Abstract
Context: Perinatal depression has a prevalence of 10% with devastating consequences for mother and baby. The prospective identification of those at risk for postpartum (PPD) or prenatal (PND) depression has led to biomarker searches in pregnancy. There are conflicting reports of associations between midpregnancy placental CRH (pCRH) and PPD or PND.
Objective: The objective of the study was to quantify the association of maternal pCRH with PPD and PND.
Design: This was a prospective cohort study (the Pregnancy, Infection, and Nutrition Study).
Setting: The study was conducted at a prenatal clinics at the University of North Carolina at Chapel Hill.
Patients: Patients included 1230 pregnant women.
Main Outcome Measures: The relationship between pCRH at less than 20 wk and 24–29 wk and maternal depression assessed in pregnancy [Center for Epidemiologic Studies Depression Scale (CES-D)] and postpartum (12 wk and 1 yr) with the Edinburgh Postnatal Depression Scale (EPDS).
Results: At 24–29 wk, 24.8% of women had CES-D score of 17 or greater, and 9.7% had a CES-D score of 25 or greater. At 12 wk postpartum, 18.2% of women had an EPDS score of 10 or greater and 7.6% had an EPDS score of 13 or greater. CRH measures at less than 20 wk and 24–29 wk were inversely correlated with a CES-D score at 24–29 wk (n = 1080, P < 0.05 and P < 0.01, respectively). Pregnancy pCRH was not correlated with the EPDS score at 12 wk (n = 484) or 1 yr postpartum (n = 391). In covariate-adjusted models, higher pCRH was not associated with a CES-D of 17 or greater at 24–29 wk (odds ratio 0.88 per sd change in pCRH at 24–29 wk, 95% confidence interval 0.76–1.03). There was no association between log CRH at 24–29 wk and PPD (covariate-adjusted odds ratio per sd 0.99, 95% confidence interval 0.69–1.42).
Conclusion: Higher midpregnancy pCRH was not associated with an increased risk of PND or PPD.
The prospective identification of postpartum depression (PPD) has led to biomarker searches in pregnancy [i.e., corticotrophin-releasing hormone (CRH)]; higher mid-pregnancy placental CRH was not associated with increased risk of PPD.
Perinatal depression, defined as major depressive episodes (1) that occur either during pregnancy or within the first 6 months postpartum, can have devastating consequences for the mother, her children, and her family (2,3,4). Perinatal depression and in particular postpartum depression (PPD) has a prevalence of approximately 10%, making it one of the most common complications of both the prenatal and postpartum period (5,6,7,8). Onset of PPD is usually within the first few months after birth. Distinguishing characteristics of PPD may include severe anxiety, agitation, suicidal thoughts, and fears of hurting and/or lack of interest in the newborn (7).
As with most forms of psychiatric illness, identification of PPD occurs after the onset of symptoms. Although specific groups face increased risks (i.e. women with a previous history of PPD or history of other mood disorder) (9), many women develop PPD without a previous history of depression or prodrome. The best intervention currently available is routine screening of all postpartum women, resulting in the diagnosis after the onset of symptoms and associated morbidity. Although validated screening instruments developed specifically for use during the perinatal period are available [e.g. Edinburgh Postnatal Depression Scale (EPDS) (10) or PPD Screening Scale (11)], these instruments do not identify prospectively women at risk for the development of PPD. Thus, the prospective identification of those at risk for perinatal depression is an important goal and has led to the search for biological markers.
Although the etiology of perinatal depression remains unclear, there are two lines of evidence that implicate hormonal dysregulation. First, despite normal levels of reproductive hormones, there is some evidence to suggest that women with PPD have an abnormal response to changes in reproductive steroid levels (12). Second, there is increasing evidence that abnormalities in hypothalamic-pituitary-adrenal (HPA) axis activity play a key role in the pathogenesis of major depressive disorder and PPD. A growing literature demonstrates that abnormalities in HPA axis activity are associated with reproductive-endocrine-related mood disorders in women, particularly during the time of transition from childbirth to the immediate postpartum period (13,14,15,16,17,18). One example is the blunted cortisol awakening rise that has been demonstrated in women with PPD, which may reflect a response, in vulnerable women, to the marked cortisol withdrawal that occurs after delivery (19). In addition, estrogen and progesterone also have profound interactions with the HPA axis and may trigger HPA axis abnormalities in susceptible women (17).
