Abstract
Background: Carney complex (CNC) is an autosomal dominant multiple neoplasia, caused mostly by inactivating mutations of the regulatory subunit 1A of the protein kinase A (PRKAR1A). Primary pigmented nodular adrenocortical disease (PPNAD) is the most frequent endocrine manifestation of CNC with a great inter-individual variability. Germline, protein-truncating mutations of phosphodiesterase type 11A (PDE11A) have been described to predispose to a variety of endocrine tumors, including adrenal and testicular tumors.
Objectives: Our objective was to investigate the role of PDE11A as a possible gene modifier of the phenotype in a series of 150 patients with CNC.
Results: A higher frequency of PDE11A variants in patients with CNC compared with healthy controls was found (25.3 vs. 6.8%, P < 0.0001). Among CNC patients, those with PPNAD were significantly more frequently carriers of PDE11A variants compared with patients without PPNAD (30.8 vs. 13%, P = 0.025). Furthermore, men with PPNAD were significantly more frequently carriers of PDE11A sequence variants (40.7%) than women with PPNAD (27.3%) (P < 0.001). A higher frequency of PDE11A sequence variants was also found in patients with large-cell calcifying Sertoli cell tumors (LCCSCT) compared with those without LCCSCT (50 vs. 10%, P = 0.0056). PDE11A variants were significantly associated with the copresence of PPNAD and LCCSCT in men: 81 vs. 20%, P < 0.004). The simultaneous inactivation of PRKAR1A and PDE11A by small inhibitory RNA led to an increase in cAMP-regulatory element-mediated transcriptional activity under basal conditions and after stimulation by forskolin.
Conclusions: We demonstrate, in a large cohort of CNC patients, a high frequency of PDE11A variants, suggesting that PDE11A is a genetic modifying factor for the development of testicular and adrenal tumors in patients with germline PRKAR1A mutation.
Carney complex patients report a high frequency of PDE11A variants, suggesting that PDE11A is a genetic modifying factor for tumor development in patients with germline PRKAR1A mutation.
Carney complex (CNC) is an autosomal dominant multiple neoplasia characterized by myxomas, spotty skin pigmentation, and endocrine overactivity (1) that is caused mostly by inactivating mutations of the regulatory subunit 1A of the protein kinase A (PKA) or cAMP-dependent protein kinase (PRKAR1A) (2,3). Recently, a transatlantic consortium studied 353 patients with CNC; pathogenic PRKAR1A mutations were reported in 73% of the patients, who carried a total of 80 different PRKAR1A mutations (4). Although some phenotype-genotype correlation emerged in this analysis, many questions remained unanswered. For example, the severity of primary pigmented nodular adrenocortical disease (PPNAD), the most frequent endocrine manifestation of CNC (1), varied considerably among patients with the same mutation, even between members of the same family (4).
As in other genetic disorders, we wondered what other genetic (and other) factors may influence the expression of CNC. Among the many possibilities, obvious candidates are the genes coding for phosphodiesterases (PDEs). Germline, protein-truncating mutations of PDE type 11A (PDE11A) have been described to predispose to a variety of adrenal hyperplasias, including PPNAD (5). PDE11A is highly polymorphic in the general population (6). We recently showed that inactivating PDE11A sequence variants appear to predispose to not only adrenocortical tumors (ADT) but also testicular germ cell tumors (TGCT) (6,7,8). The effect of PDE11A genetic variants appears to be limited to steroidogenic tissues, because in a recent study of sporadic GH-secreting tumors, the frequency of PDE11A missense variants was found only slightly increased compared with that in control patients (9). In vitro studies of PDE11A sequence variants that were predicted in silico to have an effect on function showed that indeed enzymatic activity was decreased, leading to higher levels of cAMP and/or cGMP in HeLa or HEK293 cells (5,6,8).
The above observations led us to investigate PDE11A as a possible gene modifier of the phenotype in patients with CNC, as has been suggested for other diseases caused by mutations in tumor suppressor genes (7,8,10). We used the same population of patients that were studied by the consortium (4) and examined in particular the possible modification by PDE11A genetic variants of the adrenal (PPNAD) and testicular [large-cell calcifying Sertoli cell tumors (LCCSCT)] phenotype of patients with germline PRKAR1A mutations. Small inhibitory RNA (siRNA) studies were then used to study a possible effect of partial PDE11A inactivation on PRKAR1A and PKA activities. The data appear to support the hypothesis that PDE11A may be a significant gene modifier of the phenotype in CNC by affecting PKA activity.
