Abstract
Context: Initiating factors leading to production of adrenal androgens are poorly defined. Cortisol is present in high concentrations within the adrenal gland, and its production rises with growth during childhood.
Objective: Our aim was to characterize the effect of cortisol and other glucocorticoids on androgen secretion from a human adrenocortical cell line and from nonadrenal cells transfected with CYP17A1 or HSD3B2.
Design/Setting: This study was performed in cultured cells, at an academic medical center.
Methods: The effects of cortisol upon steroid production in human adrenal NCI-H295R cells were measured by immunoassay, tandem mass spectrometry, and thin-layer chromatography. The effects of cortisol upon the activities of 17, 20 lyase and 3βHSD2 were measured in NCI-H295R cells and in transfected COS-7 cells.
Results: Cortisol markedly and rapidly stimulated dehydroepiandrosterone (DHEA) in a dose-dependent manner at cortisol concentrations ≥50 μm. Cortisone and 11-deoxycortisol were also potent stimulators of DHEA secretion, whereas prednisolone and dexamethasone were not. Treatment with cortisol did not affect expression of CYP17A1 or HSD3B2 mRNAs. Stimulation of DHEA secretion by cortisol was associated with competitive inhibition of 3βHSD2 activity.
Conclusions: Cortisol inhibits 3βHSD2 activity in adrenal cells and in COS-7 cells transfected with HSD3B2. Thus, it is possible that intraadrenal cortisol may participate in the regulation of adrenal DHEA secretion through inhibition of 3βHSD2. We hypothesize that a rise in intraadrenal cortisol during childhood growth may lead to inhibition of 3βHSD2 activity and contribute to the initiation of adrenarche.
Physiologic concentrations of cortisol can stimulate DHEA secretion from human adrenal cells via inhibition of 3βHSD2.
Adrenarche is the initiation of dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) secretion from the zona reticularis of the adrenal gland during childhood, first documented by Talbot in 1943 as a rise in urinary 17-ketosteroid secretion (1). Adrenarche is a gradual process beginning during the first five years of life (2,3), whereas pubarche, the appearance of pubic hair and an end result of adrenarche, normally occurs when children are eight or more years of age. Premature adrenarche is among the most common pediatric endocrine disorders, usually considered benign but occasionally the first sign of an adrenal tumor or enzyme deficiency. In some children, premature adrenarche may be a forerunner of the polycystic ovary syndrome or the metabolic syndrome (4).
The trigger for adrenarche remains unknown, and leads to, directly or indirectly, increased synthesis or decreased metabolism of DHEA, activities controlled by the enzymes 17, 20 lyase, 3-β hydroxysteroid dehydrogenase Type II (3βHSD2), and DHEA-sulfotransferase. P450c17 is the qualitative regulator of steroidogenesis, as the relative activities of its two distinct enzymes, 17α-hydroxylase and 17, 20 lyase, direct steroidogenesis toward mineralocorticoid, glucocorticoid, or androgen production (5). 17, 20 lyase activity increases during adrenarche (2,6,7), and therefore its regulation has been the focus of much research (5). The activity of 17, 20 lyase is essential for androgen production, and is enhanced by its phosphorylation (8), and by the presence in the zona reticularis of cytochrome b5 (9), an allosteric cofactor (10), and P450 oxidoreductase, an electron donor (11), all of which facilitate electron transfer (12). Both older children (13) and adults (14) have decreased expression of 3βHSD2 in the zona reticularis, which promotes adrenal androgen production, but whether this is an initiating or later adrenarchal event is not known. These factors all likely account for the zone-specific production of adrenal androgens.
Thirty years ago, cortisol was hypothesized to play a role in the initiation of adrenarche (15). Though the average serum cortisol concentration remains constant throughout life, its daily production rate increases more than 5-fold as children grow into adulthood (16). At the same time, adrenal gland growth occurs at approximately one-third the rate of body growth (17,18,19). The increasing cortisol production and differential adrenal-to-body growth rates suggested to us that the intraadrenal cortisol concentration may rise during childhood. Additionally, treatment of primary cultures of human fetal adrenal cells with high concentrations of glucocorticoid leads to increased DHEA secretion (20). Thus, we asked whether cortisol stimulates the synthesis and secretion of DHEA from human adrenocortical cells, as cortisol’s role in influencing adrenal androgen production had not been rigorously investigated previously. We report here that cortisol, in concentrations present in the human adrenal (21), markedly stimulates DHEA production in human adrenocortical cells, and that this occurs through competitive inhibition of 3βHSD2 activity.
Materials and Methods
Cell culture
To determine the effect of cortisol on adrenal steroidogenesis, we used the NCI-H295R human adrenal cell line [American Type Culture Collection (ATCC), Manassas, VA] which contains all of the enzymes and steroids of the human adrenal gland (22). NCI-H295R cells or COS-7 monkey kidney cells (also from ATCC) were grown in a humidified atmosphere (95% air-5% CO2) at 37 C in six-well plates (Corning, Corning, NY) to 50–60% confluence. NCI-H295R cells were grown in 2.5 ml RPMI-1640 media (Invitrogen, Carlsbad, CA) supplemented with 4% fetal bovine serum (FBS), 10,000 U/ml penicillin, 10,000 μg/ml streptomycin (Invitrogen), and insulin, transferrin, and selenium (ITS-A, Invitrogen). COS-7 cells were grown in 2.5 ml DMEM (Invitrogen) supplemented with 10% FBS, 10,000 U/ml of penicillin and 10,000 μg/ml streptomycin. Cells were washed and fresh media was added immediately before beginning all experiments.