Dramatic hormonal changes occur during the transition from pregnancy to the postpartum period (18). The third trimester of pregnancy is characterized by high estrogen and progesterone levels and a hyperactive HPA axis with high plasma cortisol (17,20). The HPA hyperactivity during pregnancy results from increased levels of circulating CRH of placental origin and decreasing levels of CRH-binding protein, both of which contribute to elevated levels of bioactive free CRH and consequential hypersecretion of ACTH and cortisol (12,21,22,23). At childbirth and during the transition to the postpartum period, estrogen and progesterone rapidly decline, and there is blunted HPA axis activity due to suppressed hypothalamic CRH secretion (16). These normal changes are altered in women with PPD (16). Findings from Bloch et al. (12) further suggest that abnormalities in HPA axis activity can be detected during euthymia and are unmasked by exposure to high gonadal steroid levels. These abnormalities, then, could represent either a trait measure, suggesting heritability, or a consequence of an earlier affective episode (and hence not dissimilar to major depressive disorder). Consequently, the dysregulation of the HPA axis may identify those susceptible to the onset of a reproductive steroid-related depressive episode.
Elevated placental CRH as a potential biomarker of those at risk for perinatal or postpartum depression has been an area of recent interest. The increasing production of placenta CRH (pCRH) throughout pregnancy can be measured in maternal peripheral blood (24). Within hours after childbirth, levels of pCRH quickly drop and become undetectable (25). Among euthymic women in the early postpartum period (first 3 wk), the HPA axis remains refractory to external CRH challenge. Among women with PPD, researchers have found a continued blunted response to CRH at 6–12 wk postpartum (16). Nonetheless, the role of midpregnancy pCRH as a biomarker of maternal prenatal and PPD remains unclear, and two studies conducted in this area have yielded conflicting results (26,27). Rich-Edwards et al. (26) reported that elevated placental CRH levels in midpregnancy were positively associated with prenatal depressive symptoms but were not associated with PPD. Conversely, Yim et al. (27) reported that midpregnancy pCRH was positively correlated with an increased risk of postpartum depression. Given these discrepant findings, we measured the association between midpregnancy pCRH and both prenatal and postpartum depression in our large longitudinal study of prenatal and postpartum health. Given the potential public health importance of a biomarker for predicting PPD, as well as the biologic plausibility of a link between CRH and maternal mood, we assessed the relationship between pCRH at less than 20 wk and 24–29 wk of pregnancy and maternal depression measured during pregnancy using the Center for Epidemiologic Studies Depression Scale (CES-D) and postpartum (12 wk and 1 yr) with the EPDS.
Subjects and Methods
Study population
Participants were recruited in the third phase of the Pregnancy, Infection, and Nutrition Study, a long-term prospective cohort study of risk factors for preterm birth (28,29,30). Women were recruited from prenatal clinics at the University of North Carolina at Chapel Hill Hospitals. Recruitment began in January 2001 and ended in June 2005, with a total of 2006 women recruited. The study was approved by the University of North Carolina at Chapel Hill Committee for the Protection of Human Subjects. Patients who agreed to participate gave full written informed consent. Women were enrolled at less than 20 wk gestation and had multiple follow-up assessments. Blood for CRH assay was obtained at less than 20 and 24–29 wk (n = 1483). Participants were excluded if blood was available at only one time point (n = 173), gestational age or time of day of blood draw was not available (n = 15), or CES-D (31) or EPDS (10) were not obtained (n = 63), or pregravid body mass index was missing (n = 2), leaving 1230 participants available for analysis. Women in this cohort were invited to participate in an ongoing study during the first year postpartum, and 688 women enrolled. Among those with prenatal CRH data, EPDS scores were available for 484 women at 12 wk and 391 women at 12 months postpartum.
