus 25(OH)D (model 1), 25(OH)D (model 2), or IGF-I (model 3). Table 2 also presents partial associations between BMC accrual and changes in either 25(OH)D or IGF-I after controlling for FFST. For each of the four skeletal sites, IGF-I was more strongly associated with BMC accrual compared with 25(OH)D. When IGF-I and 25(OH)D were included in the same regression equation, 25(OH)D did not have a significant predictive effect on BMC accrual at any skeletal site above and beyond that of IGF-I. When FFST was added to the models involving either logeIGF-I or loge25(OH)D, R2 values increased, demonstrating that FFST adds explanatory power to the model beyond logeIGF-I or loge25(OH)D alone. More importantly, however, in the FFST-adjusted models, the R2 values were again higher for the models involving logeIGF-I than for those involving loge25(OH)D, suggesting a greater partial association between BMC accrual and IGF-I than between BMC accrual and 25(OH)D.
Table 2.
Correlation (R2 values) explaining changes in BMC relative to plasma 25(OH)D, IGF-I, and 25(OH)D plus IGF-I in prepubertal females (n = 76) over a period of up to 9 yr
| Skeletal site | 25(OH)D + IGF-Ia | 25(OH)Db | IGF-Ic | 25(OH)D + FFSTd | IGF-I + FFSTe |
|---|---|---|---|---|---|
| Total body | 0.837 | 0.809 | 0.874 | 0.947 | 0.953 |
| Lumbar spine | 0.718 | 0.698 | 0.759 | 0.925 | 0.932 |
| Proximal femur | 0.807 | 0.771 | 0.847 | 0.921 | 0.928 |
| Forearm | 0.780 | 0.759 | 0.812 | 0.921 | 0.927 |
Linear mixed models were employed to analyze the proportion of variance that 25(OH)D and IGF-I explained on BMC accrual at four skeletal sites.
The R2 value depicts the pattern of change over time accounting for how growth in BMC might be influenced by both 25(OH)D and IGF-I. This model regressed BMC on age, age2, baseline age, baseline age2, natural log (loge) of IGF-I, the interaction of logeIGF-I and age, the interaction of logeIGF-I and age2, logeIGF-I and baseline age, logeIGF-I and baseline age2, loge25(OH)D (adjusted for season and race), the interaction of loge25(OH)D and age, the interaction of loge25(OH)D and age2, loge25(OH)D and baseline age, loge25(OH)D and baseline age2, and race.
The R2 value depicts the pattern of change over time accounting for how growth in BMC might be influenced by 25(OH)D but not IGF-I. This model is the same as the 25(OH)D plus IGF-I model (2), except it excludes all terms involving IGF-I, capturing how bone mineral accrual depends on 25(OH)D.
The R2 value depicts the pattern of change over time accounting for how growth in BMC might be influenced by IGF-I but not 25(OH)D. This model is the same as the 25(OH)D plus IGF-I model (2), except it excludes all terms involving 25(OH)D, capturing how bone mineral accrual depends on IGF-I.
The R2 value depicts the partial association between BMC accrual and changes in 25(OH)D after controlling for FFST.
The R2 value depicts the partial association between BMC accrual and changes in IGF-I after controlling for FFST.