NCI-H295R cells were exposed for 8 h (unless otherwise indicated) to cortisol (0.01 to 1000 μm), dexamethasone (0.001 to 100 μm), 11-deoxycortisol (250 μm), prednisolone (250 μm), cortisone (250 μm), or 8-bromo-cyclic AMP (8-Br-cAMP, 100 μm) (all from Sigma, St. Louis, MO) dissolved in ethanol (steroids) or water (8-Br-cAMP). Equal amounts of ethanol or water were added to control cells, with the volume of ethanol ≤2.5% of total media volume in each well. Subsequently, media were collected and frozen at −85 C for later measurement, or frozen at −20 C for thin-layer chromatography (TLC). After media removal, cells were collected by gentle trypsinization, and washed, centrifuged, and analyzed for protein determination (Bio-Rad Laboratories, Hercules, CA).
Hormone assays in cell culture
Cortisol was measured by ELISA (Alpco Diagnostics, Salem, NH). DHEA was measured by either ELISA (Alpco Diagnostics), RIA (Diagnostic Systems Laboratories, Webster, TX), or liquid chromatography-tandem mass spectroscopy (LC-MS/MS). DHEAS was measured by ELISA (Alpco Diagnostics). Androstenedione was measured by LC-MS/MS. Tritiated steroids in media were separated by TLC, quantified using a phosphorimager, and displayed using x-ray film. DHEA and androstenedione were measured by LC-MS/MS using an API-5000 triple-quadrupole mass spectrometer (Applied Biosystems, Foster City, CA), a Shimadzu Prominence HPLC System (Shimadzu Scientific Instruments, Columbia, MD), and a Supelco Acentis LC-18 HPLC column (Supelco, Bellefonte, PA). Liquid-liquid extraction was performed on 200 μl of samples, standards, and controls using diethyl ether with a deuterium-labeled internal standard. Ether extracts were dried under nitrogen at 40 C and reconstituted with 40 μl methanol; 25 μl was injected into the LC-MS/MS system. DHEA and androstenedione were eluted from the column using a gradient flow at 0.25 ml/minute with 0.05% formic acid in water and methanol. Quantification was achieved with multiple reaction monitoring (MRM) in positive ion mode. Within-day assay coefficient of variation (CV), over a range of 10 to 1000 ng/dl, averaged 8.2%, and interassay CV averaged 12.6%. Serial dilution of a spiked serum pool assured linearity with R2 = 0.992. A standard addition recovery study proved recoveries from 93.4 to 96.2%. After a functional sensitivity study, the lower limit of detection was determined to be 10 ng/dl.
Expression of CYP17A1 or HSD3B2 constructs
Full-length complementary DNAs (cDNAs) encoding CYP17A1 or HSD3B2 were synthesized, cloned into expression vectors, and transiently transfected into COS-7 cells. cDNAs encoding CYP17A1 (NM_000102) or HSD3B2 (NM_000198.3) were synthesized by PCR using Platinum Taq DNA Polymerase High Fidelity (Invitrogen), total RNA isolated from NCI-H295R cells, and primers spanning the coding regions of CYP17A1 (forward: 5′AAGCTTGCCACCATGTGGGAGCTCGTGGCTCTCTTG 3′; reverse: 5′ GCGGCCGCTTAGGTGCTACCCTCAGCCTGGG 3′) and HSD3B2 (forward: 5′ GCGGCCGCGCCACGATGGGCTGGAGCTGCCTTGTG 3′; reverse: 5′ TCTAGATTCACTGAGTCTTGGACTTCAGGGTCTCC 3′) that also included terminal restriction endonuclease linker sites. PCR products were sequenced to confirm their correct identities, and separately ligated into pRc/CMV (Invitrogen) with the sense strand downstream from the CMV promoter. Using FuGENE 6 (Roche, Mannheim, Germany), COS-7 cells were transiently transfected with the CYP17A1 or HSD3B2 constructs, or with the same amount of empty vector for controls. Experiments were performed 24 to 48 h after transfection.
Assays of steroidogenesis
To investigate the effect of cortisol on steroidogenic enzyme activity, NCI-H295R cells or transfected COS-7 cells were treated with radiolabeled steroid precursors, [3H] (7) pregnenolone (12.6 Ci/mmol, Perkin-Elmer, Waltham, MA) or [3H] (1,2,6,7) DHEA (94.5 Ci/mmol, Perkin-Elmer), 500,000 to 1,500,000 counts per minute per well. Cortisol was added to cells 2 h before the addition of radiolabeled precursors. Media were collected either 1 or 6 h after radiolabeled steroids were added to COS-7 or NCI-H295R cells, respectively (unless otherwise noted). For determination of apparent kinetic constants, transfected COS-7 cells were incubated with 0.5, 2.0, 4.0, or 7.0 μm unlabeled steroid.