Patients and Methods
Patients and control subjects
The institutional review boards of the participating research centers have approved the genetic and clinical studies of patients with CNC. All patients and controls signed informed consent for genetic testing and for the analysis of the collected data. Of the 353 patients that were reported by the consortium (4), we selected for inclusion in this study those who met the diagnostic criteria for CNC (4,11) and were found to have a pathogenic PRKAR1A mutation (11); there were 150 patients that met these criteria and for whom DNA was available. We also tested 26 CNC patients without PRKAR1A mutations.
Control subjects were recruited by Cochin Hospital, Paris, France, as part of a clinical protocol that studies the genetic predisposition to endocrine tumors. All 279 controls were examined by a senior endocrinologist to exclude personal or family history, and any clinical signs suggestive of a genetic syndrome or any endocrine neoplasia, including pituitary, thyroid or adrenal tumors. The control group was matched in the distribution of gender and age (±5 yr) with that of the CNC patients.
DNA preparation and sequencing studies
DNA was extracted from blood samples as described previously (7). Sequencing of the PRKAR1A gene (Ensembl protein coding gene ENSG00000108946) in our patients has been described before (4). For all 150 PRKAR1A mutation carriers and the 279 controls, the 20 coding exons (exons 3–23) and flanking intronic sequences of the PDE11A gene (Ensembl protein coding gene ENSG00000128655) were amplified by PCR using specific primers, as previously described (7). All amplified samples were examined by agarose gel electrophoresis to confirm successful amplification of each exon. Direct sequencing of the purified fragments was then performed using the Genetic Sequencer ABI3100 Applied Biosystems (Foster City, CA) apparatus.
siRNA studies
Human embryonic kidney HEK293 cells were cultured as previously described (4) in 12-well plates (3 × 105 cells per well) and were transfected with Lipofectamine 2000 reagent (Lipo2000; Invitrogen Life Technologies, Cergy Pontoise, France) following the manufacturer’s instructions. A cAMP/PKA pathway reporter construct driving the expression of luciferase gene was used for all expression studies: 4x cAMP response element (CRE)-Luc (Stratagene, La Jolla, CA) with a basic promoter element (TATA box) joined to four CRE repeats. The Rous sarcoma virus (RSV)-Renilla construct, containing the RSV promoter-enhancer inserted upstream of the coding sequence of the Renilla luciferase (Promega Corp., Charbonnière, France), was used as a control for transfection efficiency. The sequence of the siRNA that was used for targeting PRKAR1A was UGAAUGGGCAACCAGUGUUdTdT (siR1A), for PDE11A was ACUAUCGGAUGGUUCUAUAdTdT (siPDE11A), and for the siRNA control was CAGUCGCGUUUGCGACUGGdTdT (siCtr) (MWG, Ebersberg, Germany). Cells were cotransfected with 20 pmol of siR1A and/or 60 pmol of siPDE11A and adjusted to 80 pmol final with the siCtr, 10 ng of the RSV-Renilla, and 250 ng of the luciferase reporter construct and lysed, and both firefly and Renilla luciferase activities were sequentially measured with the Dual Luciferase Reporter Assay System (Promega). Results are expressed as firefly luciferase activity normalized to Renilla luciferase activity of the same sample. The experiment was performed in triplicate.
Statistical analysis
Quantitative variables were described using means and sd. Qualitative variables were described using percentages. Conditional polytomous logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for carrying PDE11A mutations among the groups of CNC patients (with or without PPNAD/LCCST) relative to the gender/age-matched control group. The χ2, Pearson, or Fisher test (as appropriate) was used to compare PDE11A mutation rates between groups of patients. Two-tailed P values were used. P values <0.05 were considered to indicate significance.
For siRNA studies, data are expressed as means ± sd. Statistical analysis was performed using the Student’s t test, and significance was set at P < 0.05.