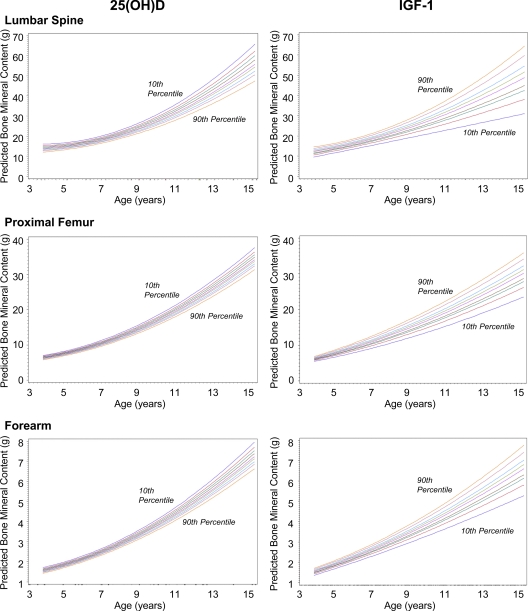
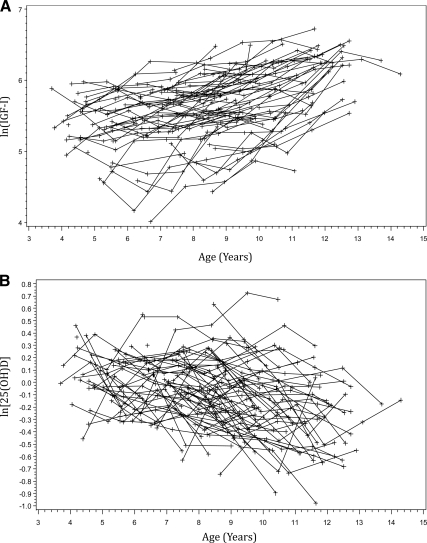
Figure 1 illustrates the predicted curves of BMC accrual based on either baseline loge25(OH)D or logeIGF-I for a subject who entered the study at age 4 yr (i.e. the minimum age represented in our sample). Each of the nine sequential curves (10th, 20th, 30th … up to the 90th) represents a percentile of either loge25(OH)D or logeIGF-I in the sample. The respective percentiles of logeIGF-I and loge25(OH)D are 5.04 and −0.45 (10th), 5.30 and −0.32 (20th), 5.46 and −0.23 (30th), 5.54 and −0.15 (40th), 5.69 and −0.07 (50th), 5.78 and −0.01 (60th), 5.89 and 0.07 (70th), 6.07 and 0.14 (80th), and 6.24 and 0.26 (90th). The respective percentiles of absolute values of IGF-I and 25(OH)D are 154.6 and 61.4 (10th), 200.0 and 70.1 (20th), 234.4 and 76.6 (30th), 255.4 and 83.3 (40th), 294.5 and 90.5 (50th), 325.6 and 95.6 (60th), 362.4 and 103.5 (70th), 435.2 and 111.1 (80th), and 515.2 and 125.4 (90th). The above back transformation for 25(OH)D was adjusted for season and race, so the corresponding decile of 25(OH)D is exp{the decile of log[25(OH)Dadj] + adjustment factor}. As an example, the adjustment for White participants measured in the summer was 4.5709, reflected in the above back-transformed absolute values for 25(OH)D deciles. The plots demonstrate the positive association of IGF-I and the negative association of 25(OH)D and BMC accrual. The predictive curve for the 90th percentile of IGF-I corresponds with the greatest BMC accrual and trends in a stepwise fashions down to the 10th percentile, whereas the predictive curve for the 10th percentile of 25(OH)D corresponds with the greatest BMC accrual and trends in a stepwise fashions down to the 90th percentile. The spread among these curves is greater for the logeIGF-I vs. the loge25(OH)D plots, showing graphically that IGF-I influences these growth curves more strongly than does 25(OH)D. There was no statistically significant correlation observed between logeIGF-I and loge25(OH)D (r = −0.101; P = 0.101). Figure 2A represents the changes in serum IGF-I at a time when many girls had entered into puberty. Figure 2B shows serum 25(OH)D levels decreasing with age.
Figure 1.
Predicted curves for BMC accrual over 9 yr. A linear mixed-effects model shows predicted curves between BMC accrual and either baseline loge25(OH)D or logeIGF-I for a subject who entered the study at age 4 yr. Each sequential curve represents a percentile (10th, 20th, 30th … up to the 90th) of either loge25(OH)D or logeIGF-I in the sample. The spread among these nine curves is greater for the logeIGF-I vs. the loge25(OH)D plots, showing graphically that IGF-I influences these growth curves more strongly than does 25(OH)D. The plots also demonstrate the greater positive association of IGF-I and BMC accrual vs. the negative association of 25(OH)D and BMC accrual.