Study outcome measures
The CES-D was used to measure depression during pregnancy concurrent with the CRH blood sample at less than 20 wk and 24–29 wk of gestation, and the EPDS to measure postpartum depression at 12 wk and 12 months postpartum. The CES-D is widely used and is a validated 20-item self-report scale designed to measure depressive symptomatology in the general population (31). A cutoff score of 17 or greater has been strongly correlated with a diagnosis of major depression in the general population (32). The EPDS was developed specifically for assessing postpartum depression and relies much less than standard depression screens on somatic or physical questions (10). It also has multiple questions that assess anxiety symptoms commonly seen in the postpartum period (33). The EPDS is a 10-item self-report screening scale that is validated and commonly used to assess postpartum depression. The response format is frequency based. A cutoff score of 12 or greater on the EPDS has consistently been correlated with a clinical diagnosis of major depressive disorder when compared with a structured clinical interview (10,34). EPDS scores of 10–12 have been associated with an accurate diagnosis of minor depressive disorder (34).
In a comprehensive systematic review of screening tools for perinatal depression, both the CES-D and the EPDS appeared to be able to identify depressive illness in pregnant and postpartum women with a degree of accuracy similar to that for depression screen results in other nonpsychiatric settings (7). The estimates of sensitivity and specificity were equivalent to those that have been reported in primary care settings. In particular, specificity was noted to be relatively good, suggesting a relatively good positive predictive value (7). We defined probable prenatal depression as CES-D score of 17 or greater because some symptoms of pregnancy overlap with CES-D items (35), and we defined probable postpartum depression as EPDS score of 13 or greater. Because CRH measurements were not normally distributed, they were log transformed before analysis.
Hormone measures
At each of the two clinic visits, blood was collected to measure CRH in tubes that were chilled and kept on ice. Most of the clinic visits and blood draws were conducted in the morning (67.2% of participants at <20 wk and 63.3% of participants at 24–29 wk). However, unlike cortisol, CRH production and secretion do not follow a circadian rhythm; CRH is continuously produced and released during pregnancy by the placenta in a pulsatile fashion, so sample collection does not need to occur at a specific time of day (36). Tubes contained EDTA (1 mg/ml of blood) and aprotinin (500 kIU/ml of blood) and were centrifuged at 0 C. The plasma was decanted from the tube, aliquoted into four cryogenic storage tubes, and stored at −70 C until extraction. Fifty-microliter plasma samples were assayed for CRH using a competitive enzyme immunoassay. The assay had a minimum detection limit of 0.08 ng/ml, and a range of 0–25 ng/ml. Average intra- and interassay coefficients of variation were less than 5% and less than 14%, respectively. Samples were tested at Salimetrics, LLC (State College, PA). A subset of samples was run in duplicate for quality control.
Statistical analysis
To quantify the association between maternal serum CRH and sociodemographic variables, we used ANOVA to measure mean log CRH by maternal age, race, education, marital status, income, body mass index before pregnancy, parity, and gestational age at delivery (<37 wk vs. 37 or more weeks). Yim et al. (27) reported Pearson correlations between measures of CRH and maternal mood. To produce comparable results, we used Pearson correlation to measure the association between log CRH at 20 and 24–29 wk and maternal mood, measured by CES-D during pregnancy measured at the same time as the CRH assay and EPDS at 12 wk and 12 months postpartum (27).
Rich-Edwards et al. (26) reported mean log CRH levels among women with and without depressed mood. To produce comparable results, we compared mean log CRH levels at less than 20 and 24–29 wk among women with and without depressed mood at less than 20, 24–29, and 12 wk postpartum, adjusting for gestational age at the time of the blood sample, maternal age, and morning vs. afternoon blood draw time. We then measured mean log CRH, further adjusting for sociodemographic variables.
Rich-Edwards et al. (26) further reported differences in the odds of probable prenatal and postnatal depression per sd difference in log CRH. We similarly used logistic regression to calculate the odds ratio of depression at 24–29 and 12 wk postpartum per sd change in log CRH at 24–29 wk. For all analyses, we included time of day of blood draw, although we found that it did not alter our results.