Measurement of messenger RNA expression by quantitative PCR
Quantitative PCR was performed on CYP17A1 and HSD3B2 mRNAs isolated from NCI-H295R cells after an 8-h exposure to cortisol (500 μm) or vehicle. Total RNA was isolated using TRIzol reagent (Invitrogen). RNA samples were treated with DNase (QIAGEN, Valencia, CA) to eliminate genomic DNA contamination and were purified using the RNeasy Mini kit (QIAGEN). RNA was reverse-transcribed using iScript cDNA Synthesis kit (Bio-Rad). 500 ng of total RNA was used with 1 μl iScript, 4 μl iScript reaction mix, in a total volume of 20 μL, at 42C for 30 min. Quantitative PCR was performed using 2 μl of cDNA and iQ SybrGreen RT-PCR Supermix (Bio-Rad) in a total volume of 25 μl using the following protocol: 95 C × 3 min, [(95 C × 10 sec, 60 C × 40 sec) × 45 cycles]. Primers specific for CYP17A1 mRNA (forward: 5′ AGCCGCACACCAACTATCAGTGAC 3′; reverse: 5′ TCACCGATGCTGGAGTCAACGTTG 3′, spanning intron 6), HSD3B2 mRNA (forward: 5′ TTGGACAAGGCCTTCAGACA 3′; reverse: 5′ ACAGGCGGTGTGGATGAC 3′, spanning intron 2), and 18S ribosomal RNA (rRNA) (forward: 5′ AGTCCCTGCCCTTTGTACACA 3′; reverse: 5′ CGATCCGAGGGCTCACTA 3′) were used, the latter as an internal control. CYP17A1 and HSD3B2 mRNA content were calculated using the ΔCT method (23).
Statistical analysis
Data are presented as the mean of three to five replicates per group, with most experiments repeated one to three times with confirmatory results. Data were analyzed using the Student’s t test (unpaired, 2-tailed) when comparing two groups, or ANOVA, followed by Tukey post hoc analysis, when comparing more than two groups. Error bars denote se of the mean. Data analysis was performed with SPSS software, version 17 (Chicago, IL). A difference was considered statistically significant if the P value was less than 0.05. Maximum velocity (Vmax) and Michaelis constants (Km) were calculated using GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA).
Results
Effect of cortisol and its analogs on adrenal secretion of DHEA and DHEAS
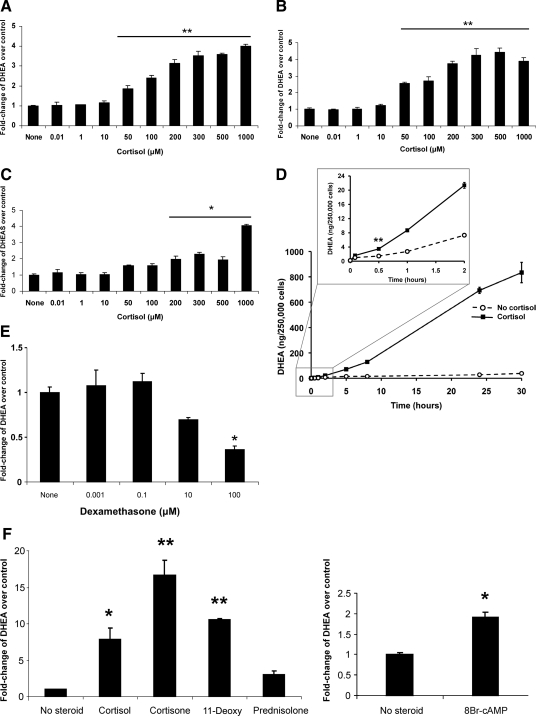
Cortisol stimulated DHEA secretion from NCI-H295R cells in a dose-dependent manner at concentrations ≥50 μm (Fig. 1A). Due to the unconventionally high concentrations of cortisol, we were concerned that it might cross-react in the DHEA assay, leading to artifactual detection of DHEA. We therefore measured the cross-reactivity of cortisol (500 μm) in the DHEA RIA and found it to be <0.0001%. Even at its highest concentration, cortisol’s cross reactivity can account for at most 0.1% of the measured DHEA in the RIA. Furthermore, we used LC-MS/MS to confirm that cortisol stimulated DHEA secretion at concentrations ≥50 μm (Fig. 1B) with a dose-response pattern similar to that detected by RIA. Because a rise in DHEA secretion might be due to inhibition of DHEA-sulfotransferase, we examined the effect of cortisol treatment on DHEAS secretion. Cross-reactivity of cortisol was experimentally measured in the DHEAS assay and found to be <0.0001%. We found that the cortisol-stimulated rise in DHEA was not accompanied by a fall in DHEAS and that DHEAS secretion rose at cortisol concentrations ≥200 μm (Fig. 1C). The data in Fig. 1C are not consistent with inhibition of DHEA-sulfotransferase by cortisol. In a time-course study, we detected stimulation of DHEA secretion soon after the addition of cortisol, with a significant rise by 30 min (Fig. 1D). In contrast to cortisol, we found that dexamethasone, a potent mediator of glucocorticoid-dependent transcription, did not stimulate DHEA secretion, and at 100 μm, dexamethasone had an inhibitory effect (Fig. 1E). We also measured endogenous cortisol secretion into media exposed to untreated NCI-H295R cells for 8 h and found it to be 0.07 μm (3.4 μg/dl).
Figure 1.