Figure 2.
A, Subject-specific profiles of ln(IGF-I) vs. age in prepubertal females (n = 76) over a period of up to 9 yr; B, subject-specific profiles of ln[25(OH)D] vs. age in prepubertal females (n = 74) over a period of up to 9 yr.
Discussion
One of the classical manifestations of IGF-I deficiency in children is impaired skeletal growth (41). Children with skeletal diseases characterized by IGF-I deficiency have reduced bone mass and small stature (42). In healthy children, much less is known about the influence of IGF-I on bone mineral accrual. The main finding in the present investigation was that in healthy adolescents, IGF-I is more strongly associated with BMC accrual in comparison with 25(OH)D at all four skeletal sites. Inclusion of both IGF-I and 25(OH)D in the same regression model did not show a significant predictive effect of 25(OH)D on BMC accrual above and beyond that of IGF-I. These findings support our hypothesis that IGF-I is a better predictor of BMC accrual than vitamin D in healthy, prepubertal adolescent females. In addition, this study revealed that the rate of BMC accrual was inversely associated with baseline 25(OH)D but was positively associated with baseline IGF-I.
Because we previously found that the decline in 25(OH)D with age is eliminated after controlling for FFST (34), and it is well established that IGF-I has anabolic actions not only on bone, but also on muscle tissue during childhood growth, we sought to examine the explanatory power of the IGF-I and 25(OH)D models taking into account FFST. Although FFST was added to our study as a secondary aim, the importance of this variable was confirmed by our results; i.e. the explanatory value was increased when FFST was added to both the IGF-I and the 25(OH)D models. Furthermore, the R2 values were higher when adding FFST to the IGF-I model vs. when 25(OH)D was added. These results suggest that whereas 25(OH)D does not have any explanatory power above and beyond that of IGF-I, FFST does have explanatory power on BMC accrual beyond that of IGF-I.
Despite our findings that 25(OH)D concentrations are inversely associated with the rate of BMC accrual, it is not known whether higher 25(OH)D concentrations lead to more favorable changes in bone size and strength. Consistent with our results, in a study following healthy adolescents over a 2-yr period, Tylavsky et al. (43) reported that 25(OH)D was inversely related to gain in total body bone area, BMC, and areal BMD. It is important to note that the majority of the subject population had sufficient baseline vitamin D status. A 12-month vitamin D intervention study conducted in Lebanese female adolescents with low mean baseline 25(OH)D reported significant negative correlations between baseline 25(OH)D and the 1-yr percent change in BMC at the spine, femoral neck, and radius (5). These negative correlations demonstrated that subjects with low baseline 25(OH)D values responded to supplementation to a greater extent than those with higher baseline 25(OH)D values. Taken together, these studies and our findings indicate that, unlike adults, low 25(OH)D status in children and adolescents may be reflective of the potential for greater bone mineral gains.
Although cell culture and animal studies have provided the majority of the evidence supporting the role of IGF-I in bone mineral accrual during growth (12,13,14,44), few studies have been conducted in children and adolescents to confirm this relationship. Rates of IGF-I secretion and bone mineral accrual parallel each other by increasing during pubertal maturation, peaking in the years surrounding peak height velocity, and declining thereafter (19,45); however, these positive correlations between IGF-I and bone mass are based primarily on cross-sectional studies. We present the first prospective investigation to demonstrate a significant positive correlation between changes in IGF-I and BMC. To our knowledge, only one other study has prospectively analyzed IGF-I and changes in BMC. Cadogan et al. (46) did not find a significant relationship between IGF-I and change in BMC and BMD over a 2-yr period in 37 healthy adolescent females aged 12 yr. The discrepancy in findings between this study (46) and the present study may be explained by a smaller sample size, shorter study duration, and a more advanced maturation stage of the participants [the majority (68%) of the 37 females in the Cadogan et al. (46) study reached menarche]. Because IGF-I may also influence geometrical properties of bone (41), future studies should examine the mechanisms by which IGF-I concentrations influence bone mineral accrual and the structural indices of bone. Likewise, IGF-I plays a role in regulating muscle mass during growth and aging. The increases in BMC accrual associated with higher circulating levels of IGF-I observed in the current study may be in part mediated by muscle. In support of this mechanism of action, prospective studies show increases in muscle precede peak BMC accrual at the total body (47,48).