Our collection of serum samples at two time points allowed us to further quantify the trajectory of serum CRH from less than 20 wk to 24–29 wk among women with and without prenatal or postnatal depression. We used a linear mixed model to calculate the random slope of log CRH between the first and second study visit for each individual, adjusted for maternal age, self-reported race, parity, education, marital status, income, and body mass index. We then used linear regression to model the association between subject-specific CRH slopes and maternal mood at the second study visit (CES-D score) and at 12 wk postpartum (EPDS score) in unadjusted models and in models adjusted for maternal age, self-reported race, parity, education, marital status, income, and body mass index. All analyses were performed using SAS version 9.2 (Cary, NC).
Results
Among the 1230 participants with two measures of CRH, maternal mood measures were available for 1178 women at less than 20 wk, 1080 women at 24–29 wk, and 484 women at 12 wk and 391 at 12 months postpartum. At the two prenatal assessments, 24.1 and 24.8% of women had a CES-D score of 17 or greater, and 11.0 and 9.7% had CES-D scores of 25 or greater. At the 12-wk postpartum follow-up assessment, 18.2% of women had an EPDS score of 10 or greater, and 7.6% had an EPDS score of 13 or greater.
When we examined mean log CRH levels at 24–29 wk by sociodemographic characteristics (Table 1), we found slightly higher levels among women who were more educated (P < 0.05) and who had a lower body mass index before pregnancy (P < 0.01). We also found higher CRH levels among black women, compared with women who were white or of another race (P < 0.05).
Table 1.
Mean log CRH at 24–29 wk gestation, by sociodemographic characteristics, adjusting for maternal age and gestational age at time of blood sample
| n | % | Mean | sd | Pa | |
|---|---|---|---|---|---|
| Age group (yr) | 0.37 | ||||
| Less than 25 | 267 | 21.7 | 6.29 | 0.52 | |
| 25 to <29 | 252 | 20.5 | 6.25 | 0.50 | |
| 29 to <33 | 372 | 30.2 | 6.31 | 0.50 | |
| 33+ | 339 | 27.6 | 6.32 | 0.50 | |
| Race/ethnicity | 0.03 | ||||
| Black | 220 | 17.9 | 6.21 | 0.53 | |
| Other | 104 | 8.5 | 6.31 | 0.48 | |
| White | 906 | 73.7 | 6.31 | 0.50 | |
| Education | 0.02 | ||||
| High school or less | 255 | 20.7 | 6.21 | 0.51 | |
| Some college | 230 | 18.7 | 6.30 | 0.52 | |
| College | 352 | 28.6 | 6.31 | 0.49 | |
| Graduate | 393 | 32.0 | 6.34 | 0.51 | |
| Marital status | 0.06 | ||||
| Married | 943 | 76.7 | 6.31 | 0.50 | |
| Not married | 287 | 23.3 | 6.25 | 0.51 | |
| Income | 0.52 | ||||
| 200% of poverty line or less | 294 | 23.9 | 6.27 | 0.57 | |
| Greater than 200% of poverty line | 895 | 72.8 | 6.30 | 0.49 | |
| Missing | 41 | 3.3 | 6.34 | 0.42 | |
| Body mass index prior to pregnancy (kg/m2) | <0.01 | ||||
| Less than 25 | 742 | 60.3 | 6.33 | 0.50 | |
| 25 to 30 | 216 | 17.6 | 6.31 | 0.54 | |
| Greater than 30 | 272 | 22.1 | 6.18 | 0.47 | |
| Parity | 0.79 | ||||
| Para 0 | 598 | 48.6 | 6.30 | 0.50 | |
| Para 1 | 414 | 33.7 | 6.30 | 0.50 | |
| Para 2+ | 218 | 17.7 | 6.27 | 0.53 | |
| Gestational age at delivery | 0.07 | ||||
| 37 weeks or more | 1073 | 87.2 | 6.29 | 0.51 | |
| Less than 37 weeks | 157 | 12.8 | 6.37 | 0.51 | |
| Time of day of blood draw | 0.06 | ||||
| Morning | 778 | 63.3 | 6.32 | 0.52 | |
| Afternoon | 452 | 36.7 | 6.26 | 0.49 |
Partial F test.