Effect of cortisol upon DHEA secretion from human adrenal cells. Dose-response of DHEA secretion from NCI-H295R cells, measured by RIA (A) or by liquid chromatography tandem mass spectrometry (LC/MS-MS) (B), after exposure to cortisol for 8 h. In both panels, compared with no steroid treatment, cortisol ≥50 μm caused a significant increase in DHEA secretion. Absolute DHEA measurements, in ng/mg protein, for the no steroid treatments were 52.9 (A) and 14.3 (B), respectively. C, Dose-response of DHEAS secretion from NCI-H295R cells, measured by ELISA. D, Time course of cortisol (500 μm) stimulation of DHEA secretion, measured by RIA. The inset highlights the first 2 h of treatment, with a significant increase in DHEA at and beyond 30 min. E, DHEA secretion was not stimulated after exposure for 8 h to a wide range of dexamethasone concentrations and was significantly inhibited at the highest concentration. F, Compared with no steroid treatment, exposure to cortisone or 11-deoxycortisol (11-Deoxy) stimulated DHEA secretion, whereas exposure to prednisolone was no different than untreated cells. Treatment with 8-bromo-cAMP also showed stimulation of DHEA production. F, Cells were exposed to steroids (250 μm) or 8-bromo-cAMP (100 μm) for 24 h. DHEA measurements in E and F were done by ELISA. Each value is expressed as the mean of three replicates per group. Each experiment, except the time course (D), was performed 2–4 times with similar results. The time course (D) was repeated once, though with fewer time points, and yielded similar results. *, P < 0.005; **, P < 0.001.
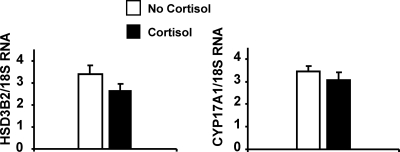
To further explore requirements for cortisol’s effect on NCI-H295R cells, we studied additional glucocorticoids with various degrees of transcriptional activity, including cortisone (transcriptionally inactive), 11-deoxycortisol (weakly active), and prednisolone (active). Cortisone and 11-deoxycortisol were even more potent than cortisol in stimulating DHEA secretion, whereas prednisolone was inactive (Fig. 1F). The cross-reactivities of cortisol and 11-deoxycortisol in the DHEA ELISA assay were undetectable and 0.027%, respectively. As a positive control, we used 8-Br-cAMP, a second messenger activator of the protein kinase A pathway, to stimulate DHEA secretion in NCI-H295R cells (Fig. 1F), as others have done previously (24,25). Measurement of mRNAs encoding two key regulators of adrenal androgen steroidogenesis, CYP17A1 and HSD3B2, revealed no significant differences following cortisol treatment of NCI-H295R cells (Fig. 2).
Figure 2.
Effect of cortisol on HSD3B2 and CYP17A1 mRNA in human adrenal cells. HSD3B2 and CYP17A1 mRNA expression did not change after treatment with cortisol (500 μm) for 8 h. Each value is expressed as the mean of 5 or 6 replicates.
Effect of cortisol on adrenal androgenic enzyme activities
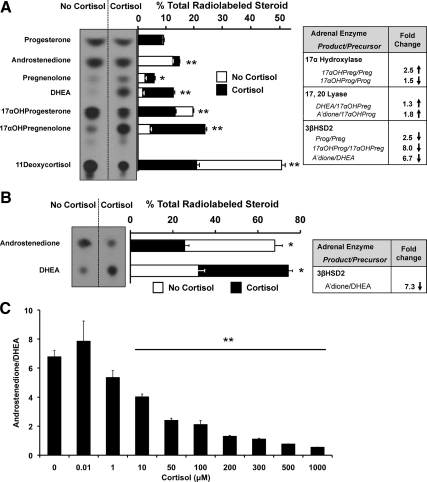
Using tritiated steroid precursors, we next sought to identify the enzyme(s) whose activity, when affected by cortisol, mediates the rise in DHEA secretion from human adrenal cells. We considered two possible mechanisms to explain cortisol’s effect on DHEA secretion from NCI-H295R cells: stimulation of 17, 20 lyase or inhibition of 3βHSD2. We first analyzed cortisol’s effect on 17, 20 lyase function (Fig. 3A and Supplemental Table 1, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org), because this enzyme plays a key role in androgen synthesis. Using [3H]-pregnenolone, we were surprised to find only a modest (1.3-fold) stimulation of 17, 20 lyase, through measurement of the conversion of 17αOH-pregnenolone to DHEA. Slightly greater stimulation of 17, 20 lyase conversion of 17αOH-progesterone to androstenedione was observed (Fig. 3A and Supplemental Table 1), although this pathway is unlikely to be important in the human adrenal (26). In contrast, cortisol treatment caused a 7- to 8-fold inhibition of 3βHSD2-catalyzed synthesis of both 17αOH-progesterone from 17αOH-pregnenolone and of androstenedione from DHEA, and a more modest 2.5-fold reduction in the conversion of pregnenolone to progesterone (Fig. 3A and Supplemental Table 1). The accumulation of Δ-5 steroids (pregnenolone, 17αOH-pregnenolone, and DHEA) at the expense of their Δ-4 products (progesterone, 17αOH-progesterone, and androstenedione) indicates inhibition of 3βHSD2 in the presence of cortisol.
Figure 3.
Effect of cortisol upon steroidogenic enzyme activities in human adrenal cells. Representative TLC and quantification of specific metabolic products of [3H]-pregnenolone (A) or [3H]-DHEA (B) in cells exposed to cortisol (500 μm), expressed as the percentage of total radiolabeled steroids. Media were collected 6 h after adding [3H]-pregnenolone or 2.5 h after adding [3H]-DHEA. In both panels, tables display the ratios of product to precursor steroids and the corresponding changes in enzyme activities. Using either steroid precursor, cortisol markedly inhibited 3βHSD2 activity. C, 3βHSD2 activity, calculated from the ratio of secreted unlabeled androstenedione to unlabeled DHEA, measured by tandem mass spectrometry. 3βHSD2 activity showed dose-dependent inhibition at cortisol concentrations ≥10 μm. 17αOHPreg, 17αOH-pregnenolone; Preg, pregnenolone; 17αOHProg, 17αOH-progesterone; Prog, progesterone; DHEA, dehydroepiandrosterone; A’dione, androstenedione. *, P < 0.01; **, P < 0.001.