Results
Clinical description of patients with CNC caused by PRKAR1A mutations
A total of 150 CNC patients were studied. They all carried a germline inactivating mutation of the PRKAR1A gene (Table 1). Seventy-four patients (49.3%) were unrelated probands, and 76 (50.7%) were relatives of an index case. A total of 110 patients (74%) were female, and 40 were male (26%). Among the endocrine manifestations, PPNAD was the most common diagnosis; it was clinically proven in 104 patients (69.3%). In 15 patients (10%), PPNAD was the only manifestation of CNC. The mean ± sd age at diagnosis of PPNAD was 26 ± 13.3 yr (range 2–65). Forty-six patients (30.6%) presented with some other manifestation of CNC but not PPNAD. The most frequent was LCCSCT, a tumor that was present in 18 of 40 men (45%). Thyroid adenomas were present in 52 patients (34%), whereas thyroid cancer was diagnosed in seven (4%). Ovarian lesions were seen in 23 of 110 female patients (21%). GH-producing pituitary adenomas were diagnosed in 20 patients (13%). Pigmented lesions were the most frequent nonendocrine component of CNC that was seen in our patients; lentiginosis was observed in 101 patients (67%), and other pigmented lesions (as blue, Spitz, and compound nevi and café-au-lait spots) were seen in 70 (46%). Cardiac myxoma was diagnosed in 60 patients (40%), whereas skin and breast myxomatosis were seen in 38 (25%) and 28 patients (18%), respectively. Psammomatous melanotic schwannoma was found in 16 patients (10%).
Table 1.
Main clinical characteristics and PDE11A mutations in the 38 patients with CNC due to PRKAR1A mutations
| Patient | Sex | PPNAD (Y/N) | Age at PPNAD diagnosis (yr) | LCCSCT (Y/N) | PRKAR1A mutation cDNA | PDE11A mutations cDNA | PDE11A mutations protein | Consequence of PPDE11A mutation |
|---|---|---|---|---|---|---|---|---|
| 1 | M | Y | 46 | Y | c.974-1G→A | c.2618T→C | I873T | Missense |
| 2 | M | Y | 25 | Y | c.502 + 1G→A | c.2599C→G | R867G | Missense |
| 3 | M | Y | 41 | Y | c.1083delA | c.2180A→G | Y727C | Missense |
| 4 | M | Y | 35 | Y | c.491-492delTG | c.171Tdel | T58PfsX41 | Codon stop |
| 5 | M | Y | 14 | Y | c.891 + 3A→G | c.2599C→G | R867G | Missense |
| 6 | M | Y | 27 | Y | c.491-492delTG | c.171Tdel | T58PfsX41 | Codon stop |
| 7 | M | Y | 57 | Y | c.891 + 3A→G | c.2599C→G | R867G | Missense |
| 8 | M | Y | 9 | Y | c.528-531delGATTins11 | c.2632A→G | M878V | Missense |
| 9 | M | Y | 16 | Y | c.682C→T | c.2599C→G | R867G | Missense |
| 10 | M | Y | 13 | N | c.709(-7-2)del6 | c.2180A→G | Y727C | Missense |
| 11 | M | Y | 20 | N | c.769 + 5G→C | c.1045G→A | A349T | Missense |
| 12 | F | Y | 20 | c.709(-7-2)del6 | c.919C→T | R307X | Codon stop | |
| 13 | F | Y | 17 | c.709(-7-2)del6 | c.2411G→A | R804H | Missense | |