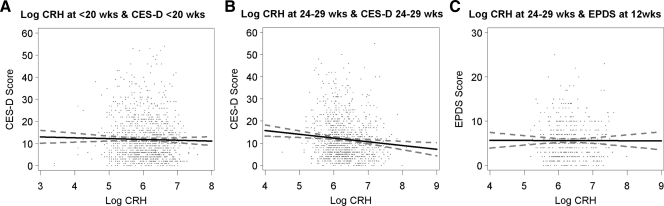
Using Pearson correlations (Table 2), we measured the association between serum CRH and maternal mood. CRH at less than 20 wk was inversely associated with CES-D score at 24–29 wk, and CRH at 24–29 wk was inversely associated with CES-D score at both less than 20 wk and 24–29 wk. We found no relationship between serum CRH during pregnancy and EPDS at 12 wk or 1 yr postpartum (CRH <20 wk, P = 0.97 and 0.67; 24–29 wk, P = 0.95 and 0.11, respectively). CES-D score during pregnancy was highly correlated with EPDS score at both 12 wk and 1 yr postpartum (P < 0.0001). In Fig. 1, we present scatter plots illustrating maternal log CRH and CES-D and EPDS scores.
Table 2.
Pearson correlations coefficients between CES-D score and log CRH at less than 20 and 24–29 wk of pregnancy and depressive symptoms at 24–29 wk of pregnancy and at 12 wk and 1 yr postpartum
| CES-D at <20 wk | CES-D at 24–29 wk | EPDS at 12 wk pp | EPDS at 1 yr pp | |
|---|---|---|---|---|
| Log CRH at <20 wk | −0.02263 | −0.06680a | 0.00172 | −0.02188 |
| 1178 | 1080 | 484 | 391 | |
| Log CRH at 24–29 wk | −0.11105b | −0.09447b | −0.00282 | −0.08132 |
| 1178 | 1080 | 484 | 391 | |
| CES-D at <20 wk | 0.63056b | 0.40265b | 0.38792b | |
| 1043 | 459 | 375 | ||
| CES-D at 24–29 wk | 0.52178b | 0.46001b | ||
| 438 | 359 |
Pearson r, n for correlation. pp, Postpartum.
P < 0.05.
P < 0.01.
Figure 1.
Correlations between serum CRH and maternal mood at less than 20, 24–29, and 12 wk postpartum. A, Log CRH at less than 20 wk of pregnancy and CES-D at less than 20 wk of pregnancy. B, Log CRH at 24–29 wk and CES-D at 24–29 wk of pregnancy. C, Log CRH at 24–29 wk of pregnancy and EPDS at 12 wk postpartum.
We then used multivariate linear regression to measure mean log CRH values among women with and without moderate to severe prenatal or postpartum depression, adjusting for sociodemographic variables (Table 3). We found a moderate inverse association between maternal CES-D score of 17 or greater and log CRH at 24–29 wk, with lower log CRH levels among depressed women [gestational, time of day, and maternal age adjusted mean 6.32 (95% confidence interval [CI] 6.29–6.36) vs. 6.25 (6.19–6.31), P = 0.04]. With further adjustment for sociodemographic factors, this association was diminished (P = 0.11). We found no association between mean log CRH and maternal mood at less than 20 wk or at 12 wk postpartum. Women who gave birth before 37 wk gestational age had higher CRH levels. When we excluded these women in a sensitivity analysis, we similarly did not find an association between CRH and risk for PPD.
Table 3.