The negative correlation between IGF-I and 25(OH)D observed in the present study was not statistically significant, but it has been proposed that higher circulating levels of IGF-I concentrations lead to lower concentrations of 25(OH)D because IGF-I stimulates the hydroxylation to the active 1,25(OH)2D form. Kasukawa et al. (49) showed this relationship using IGF-I KO mice. The IGF-I KO mice exhibited a significant decrease in serum 1,25(OH)2D concentrations (24%, P < 0.05) compared with wild-type controls. It was also reported that the IGF-I KO mice exhibited a 70% decrease in kidney vitamin D receptor expression. Bianda et al. (50) observed this association between the two hormones in a group of young healthy male subjects. After a 5-d sc IGF-I infusion, 1,25(OH)2D and osteocalcin levels increased significantly (P < 0.06 and P < 0.02, respectively). Although circulating 25(OH)D is recognized as the best functional indicator of vitamin D status in adults, a limitation of the present study was that 1,25(OH)2D was not assessed. In focusing only on 25(OH)D, information on the functional role of vitamin D during childhood bone growth may not have been captured. For example, Abrams et al. (51) reported that higher 1,25(OH)2D concentrations were significantly correlated with fractional calcium absorption in 93 healthy adolescents, whereas 25(OH)D was not significantly associated with fractional calcium absorption. Future studies should include additional functional indicators, such as 1,25(OH)2D concentrations, to develop a greater understanding of vitamin D metabolism during growth.
It is important to note in the present study that the majority of participants had sufficient vitamin D status. The associations between changes in IGF-I and bone mineral accrual may vary in children and adolescents with insufficient levels of vitamin D. Further investigation is needed regarding these hormones in a pediatric population heterogeneous in vitamin D status. Although estradiol was not assessed in the current study and may have affected the findings, reference values indicate that estradiol levels markedly increase at approximately 14 yr of age (52). Only two of the samples were collected from females aged 14 yr, and the majority of the participants in the study were prepubertal, with only nine participants having reached menarche.
In summary, change in IGF-I concentrations was the strongest predictor of BMC accrual at all skeletal sites over a 9-yr period in prepubertal females. Marginally, vitamin D was also a predictor of BMC gain, but in an inverse manner, such that subjects with lower 25(OH)D levels showed the greatest BMC gain. Additional clinical studies are needed to 1) determine how vitamin D affects bone growth and mineralization, 2) examine the role of IGF-I on bone structure, and 3) delineate the relationships of IGF-I and 1,25(OH)2D and their effects on bone growth in children.
Acknowledgments
We gratefully acknowledge the study participants and families for their enthusiasm and commitment to this project.
Footnotes
This work was supported by National Institute on Child Health and Human Development Grant 1 RO1 HD 35592.
Disclosure Summary: None of the authors had any personal or financial conflicts of interest and have nothing to disclose.
First Published Online October 20, 2010
Abbreviations: BMC, Bone mineral content; BMD, bone mineral density; BMI, body mass index; CV, coefficient of variation; DXA, dual energy x-ray absorptiometry; FFST, fat-free soft tissue; ICC, intraclass correlation coefficient; KO, knockout; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxycholecalciferol.