Next, using [3H]-DHEA as the substrate for 3βHSD2, we demonstrated a 7-fold reduction in enzymatic activity by cortisol (Fig. 3B and Supplemental Table 1). In parallel with its effect on DHEA secretion (Fig. 1, A and B), cortisol’s inhibition of 3βHSD2 activity was dose-dependent at concentrations ≥10 μm, as determined by the ratio of secreted androstenedione to DHEA, with higher cortisol concentrations causing 7-fold inhibition (Fig. 3C).
Competitive inhibition of 3βHSD2 by cortisol
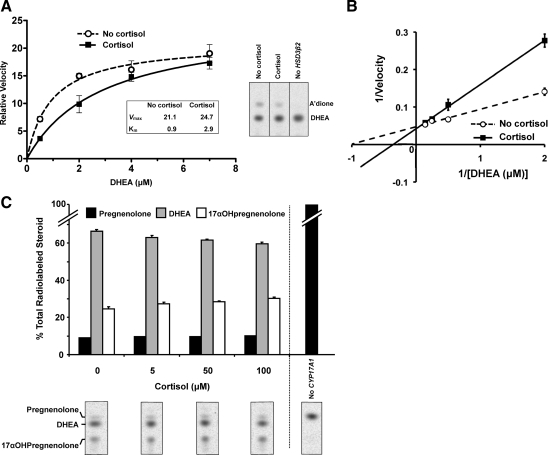
To determine the nature of cortisol’s inhibitory effect on 3βHSD2, we expressed HSD3B2 in COS-7 cells, a cell line which does not contain the glucocorticoid receptor (27,28). In addition, untransfected COS-7 cells do not express HSD3B2 and therefore cannot convert DHEA to androstenedione (29) (Fig. 4A). After transient transfection with HSD3B2, [3H]-DHEA was converted to androstenedione, and we found that cortisol competitively inhibited the 3βHSD2 activity (Fig. 4, A and B), with a 3-fold increase in the apparent Km for DHEA (P < 0.001). The Km for DHEA in the absence of cortisol (0.9 μm, Fig. 4A) is close to the previously reported value of 1.6 μm (30) at a similar concentration to DHEA levels in the human adrenal cortex (21). At this DHEA concentration, we showed that cortisol reduced 3βHSD2 velocity by approximately 50% (Fig. 4A).
Figure 4.
Effect of cortisol on steroidogenic enzyme activities in nonadrenal cells engineered to express HSD3B2 or CYP17A1. Representative TLC and quantification of metabolic products of [3H]-DHEA in cells transiently transfected with HSD3B2 (A and B) or of [3H]-pregnenolone in cells transiently transfected with CYP17A1 (C). In the absence of transfection of either gene, no metabolism of the labeled steroid was observed. Substrate-velocity (A) and Lineweaver-Burk (B) plots were used to obtain Michaelis-Menten kinetic constants for 3βHSD2 metabolism of DHEA in the absence and presence of cortisol (50 μm). There was little change in relative maximal velocity, Vmax (P = 0.28), and a 3-fold increase in the apparent Michaelis constant, Km (P < 0.001), with the addition of cortisol, indicating that cortisol is a competitive inhibitor of 3βHSD2. In the representative TLC chromatogram in A, the DHEA substrate concentration was 0.5 μm. C, Increasing amounts of cortisol were associated with small but significant increases in 17αOH-pregnenolone and decreases in DHEA (P = 0.006 and P = 0.003, respectively for effect of cortisol), indicating a modest decrease in 17, 20 lyase activity.
We also detected a small effect of cortisol upon the 17α-hydroxylase and 17, 20 lyase activities of CYP17 using COS-7 cells transfected with CYP171A (Fig. 4C). Metabolism of [3H]-pregnenolone did not occur in untransfected cells (Fig. 4C). After transient transfection with CYP171A, pregnenolone was converted to 17αOH-pregnenolone and DHEA. Exposure to cortisol did not stimulate DHEA production and instead caused a modest dose-dependent inhibition of 17, 20 lyase activity (Fig. 4C and Supplemental Table 2).
Discussion
We have shown that cortisol, at physiologic intraadrenal concentrations (21), is capable of marked competitive inhibition of adrenal 3βHSD2 in a cell culture model. These results are the first to demonstrate that cortisol can stimulate DHEA and DHEAS secretion from adrenal cells.
Our results indicate that the cortisol-mediated stimulation of DHEA secretion is unlikely to be due to a transcriptional mechanism based upon the following: its onset is rapid; the cortisol concentration required to stimulate DHEA secretion is far greater than that needed for glucocorticoid receptor binding; dexamethasone and prednisolone are inactive or minimally active, whereas cortisone and 11-deoxycortisol are more active than cortisol; the rise in DHEA occurs without changes in CYP17A1 or HSD3B2 mRNA levels; and cortisol inhibits 3βHSD2 activity in COS-7 cells that lack glucocorticoid receptors. Dexamethasone’s inhibition of DHEA secretion at high concentrations is consistent with its known competitive inhibition of both 17-hydroxylase and 17, 20 lyase activities at high concentrations (31).