| 14 | F | Y | 27 | c.709(-7-2)del6 | c.2180A→G | Y727C | Missense | |
| 15 | F | Y | 8 | c.891 + 3A→G | c.2632A→G | M878V | Missense | |
| 16 | F | Y | 15 | c.763-764delAT | c.2180A→G | Y727C | Missense | |
| 17 | F | Y | 26 | c.550(-9-2)del8 | c.2411G→A | R804H | Missense | |
| 18 | F | Y | 11 | c.845-846 ins A | c.2411G→A | R804H | Missense | |
| 19 | F | Y | 27 | c.709(-5-107)del 103 | c.2180A→G | Y727C | Missense | |
| 20 | F | Y | 21 | c.709(-7-2)del6 | c.1142G→T | E382X | Codon stop | |
| 21 | F | Y | 9 | c.279-282delTAGG | c.2180A→G | Y727C | Missense | |
| 22 | F | Y | 16 | c.440 + 1G→A | c.171Tdel | T58PfsX41 | Codon stop | |
| 23 | F | Y | 30 | c.865 G→T | c.2180A→G | Y727C | Missense | |
| 24 | F | Y | 2 | c.491-492delTG | c.2411G→A | R804H | Missense | |
| 25 | F | Y | 42 | c.738T→G | c.824C→A | S275X | Codon stop | |
| 26 | F | Y | 12 | c.353-365del13 | c.2180A→G | Y727C | Missense | |
| 27 | F | Y | 12 | c.709(-7-2)del6 | c.652C→T | L218F | Missense | |
| 28 | F | Y | 42 | c.502 + 1G→A | c.2180A→G | Y727C | Missense | |
| 29 | F | Y | 19 | c.43-58del16 | c.2632A→G | M878V | Missense | |
| 30 | F | Y | 35 | c.491-492delTG | c.2180A→G | Y727C | Missense | |
| 31 | F | Y | 22 | c.491-492delTG | c.2180A→G | Y727C | Missense | |
| 32 | F | Y | 24 | c.491-492delTG | c.2180A→G | Y727C | Missense | |
| 33 | F | N | c.763-764delAT | c.2180A→G | Y727C | Missense | ||
| 34 | F | N | c.408-412delGCTGT | c.2180A→G | Y727C | Missense | ||
| 35 | F | N | c.709(-7-2)del6 | c.2599C→G | R867G | Missense | ||
| 36 | F | N | c.709(-7-2)del6 | c.1045G→A | A349T | Missense | ||
| 37 | F | N | c.709(-7-2)del6 | c.1045G→A | A349T | Missense | ||
| 38 | F | N | c.709(-7-2)del6 | c.919C→T | R307X | Codon stop |
F, Female; M, male; N, no; Y, yes.
PDE11A sequence defects in CNC patients with PRKAR1A mutations and controls
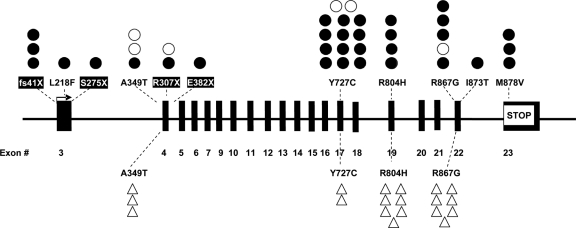
By sequencing the entire PDE11A gene in 150 CNC patients, we found 38 point mutations (25.3%) leading to missense substitutions (31 patients) or premature stop codon and nonsense mRNA (seven patients), all of them in the heterozygous state (Table 1 and Fig. 1). Four of these sequence defects (I873T, E382X, S275X, and L218F) have never been reported previously. The distribution of PDE11A sequence variants was similar between related and unrelated patients; 18 mutation carriers were observed in the group of 74 unrelated patients (24.3%), and 20 in the group of 76 relatives (26.3%).
Figure 1.
Localization of codon stop mutations (black boxes) and missense variants (no boxes) on the 23 exons of the PDE11A gene (coding starts from exon 3 and ends with exon 23). •, CNC patients with PPNAD; ○, CNC patients without PPNAD; ▵, controls.