References
- Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B 2004 Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med 116:634–639 [DOI] [PubMed] [Google Scholar]
- Weaver CM, Fleet JC 2004 Vitamin D requirements: current and future. Am J Clin Nutr 80(6 Suppl):1735S–1739S [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Bischoff-Ferrari HA 2007 Therapy of osteoporosis with calcium and vitamin D. J Bone Miner Res 22(Suppl 2):V59–V63 [DOI] [PubMed] [Google Scholar]
- Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R 2000 Calcium accretion in girls and boys during puberty: a longitudinal analysis. J Bone Miner Res 15:2245–2250 [DOI] [PubMed] [Google Scholar]
- El-Hajj Fuleihan G, Nabulsi M, Tamim H, Maalouf J, Salamoun M, Khalife H, Choucair M, Arabi A, Vieth R 2006 Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab 91:405–412 [DOI] [PubMed] [Google Scholar]
- Docio S, Riancho JA, Pérez A, Olmos JM, Amado JA, González-Macías J 1998 Seasonal deficiency of vitamin D in children: a potential target for osteoporosis-preventing strategies? J Bone Miner Res 13:544–548 [DOI] [PubMed] [Google Scholar]
- Schou AJ, Heuck C, Wolthers OD 2003 Vitamin D supplementation to healthy children does not affect serum osteocalcin or markers of type I collagen turnover. Acta Paediatr 92:797–801 [PubMed] [Google Scholar]
- Viljakainen HT, Natri AM, Kärkkäinen M, Huttunen MM, Palssa A, Jakobsen J, Cashman KD, Mølgaard C, Lamberg-Allardt C 2006 A positive dose-response effect of vitamin D supplementation on site-specific bone mineral augmentation in adolescent girls: a double-blinded randomized placebo-controlled 1-year intervention. J Bone Miner Res 21:836–844 [DOI] [PubMed] [Google Scholar]
- Conover CA 2000 Insulin-like growth factors and the skeleton. In: Canalis E, ed. Skeletal growth factors. Philadelphia: Lippincott Williams, Wilkins; 101–116 [Google Scholar]
- Howard G, Spencer E 1991 IGF-parathyroid hormone interactions on bone in vitro and in vivo. New York: Elsevier [Google Scholar]
- Conover CA 2000 In vitro studies of insulin-like growth factor I and bone. Growth Horm IGF Res 10(Suppl B):S107–S110 [DOI] [PubMed] [Google Scholar]
- Hayden JM, Mohan S, Baylink DJ 1995 The insulin-like growth factor system and the coupling of formation to resorption. Bone 17:93S–98S [DOI] [PubMed] [Google Scholar]
- Canalis E 1993 Insulin like growth factors and the local regulation of bone formation. Bone 14:273–276 [DOI] [PubMed] [Google Scholar]
- Mohan S, Richman C, Guo R, Amaar Y, Donahue LR, Wergedal J, Baylink DJ 2003 Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and -independent mechanisms. Endocrinology 144:929–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenkamp MJ, Karperien M, Pereira AM, Hilhorst-Hofstee Y, van Doorn J, Chen JW, Mohan S, Denley A, Forbes B, van Duyvenvoorde HA, van Thiel SW, Sluimers CA, Bax JJ, de Laat JA, Breuning MB, Romijn JA, Wit JM 2005 Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. J Clin Endocrinol Metab 90:2855–2864 [DOI] [PubMed] [Google Scholar]
- Abuzzahab MJ, Schneider A, Goddard A, Grigorescu F, Lautier C, Keller E, Kiess W, Klammt J, Kratzsch J, Osgood D, Pfäffle R, Raile K, Seidel B, Smith RJ, Chernausek SD 2003 IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. N Engl J Med 349:2211–2222 [DOI] [PubMed] [Google Scholar]
- Inagaki K, Tiulpakov A, Rubtsov P, Sverdlova P, Peterkova V, Yakar S, Terekhov S, LeRoith D 2007 A familial insulin-like growth factor-I receptor mutant leads to short stature: clinical and biochemical characterization. J Clin Endocrinol Metab 92:1542–1548 [DOI] [PubMed] [Google Scholar]