The high degree of structural similarity among cortisol, cortisone, and 11-deoxycortisol, despite their divergent transcriptional activities, suggests that they may operate via direct interaction with an enzyme in the adrenal androgen biosynthetic pathway. The kinetic effect of cortisol on 3βHSD2 activity, the high steroid concentration required for this effect (many times greater than the Km for DHEA), the ability of both cortisol and cortisone to stimulate adrenal DHEA secretion despite their different glucocorticoid potencies, and the structural similarity between these two steroids (which differ by a single hydrogen atom on C11 of the steroid nucleus) all suggest that the effect on 3βHSD2 may be through competitive inhibition of the enzyme’s dehydrogenase and/or isomerase activities. Intriguingly, the steroids which stimulate DHEA secretion through inhibition of 3βHSD2—cortisol, cortisone, and 11-deoxycortisol—all share an A ring steroid structure with the Δ-4 steroids, progesterone, 17αOH-hydroxyprogesterone, and androstenedione. In contrast, dexamethasone and prednisolone, which do not stimulate DHEA secretion, have different A ring structures. Therefore, it is possible that the steroids that inhibit 3βHSD2 may mimic the Δ-4 end product inhibition of 3βHSD2 activity that has been observed by others (32,33). Medroxyprogesterone acetate (MPA) shares this Δ-4 A ring steroid structure and has been shown to inhibit 3βHSD2 (31). However, despite estradiol not sharing this Δ-4 A ring structure, it does competitively inhibit 3βHSD2 (34) via a mechanism that does not require the estrogen receptor (35). Further studies are required to discover the additional structural factors that cause competitive inhibition of 3βHSD2 by intraadrenal and other steroids.
In the fetus, suppression of adrenal 3βHSD2 activity by the high concentrations of circulating estradiol, in concert with low 3βHSD2 expression in the fetal adrenal, may facilitate production of C19 steroids during fetal life (35). However, estradiol is unlikely to affect postnatal adrenal steroidogenesis at a time when circulating estradiol concentrations are much lower. On the other hand, we have demonstrated that cortisol, at concentrations present in the postnatal adrenal (21), causes competitive inhibition of 3βHSD2 activity.
In 1980, Anderson hypothesized that a rise in intraadrenal cortisol, occurring by an unknown mechanism and acting through an undefined pathway, may trigger adrenarche (15). Winter and his colleagues had considered that inhibition of 3βHSD2 by intraadrenal steroids may contribute to the initiation of adrenarche (32), but showed no effect with 1 μm cortisol, the highest dose they tried, despite their prior finding of a 50-fold higher cortisol concentration in adrenal homogenates (21). During the same period, Rabe et al. (36) showed that cortisol, in amounts used in our study, inhibited human placental 3βHSD1 activity by 50%. In our preliminary analysis of human adrenal glands, we found an average peak intraadrenal cortisol concentration of 114 ± 36 μm (Topor, L. S., and J. A. Majzoub, unpublished results).
Most of our steroid measurements were performed using immunoassays, with confirmatory samples analyzed by LC/MS-MS. LC/MS-MS offers superior sensitivity and specificity compared with immunoassay (37). The use of different assays led to similar magnitudes of DHEA stimulation by cortisol. The cortisol dose-response results are consistent between the two assay methods along the full range of cortisol concentrations, demonstrating the lack of cortisol cross-reactivity in the DHEA immunoassay.
Several investigators have observed a major increase in adrenal 17, 20 lyase activity during human adrenarche (2,6,7), and much effort has therefore been focused upon regulation of this enzyme (5). The activity of 17, 20 lyase in the zona reticularis is enhanced by many factors (8,9,10,11) not addressed in our study, all of which contribute to increased 17, 20 lyase activity during adrenarche. As our study was conducted in cultured cells, the potential effects of inhibition of 3βHSD2 activity in vivo, including secondary stimulation of ACTH secretion, could not be assessed. This study is also limited by the use of an in vitro cell culture model, which does not include the zonation of the adrenal gland. The steroid production and blood flow of the adrenal gland lead to differential steroid concentrations in the three cortical zones, while our in vitro system could not account for blood flow or diffusion of steroids. Additionally, the NCI-H295R cells are neoplastic, with limited responsiveness to ACTH but retained responsiveness via cAMP-dependent pathways (22). Despite these limitations, as the NCI-H295R cell line contains the enzymes necessary for adrenal steroidogenesis (22), it provides a suitable and practical environment for studying androgen steroidogenesis.
Other studies have shown that decreased 3βHSD2 activity in the cortisol biosynthetic pathway is characteristic of adrenarche (2,3,6,7) and is even more evident in premature adrenarche (38,39). These prior studies, along with our findings, lead us to speculate that during adrenarche, a mild 3βHSD2 block in cortisol secretion could result in up-regulation of the pituitary-adrenal axis, including increased stimulation by ACTH, leading to the previously observed enhanced 17, 20 lyase activity (2,6,7) and growth of the zona reticularis (19). Rigorous quantification of ACTH secretory dynamics, as has been carried out in adults (40), has not yet been performed in children. Future studies in children are necessary to determine whether rising cortisol and ACTH production are associated with inhibition of 3βHSD2 and subsequent initiation of adrenarche.
In summary, cortisol stimulates marked production and secretion of DHEA in human adrenal cells through competitive inhibition of 3βHSD2 activity. As this occurs at physiological intraadrenal cortisol concentrations, it is possible that intraadrenal cortisol may participate in the regulation of adrenal DHEA secretion in humans. Furthermore, if intraadrenal cortisol rises during childhood, this could lead to the inhibition of 3βHSD2 activity and thereby contribute to the initiation of adrenarche.