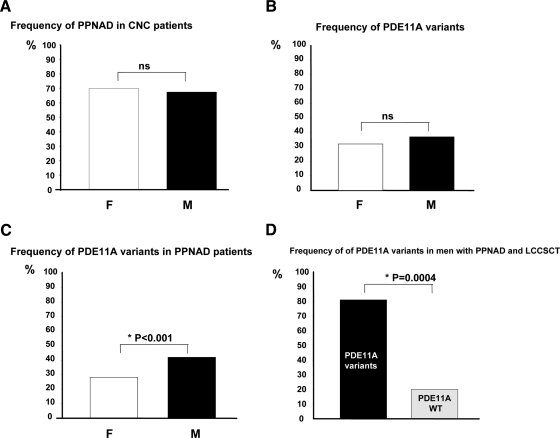
We found no nonsense PDE11A mutations in the 279 controls. In the same group, four missense variants in a total of 19 individuals (6.8%) were found, all of which have been reported previously in the general population: R804H (seven individuals), R867G (seven), A349T (three), and Y727C (two) (Fig. 1). All variants were present in heterozygote state. Thus, there was an overall significantly higher frequency of PDE11A mutations in CNC patients compared with those in the 279 control subjects (25.3 vs. 6.8%, P < 0.0001). The OR for a PDE11A defect to be with a PRKAR1A mutation was 3.85 (CI = 1.07–2.82; P = 0.0004). Because there was no difference in PPNAD frequency between the two genders (70 vs. 67.5%, Fig. 2A), there were no differences in the frequency of PDE11A sequence variants between men and women (Fig. 2B), and the presence of the PDE11A defect was not associated with the status of an index case; PDE11A sequence variants were seen in 24.3% of unrelated (index) cases and 26.3% of related patients (vs. 6.8% of controls, P < 0.0001).
Figure 2.
Relationship between PDE11A variants and clinical characteristics of patients with CNC. A, Frequency of PPNAD in CNC patients according to the gender: white bar, women; black bar, men. B, Frequency of PDE11A sequence variants in women (white bar) and men (black bar) with CNC. C, Gender distribution of PDE11A sequence variants in CNC patients with PPNAD: white bar, women; black bar, men. *, P < 0.001. D, Frequency of men with PPNAD and LCCSCT with PDE11A sequence variants (black bar), and men with PPNAD and LCCSCT without PDE11A sequence variants (gray bar). *, P = 0.0004.
PDE11A sequence defects in CNC patients without PRKAR1A mutations
By sequencing the entire PDE11A gene in 26 CNC patients without PRKAR1A mutations, we found eight (30.7%) point mutations (seven missense and one codon stop mutation); this was not significantly different compared with the frequency among CNC patients with PRKAR1A mutations.
PDE11A sequence variants in PRKAR1A mutation carriers and PPNAD
CNC patients with PPNAD (n = 79) had a higher frequency of PDE11A sequence variants than the controls (27.8 vs. 6.8%, OR = 5.28; CI = 2.56–10.96; P = 0.0001). CNC patients without PPNAD (n = 39) had a 10.3% frequency of PDE11A sequence variants, a percentage that was not significantly different from the controls (OR = 1.56; CI = 0.52–4.68). In addition, all of the PDE11A sequence variants present in CNC patients without PPNAD were missense, and most had previously been described and were also present in the group of controls (i.e. R867 and A349T). When the two groups of CNC patients were compared, patients with PPNAD were significantly more frequently carriers of PDE11A sequence variants (30.8 vs. 13%, P = 0.025). Men with PPNAD were significantly more frequently carriers of PDE11A sequence variants (40.7%) than women with PPNAD (27.3%) (P < 0.001, Fig. 2C).
PDE11A sequence variants in PRKAR1A mutation carriers and LCCSCT
A higher frequency of PDE11A sequence variants was found in CNC patients with LCCSCT (nine of 18) compared with those without LCCSCT (two of 20) (50 vs. 10%, P = 0.0056). Moreover, CNC men with LCCSCT (just like those with PPNAD) had significantly higher frequency of PDE11A sequence variants than age-matched control men with a fairly high OR (OR = 20.33; CI = 0.46–9.04; P < 0.0001). PDE11A sequence variants were significantly associated with the copresence of PPNAD with LCCSCT; nine of 11 men (81%) with both tumors had a PDE11A sequence variant, whereas only six of 29 patients without a PDE11A sequence variant (20%) had both PPNAD and LCCSCT (P < 0.004, Fig. 2D).
Other CNC manifestations
No association was found between the presence of PDE11A sequence variants and any of the other CNC manifestations that we studied, such as pituitary and thyroid adenomas, thyroid cancer, ovarian and pigmented lesions, and cardiac, skin, and breast myxomas.