- Woods KA, Camacho-Hübner C, Savage MO, Clark AJ 1996 Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med 335:1363–1367 [DOI] [PubMed] [Google Scholar]
- Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jørgensen K, Müller J, Hall K, Skakkebaek NE 1994 Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab 78:744–752 [DOI] [PubMed] [Google Scholar]
- Juul A, Holm K, Kastrup KW, Pedersen SA, Michaelsen KF, Scheike T, Rasmussen S, Müller J, Skakkebaek NE 1997 Free insulin-like growth factor I serum levels in 1430 healthy children and adults, and its diagnostic value in patients suspected of growth hormone deficiency. J Clin Endocrinol Metab 82:2497–2502 [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Conover C 1997 Growth hormone/insulin-like growth factor-I axis in aging: a summary of a National Institutes of Aging-Sponsored Symposium. J Clin Endocrinol Metab 82:3919–3922 [DOI] [PubMed] [Google Scholar]
- Rusinska A, Chlebna-Sokol D 2006 Insulin-like growth factor-I and mineral metabolism markers in children with idiopathic decrease in bone mass. Clin Chim Acta 366:257–263 [DOI] [PubMed] [Google Scholar]
- Kanbur NO, Derman O, Kinik E 2005 The relationships between pubertal development, IGF-1 axis, and bone formation in healthy adolescents. J Bone Miner Metab 23:76–83 [DOI] [PubMed] [Google Scholar]
- Libanati C, Baylink DJ, Lois-Wenzel E, Srinvasan N, Mohan S 1999 Studies on the potential mediators of skeletal changes occurring during puberty in girls. J Clin Endocrinol Metab 84:2807–2814 [DOI] [PubMed] [Google Scholar]
- Léger J, Mercat I, Alberti C, Chevenne D, Armoogum P, Tichet J, Czernichow P 2007 The relationship between the GH/IGF-I axis and serum markers of bone turnover metabolism in healthy children. Eur J Endocrinol 157:685–692 [DOI] [PubMed] [Google Scholar]
- Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A 1999 The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab 84:4489–4496 [DOI] [PubMed] [Google Scholar]
- Gómez JM 2006 The role of insulin-like growth factor I components in the regulation of vitamin D. Curr Pharm Biotechnol 7:125–132 [DOI] [PubMed] [Google Scholar]
- Rucker D, Ezzat S, Diamandi A, Khosravi J, Hanley DA 2004 IGF-I and testosterone levels as predictors of bone mineral density in healthy, community-dwelling men. Clin Endocrinol (Oxf) 60:491–499 [DOI] [PubMed] [Google Scholar]
- Laing EM, Wilson AR, Modlesky CM, O'Connor PJ, Hall DB, Lewis RD 2005 Initial years of recreational artistic gymnastics training improves lumbar spine bone mineral accrual in 4- to 8-year-old females. J Bone Miner Res 20:509–519 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Office of Extramural Research Accessed 10 November 2009 NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. http://grants.nih.gov/grants/funding/women_min/guidelines_amended_10_2001.htm [Google Scholar]
- Tanner J 1962 Growth and adolescence. 2nd ed. Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- Lohman TG, Roche AF, Martorell R 1988 Anthropometric standardization reference manual. Champaign, IL: Human Kinetics [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL 2002 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11:1–190 [PubMed] [Google Scholar]
- Willis CM, Laing EM, Hall DB, Hausman DB, Lewis RD 2007 A prospective analysis of plasma 25-hydroxyvitamin D concentrations in white and black prepubertal females in the southeastern United States. Am J Clin Nutr 85:124–130 [DOI] [PubMed] [Google Scholar]
- Taylor RW, Goulding A 1998 Validation of a short food frequency questionnaire to assess calcium intake in children aged 3 to 6 years. Eur J Clin Nutr 52:464–465 [DOI] [PubMed] [Google Scholar]