Supplementary Material
Acknowledgments
We thank H. B. Kim, M.D., for providing human adrenal tissue and Henry Feldman, Ph.D., for assistance with statistical evaluation. We also thank Andrew Dauber, M.D., and especially David Breault, M.D., Ph.D., for helpful discussions.
Footnotes
This work was supported by the Clinical Investigator Training Program, Harvard/MIT Health Sciences and Technology - Beth Israel Deaconess Medical Center, in collaboration with Pfizer Inc. and Merck & Co. (to L.S.T.), the National Institutes of Health (to J.A.M.), and the Timothy Murphy Fund.
Present address for M.A.: Nagoya University Graduate School of Medicine, Nagoya, Japan.
Disclosure Summary: The authors have nothing to declare.
First Published Online October 13, 2010
Abbreviations: CV, Coefficient of variation; DHEA, dehydroepiandrosterone; DHEAS, DHEA-sulfate; FBS, fetal bovine serum; LC-MS/MS, liquid chromatography-tandem mass spectroscopy; MRM, multiple reaction monitoring; TLC, thin-layer chromatography.
References
- Talbot NR, Butler AM, Berman RA, Rodriguez PM, MacLachlan EA 1943 Excretion of 17-ketosteroids by normal and by abnormal children. AMA Am J Dis Child 65:364–375 [Google Scholar]
- Palmert MR, Hayden DL, Mansfield MJ, Crigler Jr JF, Crowley Jr WF, Chandler DW, Boepple PA 2001 The longitudinal study of adrenal maturation during gonadal suppression: evidence that adrenarche is a gradual process. J Clin Endocrinol Metab 86:4536–4542 [DOI] [PubMed] [Google Scholar]
- Remer T, Boye KR, Hartmann MF, Wudy SA 2005 Urinary markers of adrenarche: reference values in healthy subjects, aged 3–18 years. J Clin Endocrinol Metab 90:2015–2021 [DOI] [PubMed] [Google Scholar]
- Ibáñez L, Díaz R, López-Bermejo A, Marcos MV 2009 Clinical spectrum of premature pubarche: links to metabolic syndrome and ovarian hyperandrogenism. Rev Endocr Metab Disord 10:63–76 [DOI] [PubMed] [Google Scholar]
- Miller WL 2009 Androgen synthesis in adrenarche. Rev Endocr Metab Disord 10:3–17 [DOI] [PubMed] [Google Scholar]
- Rich BH, Rosenfield RL, Lucky AW, Helke JC, Otto P 1981 Adrenarche: changing adrenal response to adrenocorticotropin. J Clin Endocrinol Metab 52:1129–1136 [DOI] [PubMed] [Google Scholar]
- Kelnar CJ, Brook CG 1983 A mixed longitudinal study of adrenal steroid excretion in childhood and the mechanism of adrenarche. Clin Endocrinol (Oxf) 19:117–129 [DOI] [PubMed] [Google Scholar]
- Zhang LH, Rodriguez H, Ohno S, Miller WL 1995 Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc Natl Acad Sci USA 92:10619–10623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapes S, Corbin CJ, Tarantal A, Conley A 1999 The primate adrenal zona reticularis is defined by expression of cytochrome b5, 17alpha-hydroxylase/17,20-lyase cytochrome P450 (P450c17) and NADPH-cytochrome P450 reductase (reductase) but not 3beta-hydroxysteroid dehydrogenase/delta5-4 isomerase (3beta-HSD). J Clin Endocrinol Metab 84:3382–3385 [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Lee TC, Miller WL 1998 Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165 [DOI] [PubMed] [Google Scholar]
- Yanagibashi K, Hall PF 1986 Role of electron transport in the regulation of the lyase activity of C21 side-chain cleavage P-450 from porcine adrenal and testicular microsomes. J Biol Chem 261:8429–8433 [PubMed] [Google Scholar]
- Miller WL 2005 Minireview: regulation of steroidogenesis by electron transfer. Endocrinology 146:2544–2550 [DOI] [PubMed] [Google Scholar]
- Gell JS, Carr BR, Sasano H, Atkins B, Margraf L, Mason JI, Rainey WE 1998 Adrenarche results from development of a 3beta-hydroxysteroid dehydrogenase-deficient adrenal reticularis. J Clin Endocrinol Metab 83:3695–3701 [DOI] [PubMed] [Google Scholar]
- Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ 1996 The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3 beta-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab 81:3558–3565 [DOI] [PubMed] [Google Scholar]
- Anderson DC 1980 The adrenal androgen-stimulating hormone does not exist. Lancet 2:454–456 [DOI] [PubMed] [Google Scholar]
- Kenny FM, Preeyasombat C, Migeon CJ 1966 Cortisol production rate. II. Normal infants, children, and adults. Pediatrics 37:34–42 [PubMed] [Google Scholar]
- Scammon RE 1930 The measurement of the body in childhood. In: Harris JA, Jackson CM, Paterson DG, Scammon RE, eds. The measurement of man. Minneapolis: The University of Minnesota Press; 173–215 [Google Scholar]
- Stowens D 1966 Pediatric pathology. 2nd ed. Baltimore: Williams & Wilkins Co.; 3–6 [Google Scholar]
- Dhom G 1973 The prepuberal and puberal growth of the adrenal (adrenarche). Beitrage zur Pathologie 150:357–377 [DOI] [PubMed] [Google Scholar]