Functional studies
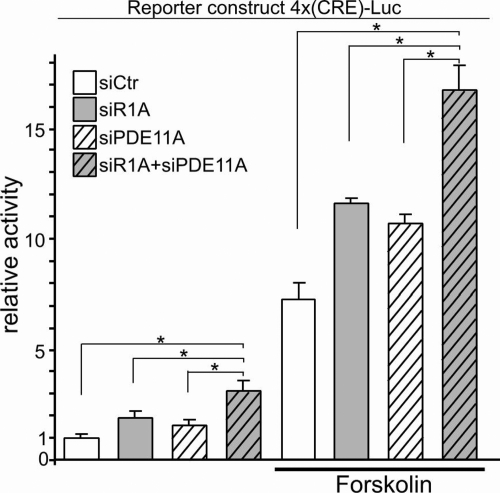
We then examined the effects of PRKAR1A and/or PDE11A silencing on PKA-dependent transcription; PRKAR1A or PDE11A inactivation in HEK293 cells increased both basal (+84% and +55% from siCtr, respectively) and FK-induced (+60% and +48% from siCtr, respectively) transcriptional activity of the reporter construct. An additive effect was observed by the concurrent inactivation of both PRKAR1A and PDE11A, both in basal conditions (+208% from siCtr) and after FK (+130% from siCtr) (Fig. 3).
Figure 3.
PRKAR1A and PDE11A silencing increase PKA-dependent transcription at baseline and after forskolin stimulation. PRKAR1A inactivation (gray bars), PDE11A inactivation (white striped bars) in HEK293 cells increases transcriptional activity of the reporter construct; coinactivation of PRKAR1A and PDE11A (gray striped bars) led to an additive effect, and similar results were found after forskolin stimulation. *, P < 0.05.
Discussion
Recently, the interest in modifier genes in the study of monogenic diseases has grown because of the recognition that most genotype-phenotype correlations could only be explained with additional genetic factors that modulate the expression of a given trait (12,13). Susceptibility genes, which are genes with functional variants that affect the cause of disease, are routinely being identified for simple Mendelian traits and, more recently, for common genetic disorders. Modifier genes are distinct from susceptibility genes, in that they usually represent relatively frequent genetic variants that affect the clinical manifestation of a disease; one of the first examples of the existence of such modifiers was in cystic fibrosis (14,15,16). In endocrinology, the first monogenic disorder to be shown to be affected by genetic variants of modifier genes was idiopathic hypogonadotropic hypogonadism (17,18). A similar polygenic model for susceptibility to TGCT has also been proposed (19,20,21). More recently, our group showed a higher frequency of PDE11A variants in patients with ADTs and TGCTs, suggesting that PDE11A can act as a genetic predisposition factor to adrenal and testicular tumor development (7,8).
The present study suggests that PDE11A can act as a modifier of the phenotype in CNC patients, at least in regard to the incidence of PPNAD. The development of a second tumor, interestingly a testicular one, LCCSCT, may also be affected by the presence of PDE11A sequence variants, although our numbers could not exclude the influence of the copresence of PPNAD in these patients. These data confirm the important role of PDE11A as a molecule with specific impact on adrenal tumorigenesis, as previously reported (7).
It was very interesting that men with PDE11A sequence variants were particularly more prone to develop PPNAD (along with LCCSCT) than women. Generally, PPNAD is more frequent in women than in men (71 vs. 29%), as we recently reported (4). The fact that in this study, which studied CNC patients carriers of PRKAR1A mutations, PDE11A variants were more frequent in men, might explain why the frequency of PPNAD was similar in men and women. This reinforces the idea that PDE11A plays an important modifier role in predisposition to PPNAD.
The genetic data are confirmed by the functional studies that demonstrated in H293 cell line an additive effect of the simultaneous inactivation of PRKAR1A and PDE11A on the transcriptional activity increase, under basal conditions and after stimulation by forskolin. These data seem to corroborate the idea that the lack of PDE11A increases the PKA-dependent transcription.
Finally, the fact that PDE11A is highly expressed in steroidogenic tissues, such as adrenal cortex and testis, may explain the specificity of this gene in increasing predisposition to adrenal and testicular tumors.