Mean log CRH (95% CI) at 24–29 wk gestation by maternal mood status during pregnancy and at 12 wk postpartum with varying levels of model adjustment
| Log CRH model 1a | Log CRH model 2b | Log CRH model 3c | |
|---|---|---|---|
| CRH measured at <20 wk | |||
| Moderate to severe prenatal depression at less than 20 wk (CES-D ≥17) | |||
| No (n = 894) | 6.01 (5.98–6.05) | 6.01 (5.98–6.05) | 6.02 (5.97–6.06) |
| Yes (n = 284) | 5.98 (5.91–6.05) | 5.98 (5.91–6.05) | 5.98 (5.91–6.06) |
| CRH measured at 24–29 wk | |||
| Moderate to severe prenatal depression at 24–29 wk (CES-D ≥17) | |||
| No (n = 812) | 6.32 (6.29–6.36)d | 6.32 (6.29–6.36) | 6.31 (6.27–6.35) |
| Yes (n = 268) | 6.25 (6.19–6.31) | 6.26 (6.20–6.32) | 6.25 (6.19–6.32) |
| Probable PPD at 12 wk after birth (EPDS ≥13) | |||
| No (n = 447) | 6.33 (6.28–6.38) | 6.33 (6.28–6.38) | 6.33 (6.27–6.38) |
| Yes (n = 37) | 6.28 (6.11–6.45) | 6.31 (6.14–6.48) | 6.31 (6.13–6.49) |
Adjusted for maternal age, time of day, and gestational age at blood draw.
Adjusted for maternal age, time of day, gestational age at blood draw, race/ethnicity, parity, education, marital status, income, and pregravid body mass index.
Adjusted for maternal age time of day, gestational age at blood draw, race/ethnicity, parity, education, marital status, income, and pregravid body mass index and restricted to women who gave birth at less than 37 wk gestation.
P < 0.05.
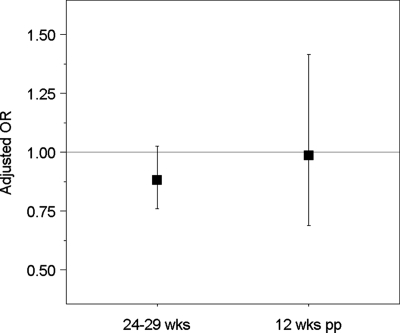
We used logistic regression to quantify the odds of prenatal or postpartum depression per sd change in log CRH at less than 20 wk and 24–29 wk of gestation (Fig. 2). In models adjusted for gestational and maternal age, higher CRH was associated with a reduced odds of CES-D of 17 or greater at 24–29 wk [odds ratio (OR) 0.86 (95% CI 0.75–0.99) per sd increase in log CRH at 24–29 wk]. With adjustment for sociodemographic confounders, this association was modestly attenuated, and confidence intervals increased (OR 0.88, 95% CI 0.76–1.03). We found no association between log CRH at 24–29 wk and postpartum depression [maternal and gestational age adjusted OR 0.90 (95% CI 0.63–1.27) per sd; sociodemographic covariate adjusted OR 0.99 (95% CI 0.69–1.42)].
Figure 2.
Adjusted OR and 95% CI for depressed mood at 24–29 wk of pregnancy, defined as CES-D of 17 or greater, and at 12 wk postpartum, defined as EPDS of 13 or greater, per sd of log CRH measured at 24–29 wk of pregnancy. Models adjusted for gestational age and time of day at blood draw, maternal age, race/ethnicity, parity, education, marital status, income, and pregravid body mass index.
When we measured the association between change in CRH level and maternal mood, we found lower CES-D scores among women with higher CRH level at less than 20 wk and greater increases in CRH between less than 20 and 24–29 wk (P < 0.01) in unadjusted models. This association was no longer present with adjustment for maternal age, self-reported race, parity, education, marital status, income, and body mass index. We found no association between CRH at less than 20 wk or change in CRH (measured by the subject specific slope) and EPDS score at 12 wk postpartum in unadjusted or multivariate-adjusted models.
Discussion
We did not observe an association between higher midpregnancy pCRH and an increased risk of either prenatal or postpartum depression, failing to replicate the findings of Yim et al. (27), who reported a critical period of midpregnancy pCRH as a sensitive and specific early diagnostic test for postpartum depression symptoms. Our findings do not support the use of midpregnancy pCRH as a screening test for those at risk of developing PPD.
However, our results are partially consistent with Rich-Edwards et al. (26), who also did not find an association between midpregnancy pCRH and postpartum depression. Unlike Rich-Edwards et al., we did not find an association between midpregnancy pCRH and prenatal depression. In fact, we observed a modest inverse association between pCRH levels and depression at 24–29 wk of pregnancy, although this association was diminished with adjustment for sociodemographic factors. Of note, the demographic characteristics of our study population are quite similar to both previous reports (>70% are Caucasian with at least some college level education), so this likely does not account for the differences in our findings.