- Kahri AI, Voutilainen R, Salmenpera M 1979 Different biological action of corticosteroids, corticosterone and cortisol, as a base of zonal function of adrenal cortex. Acta Endocrinol (Copenh) 91:329–337 [DOI] [PubMed] [Google Scholar]
- Dickerman Z, Grant DR, Faiman C, Winter JS 1984 Intraadrenal steroid concentrations in man: zonal differences and developmental changes. J Clin Endocrinol Metab 59:1031–1036 [DOI] [PubMed] [Google Scholar]
- Rainey WE, Saner K, Schimmer BP 2004 Adrenocortical cell lines. Mol Cell Endocrinol 228:23–38 [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ 2008 Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108 [DOI] [PubMed] [Google Scholar]
- Rainey WE, Bird IM, Sawetawan C, Hanley NA, McCarthy JL, McGee EA, Wester R, Mason JI 1993 Regulation of human adrenal carcinoma cell (NCI-H295) production of C19 steroids. J Clin Endocrinol Metab 77:731–737 [DOI] [PubMed] [Google Scholar]
- Mikhaylova IV, Jääskeläinen T, Jääskeläinen J, Palvimo JJ, Voutilainen R 2008 Leukemia inhibitory factor as a regulator of steroidogenesis in human NCI-H295R adrenocortical cells. J Endocrinol 199:435–444 [DOI] [PubMed] [Google Scholar]
- Lin D, Harikrishna JA, Moore CC, Jones KL, Miller WL 1991 Missense mutation serine106—-proline causes 17 alpha-hydroxylase deficiency. J Biol Chem 266:15992–15998 [PubMed] [Google Scholar]
- Giguère V, Hollenberg SM, Rosenfeld MG, Evans RM 1986 Functional domains of the human glucocorticoid receptor. Cell 46:645–652 [DOI] [PubMed] [Google Scholar]
- Low SC, Chapman KE, Edwards CR, Seckl JR 1994 ‘Liver-type’ 11 beta-hydroxysteroid dehydrogenase cDNA encodes reductase but not dehydrogenase activity in intact mammalian COS-7 cells. J Mol Endocrinol 13:167–174 [DOI] [PubMed] [Google Scholar]
- Codner E, Okuma C, Iñiguez G, Boric MA, Avila A, Johnson MC, Cassorla FG 2004 Molecular study of the 3 beta-hydroxysteroid dehydrogenase gene type II in patients with hypospadias. J Clin Endocrinol Metab 89:957–964 [DOI] [PubMed] [Google Scholar]
- Rhéaume E, Lachance Y, Zhao HF, Breton N, Dumont M, de Launoit Y, Trudel C, Luu-The V, Simard J, Labrie F 1991 Structure and expression of a new complementary DNA encoding the almost exclusive 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase in human adrenals and gonads. Mol Endocrinol 5:1147–1157 [DOI] [PubMed] [Google Scholar]
- Lee TC, Miller WL, Auchus RJ 1999 Medroxyprogesterone acetate and dexamethasone are competitive inhibitors of different human steroidogenic enzymes. J Clin Endocrinol Metab 84:2104–2110 [DOI] [PubMed] [Google Scholar]
- Byrne GC, Perry YS, Winter JS 1986 Steroid inhibitory effects upon human adrenal 3 beta-hydroxysteroid dehydrogenase activity. J Clin Endocrinol Metab 62:413–418 [DOI] [PubMed] [Google Scholar]
- Thomas JL, Berko EA, Faustino A, Myers RP, Strickler RC 1988 Human placental 3 beta-hydroxy-5-ene-steroid dehydrogenase and steroid 5—4-ene-isomerase: purification from microsomes, substrate kinetics, and inhibition by product steroids. J Steroid Biochem 31:785–793 [DOI] [PubMed] [Google Scholar]
- Winter JS, Smail PJ 1983 Effects of ACTH and estradiol on steroid production by cultured adrenal cells from an anencephalic fetus and from normal adults. Steroids 42:677–685 [DOI] [PubMed] [Google Scholar]
- Gell JS, Oh J, Rainey WE, Carr BR 1998 Effect of estradiol on DHEAS production in the human adrenocortical cell line, H295R. J Soc Gynecol Investig 5:144–148 [DOI] [PubMed] [Google Scholar]
- Rabe T, Kiesel L, Runnebaum B 1985 Regulation of human placental progesterone synthesis in vitro by naturally occurring steroids. J Steroid Biochem 22:657–664 [DOI] [PubMed] [Google Scholar]
- Dauber A, Kellogg M, Majzoub JA 2010 Monitoring of therapy in congenital adrenal hyperplasia. Clin Chem 56:1245–1251 [DOI] [PubMed] [Google Scholar]
- Lutfallah C, Wang W, Mason JI, Chang YT, Haider A, Rich B, Castro-Magana M, Copeland KC, David R, Pang S 2002 Newly proposed hormonal criteria via genotypic proof for type II 3beta-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab 87:2611–2622 [DOI] [PubMed] [Google Scholar]
- Mermejo LM, Elias LL, Marui S, Moreira AC, Mendonca BB, de Castro M 2005 Refining hormonal diagnosis of type II 3beta-hydroxysteroid dehydrogenase deficiency in patients with premature pubarche and hirsutism based on HSD3B2 genotyping. J Clin Endocrinol Metab 90:1287–1293 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Roelfsema F, Iranmanesh A, Carroll BJ, Keenan DM, Pincus SM 2009 Basal, pulsatile, entropic (patterned), and spiky (staccato-like) properties of ACTH secretion: impact of age, gender, and body mass index. J Clin Endocrinol Metab 94:4045–4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.