In conclusion, we found in a large cohort of patients with CNC due to PRKAR1A mutations (in particular with PPNAD and LCCSCT), a high frequency of PDE11A variants, supporting the notion that PDE11A may act as a genetic predisposition factor and/or modifier in adrenal and testicular tumorigenesis. These data also suggest that in CNC, as in other diseases, multiple genetic factors are responsible for the phenotypic variability, as for adrenal and testicular tumor susceptibility.
Acknowledgments
We thank the patients and their families who participated in our research studies and donated their time for this investigation.
Footnotes
This work was supported by grants from the Agence Nationale pour la Recherche (ANR06-MRAR-007 and ANR08-GENOPAT-002) and, in part, by the National Institute of Child Health and Human Development, intramural National Institutes of Health (NIH) project Z01-HD-000642-04 to C.S., and, in part, by the NIH Clinical Center.
Disclosure Summary: All the authors have nothing to declare.
First Published Online November 3, 2010
Abbreviations: ADT, Adrenocortical tumors; CI, confidence interval; CNC, Carney complex; CRE, cAMP response element; LCCSCT, large-cell calcifying Sertoli cell tumors; OR, odds ratio; PDE, phosphodiesterase; PKA, protein kinase A; PPNAD, primary pigmented nodular adrenocortical disease; RSV, Rous sarcoma virus; siRNA, small inhibitory RNA; TGCT, testicular germ cell tumor.
Institut National de la Santé et de la Recherche Médicale Unité 1016 (R.L., D.V., A.F., K.P., B.R., L.G., X.B., J.B.), Institut Cochin, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8104, Paris, France; Université Paris 5 (R.L., D.V., A.F., K.P., B.R., L.G., X.B., J.B.), Paris, France; Assistance Publique Hôpitaux de Paris (R.L., L.G., X.B., J.B.), Hôpital Cochin, Department of Endocrinology, Reference Center for Rare Adrenal Diseases, 75014 Paris, France; Section on Endocrinology and Genetics (A.H., J.S., J.M., L.D.H., F.R.F., M.L., M.N., C.A.S.), Program on Developmental Endocrinology and Genetics, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland 20892; Assistance Publique Hôpitaux de Paris (J.C.), Hôpital Cochin, Department of Biostatistics, Paris, France; Assistance Publique Hôpitaux de Paris (M.G.B., E.C.), Hôpital Cochin, Oncogenetic Unit, Paris, France; Assistance Publique Hôpitaux de Paris (M.L.R.S.), Hôpital Ambroise Paré, Department of Endocrinologie, Boulogne sur Seine and University of Versaille St. Quentin, 92210 France; and Laboratory of Molecular Genetics (F.R.F.), Pontificia Universidade Catolica do Parana, 80215-901 Curitiba, Brazil
References
- Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL 1985 The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 64:270–283 [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA 2000 Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet 26:89–92 [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Sandrini F, Monbo J, Lin JP, Carney JA, Stratakis CA 2000 Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the carney complex. Hum Mol Genet 9:3037–3046 [DOI] [PubMed] [Google Scholar]
- Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, René-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA 2009 Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab 94:2085–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, Keil M, Heyerdahl S, Matyakhina L, Libè R, Fratticci A, Kirschner LS, Cramer K, Gaillard RC, Bertagna X, Carney JA, Bertherat J, Bossis I, Stratakis CA 2006 A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet 38:794–800 [DOI] [PubMed] [Google Scholar]
- Horvath A, Giatzakis C, Robinson-White A, Boikos S, Levine E, Griffin K, Stein E, Kamvissi V, Soni P, Bossis I, de Herder W, Carney JA, Bertherat J, Gregersen PK, Remmers EF, Stratakis CA 2006 Adrenal hyperplasia and adenomas are associated with inhibition of phosphodiesterase 11A in carriers of PDE11A sequence variants that are frequent in the population. Cancer Res 66:11571–11575 [DOI] [PubMed] [Google Scholar]