Although we did observe somewhat higher midpregnancy CRH levels among women who gave birth before 37 wk gestation, our findings were not altered when we excluded this group. Our results support numerous previous reports documenting the important association between higher levels of midpregnancy CRH and increased rates of preterm birth (37,38,39,40,41). However, our results do not demonstrate that increased midpregnancy CRH is related to postpartum depression, even in those women who deliver preterm. As suggested by Kramer et al. (37), placental CRH (the source of most of the measured maternal CRH) may not be an integral part of the maternal HPA axis response. This is one potential explanation for our findings and would suggest pCRH is not an appropriate biomarker for maternal perinatal or postpartum depression.
Limitations of our study include the selective nature of the study population based on the type of prenatal clinic attended, willingness to participate in the study, and the attrition from prenatal to postpartum assessments. Measurement error is also a concern because CRH is secreted in pulsatile bursts that may influence measurements. However, our collection methods did not differ from other studies of pCRH and perinatal mood disorders. CRH binding protein directly affects CRH measures and this study did not assess the binding protein. Our study collected CRH data at two point-in-time measures only. However, for the purposes of assessing the clinical utility of CRH as a marker for perinatal depression, this is a reasonable approach because more demanding measurement protocols would not be practical in a clinical setting. Lastly, although the outcome measure of major depression was assessed with valid and reliable self-report measures (CES-D and EPDS), all rating scales have limitations, and the diagnosis of depression was not confirmed with structured clinical interviews. In addition, different rating scales were used during pregnancy (CES-D) and postpartum (EPDS).
In conclusion, in a large prospective, longitudinal study of women recruited during pregnancy and followed through 12 months postpartum, we found no evidence of a direct association between placental CRH and depressed mood, either during pregnancy or in the postpartum period. Our results suggest that CRH is not a useful biomarker of perinatal depression risk.
Footnotes
Present address for D.S.: Departments of Community Health and Obstetrics and Gynecology. Brown University, Providence, Rhode Island 02912.
The Pregnancy, Infection, and Nutrition study was supported by Grants HD37584, HD39373, and DK61981. The General Clinic Research Center was supported by the National Institutes of Health General Clinical Research Centers program of the Division of Research Resources Grant RR00046.
The work described in this paper was presented as a poster presentation at the Annual Meeting of the Society for Biological Psychiatry, May 22, 2010, New Orleans, LA.
Disclosure Summary: The authors have nothing to disclose. The authors of this manuscript do not have conflicts of interest relevant to the subject of this manuscript. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
First Published Online October 13, 2010
Abbreviations: CES-D, Center for Epidemiologic Studies Depression Scale; CI, confidence interval; EPDS, Edinburgh Postnatal Depression Scale; HPA, hypothalamic-pituitary-adrenal; OR, odds ratio; pCRH, placental CRH; PPD, postpartum depression.