- Bergman EA, Boyungs JC, Erickson ML 1990 Comparison of a food frequency questionnaire and a 3-day diet record. J Am Diet Assoc 90:1431–1433 [PubMed] [Google Scholar]
- Crawford PB, Obarzanek E, Morrison J, Sabry ZI 1994 Comparative advantage of 3-day food records over 24-hour recall and 5-day food frequency validated by observation of 9- and 10-year-old girls. J Am Diet Assoc 94:626–630 [DOI] [PubMed] [Google Scholar]
- Slemenda CW, Miller JZ, Hui SL, Reister TK, Johnston Jr CC 1991 Role of physical activity in the development of skeletal mass in children. J Bone Miner Res 6:1227–1233 [DOI] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH 2004 Applied longitudinal analysis. Hoboken, NJ: John Wiley & Sons [Google Scholar]
- Edwards LJ, Muller KE, Wolfinger RD, Qaqish BF, Schabenberger O 2008 An R2 statistic for fixed effects in the linear mixed model. Stat Med 27:6137–6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Rosen CJ 2009 Insulin-like growth factor-I and bone: lessons from mice and men. Pediatr Nephrol 24:1277–1285 [DOI] [PubMed] [Google Scholar]
- Zofková I 2003 Pathophysiological and clinical importance of insulin-like growth factor-I with respect to bone metabolism. Physiol Res 52:657–679 [PubMed] [Google Scholar]
- Tylavsky FA, Ryder KA, Lyytikainen A, Cheng S 2005 Vitamin D, parathyroid hormone, and bone mass in adolescents. J Nutr 135:2735S–2738S [DOI] [PubMed] [Google Scholar]
- Mohan S, Baylink DJ 2005 Impaired skeletal growth in mice with haploinsufficiency of IGF-I: genetic evidence that differences in IGF-I expression could contribute to peak bone mineral density differences. J Endocrinol 185:415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfqvist C, Andersson E, Gelander L, Rosberg S, Blum WF, Albertsson Wikland K 2001 Reference values for IGF-I throughout childhood and adolescence: a model that accounts simultaneously for the effect of gender, age, and puberty. J Clin Endocrinol Metab 86:5870–5876 [DOI] [PubMed] [Google Scholar]
- Cadogan J, Blumsohn A, Barker ME, Eastell R 1998 A longitudinal study of bone gain in pubertal girls: anthropometric and biochemical correlates. J Bone Miner Res 13:1602–1612 [DOI] [PubMed] [Google Scholar]
- Xu L, Nicholson P, Wang Q, Alén M, Cheng S 2009 Bone and muscle development during puberty in girls: a seven-year longitudinal study. J Bone Miner Res 24:1693–1698 [DOI] [PubMed] [Google Scholar]
- Rauch F, Bailey DA, Baxter-Jones A, Mirwald R, Faulkner R 2004 The ‘muscle-bone unit’ during the pubertal growth spurt. Bone 34:771–775 [DOI] [PubMed] [Google Scholar]
- Kasukawa Y, Baylink DJ, Wergedal JE, Amaar Y, Srivastava AK, Guo R, Mohan S 2003 Lack of insulin-like growth factor I exaggerates the effect of calcium deficiency on bone accretion in mice. Endocrinology 144:4682–4689 [DOI] [PubMed] [Google Scholar]
- Bianda T, Hussain MA, Glatz Y, Bouillon R, Froesch ER, Schmid C 1997 Effects of short-term insulin-like growth factor-I or growth hormone treatment on bone turnover, renal phosphate reabsorption and 1,25 dihydroxyvitamin D3 production in healthy man. J Intern Med 241:143–150 [DOI] [PubMed] [Google Scholar]
- Abrams SA, Griffin IJ, Hawthorne KM, Gunn SK, Gundberg CM, Carpenter TO 2005 Relationships among vitamin D levels, parathyroid hormone, and calcium absorption in young adolescents. J Clin Endocrinol Metab 90:5576–5581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay K, Andersson AM, Skakkebaek NE 2004 Estradiol levels in prepubertal boys and girls: analytical challenges. Int J Androl 27:266–273 [DOI] [PubMed] [Google Scholar]