- Libé R, Fratticci A, Coste J, Tissier F, Horvath A, Ragazzon B, Rene-Corail F, Groussin L, Bertagna X, Raffin-Sanson ML, Stratakis CA, Bertherat J 2008 Phosphodiesterase 11A (PDE11A) and genetic predisposition to adrenocortical tumors. Clin Cancer Res 14:4016–4024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Korde L, Greene MH, Libe R, Osorio P, Faucz FR, Raffin-Sanson ML, Tsang KM, Drori-Herishanu L, Patronas Y, Remmers EF, Nikita ME, Moran J, Greene J, Nesterova M, Merino M, Bertherat J, Stratakis CA 2009 Functional phosphodiesterase 11A mutations may modify the risk of familial and bilateral testicular germ cell tumors. Cancer Res 69:5301–5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peverelli E, Ermetici F, Filopanti M, Elli FM, Ronchi CL, Mantovani G, Ferrero S, Bosari S, Beck-Peccoz P, Lania A, Spada A 2009 Analysis of genetic variants of phosphodiesterase 11A in acromegalic patients. Eur J Endocrinol 161:687–694 [DOI] [PubMed] [Google Scholar]
- Pharoah PD, Dunning AM, Ponder BA, Easton DF 2004 Association studies for finding cancer-susceptibility genetic variants. Nat Rev Cancer 4:850–860 [DOI] [PubMed] [Google Scholar]
- Horvath A, Bertherat J, Groussin L, Guillaud-Bataille M, Tsang K, Cazabat L, Libé R, Remmers E, René-Corail F, Faucz FR, Clauser E, Calender A, Bertagna X, Carney JA, Stratakis CA 2010 Mutations and polymorphisms in the gene encoding regulatory subunit type 1-α of protein kinase A (PRKAR1A): an update. Hum Mutat 31:369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau JH 2001 Modifier genes in mice and humans. Nat Rev Genet 2:165–174 [DOI] [PubMed] [Google Scholar]
- Nadeau JH 2003 Modifier genes and protective alleles in humans and mice. Curr Opin Genet Dev 13:290–295 [DOI] [PubMed] [Google Scholar]
- Haston CK, Hudson TJ 2005 Finding genetic modifiers of cystic fibrosis. N Engl J Med 353:1509–1511 [DOI] [PubMed] [Google Scholar]
- Drumm ML, Konstan MW, Schluchter MD, Handler A, Pace R, Zou F, Zariwala M, Fargo D, Xu A, Dunn JM, Darrah RJ, Dorfman R, Sandford AJ, Corey M, Zielenski J, Durie P, Goddard K, Yankaskas JR, Wright FA, Knowles MR 2005 Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med 353:1443–1453 [DOI] [PubMed] [Google Scholar]
- Badano JL, Katsanis N 2002 Beyond Mendel: an evolving view of human genetic disease transmission. Nat Rev Genet 3:779–789 [DOI] [PubMed] [Google Scholar]
- Carlton VE, Harris BZ, Puffenberger EG, Batta AK, Knisely AS, Robinson DL, Strauss KA, Shneider BL, Lim WA, Salen G, Morton DH, Bull LN 2003 Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat Genet 34:91–96 [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, Li WP, Maccoll G, Eliseenkova AV, Olsen SK, Ibrahimi OA, Hayes FJ, Boepple P, Hall JE, Bouloux P, Mohammadi M, Crowley W 2007 Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest 117:457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausz C, Looijenga LH 2008 Genetic aspects of testicular germ cell tumors. Cell Cycle 7:3519–3524 [DOI] [PubMed] [Google Scholar]
- Oosterhuis JW, Looijenga LH 2005 Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer 5:210–222 [DOI] [PubMed] [Google Scholar]
- Crockford GP, Linger R, Hockley S, Dudakia D, Johnson L, Huddart R, Tucker K, Friedlander M, Phillips KA, Hogg D, Jewett MA, Lohynska R, Daugaard G, Richard S, Chompret A, Bonaïti- Pellié C, Heidenreich A, Albers P, Olah E, Geczi L, Bodrogi I, Ormiston WJ, Daly PA, Guilford P, Fosså SD, Heimdal K, Tjulandin SA, Liubchenko L, Stoll H, Weber W, Forman D, Oliver T, Einhorn L, McMaster M, Kramer J, Greene MH, Weber BL, Nathanson KL, Cortessis V, Easton DF, Bishop DT, Stratton MR, Rapley EA 2006 Genome-wide linkage screen for testicular germ cell tumour susceptibility loci. Hum Mol Genet 15:443–451 [DOI] [PubMed] [Google Scholar]