References
- American Psychiatric Association Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Murray L, Stein A 1989 The effects of postnatal depression on the infant. Baillieres Clin Obstet Gynaecol 3:921–33 [DOI] [PubMed] [Google Scholar]
- Marmorstein NR, Malone SM, Iacono WG 2004 Psychiatric disorders among offspring of depressed mothers: associations with paternal psychopathology. Am J Psychiatry 161:1588–1594 [DOI] [PubMed] [Google Scholar]
- Flynn HA, Davis M, Marcus SM, Cunningham R, Blow FC 2004 Rates of maternal depression in pediatric emergency department and relationship to child service utilization. Gen Hosp Psychiatry 26:316–322 [DOI] [PubMed] [Google Scholar]
- O'Hara MW, Swain AM 1996 Rates and risk of postpartum depression—a meta-analysis. Int Rev Psychiatry 8:37–54 [Google Scholar]
- Yonkers KA, Ramin SM, Rush AJ, Navarrete CA, Carmody T, March D, Heartwell SF, Leveno KJ 2001 Onset and persistence of postpartum depression in an inner-city maternal health clinic system. Am J Psychiatry 58:1856–1863 [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC 2005 Perinatal depression: prevalence, screening accuracy and screening outcomes. Evid Rep Technol Assess 119:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz PM, Williams SB, Callaghan WM, Bachman DJ, Whitlock EP, Hornbrook MC 2007 Clinically identified maternal depression before, during and after pregnancies ending in live births. Am J Psychiatry 164:1457–1459 [DOI] [PubMed] [Google Scholar]
- Robertson E, Grace S, Wallington T, Stewart DE 2004 Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry 26:289–295 [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R 1987 Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 150:782–786 [DOI] [PubMed] [Google Scholar]
- Beck CT, Gable RK 2001 Further validation of the Postpartum Depression Screening Scale. Nurs Res 50:155–164 [DOI] [PubMed] [Google Scholar]
- Bloch M, Rubinow DR, Schmidt PJ, Lotsikas A, Chrousos GP, Cizza G 2005 Cortisol response to ovine corticotropin-releasing hormone in a model of pregnancy and parturition in euthymic women with and without a history of postpartum depression. J Clin Endocrinol Metab 90:695–699 [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau, Murphy J, Nieman L, Rubinow DR 2000 Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 157:924–930 [DOI] [PubMed] [Google Scholar]
- Greenwood J, Parker G 1984 The dexamethasone suppression test in the puerperium. Aus N Z J Psychiatry 18:282–284 [DOI] [PubMed] [Google Scholar]
- Wisner KL, Stowe ZN 1997 Psychobiology of postpartum mood disorders. Semin Reprod Endocrinol 15:77–89 [DOI] [PubMed] [Google Scholar]
- Magiakou MA, Mastorakos G, Rabin D, Dubbert B, Gold PW, Chrousos GP 1996 Hypothalamic-cortico-releasing hormone suppression during the post-partum period: implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab 81:1912–1917 [DOI] [PubMed] [Google Scholar]
- Bloch M, Daly RC, Rubinow DR 2003 Endocrine factors in the etiology of postpartum depression. Compr Psychiatry 44:234–246 [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I 2003 Maternal and fetal hypothalamic-pituitary-adrenal axis during pregnancy and postpartum. Ann NY Acad Sci 997:136–149 [DOI] [PubMed] [Google Scholar]
- Taylor A, Glover V, Marks M, Kammerer M 2009 Diurnal pattern of cortisol output in postnatal depression. Psychoneuroendocrinology 34:1184–1188 [DOI] [PubMed] [Google Scholar]
- Noltern WE, Lindheimer MD, Rueckert PA, Oparil S, Ehrlich EN 1980 Diurnal patterns and regulation of cortisol secretion in pregnancy. J Clin Endocrinology Metab 51:466–472 [DOI] [PubMed] [Google Scholar]
- Linton EA, Perkins AV, Woods RJ, Eben F, Wolfe CD, Behan DP, Potter E, Vale WW, Lowry PJ 1993 Corticotropin releasing hormone-binding protein (CRH-BP): plasma levels decrease during the third trimester of normal human pregnancy. J Clin Endocrinol Metab 76:260–262 [DOI] [PubMed] [Google Scholar]
- Gold PW, Gabry KE, Yasuda MR, Chrousos GP 2002 Divergent endocrine abnormalities in melancholic and atypical depression: clinical and pathophysiologic implications. Endocrinol Metab Clin North Am 31:37–62, vi [DOI] [PubMed] [Google Scholar]
- Jolley SN, Elmore S, Barnard KE, Carr DB 2007 Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biol Res Nurs 8:210–222 [DOI] [PubMed] [Google Scholar]
- Sasaki A, Liotta AS, Luckey MM, Margioris AN, Suda T, Krieger DT 1984 Immunoreactive corticotropin-releasing factor is present in human maternal plasma during the third trimester of pregnancy. J Clin Endocrinol Metab 59:812–814 [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW 1998 Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive systems: clinical implications. Ann Intern Med 129:229–240 [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Mohllajee AP, Kleinman K, Hacker MR, Majzoub J, Wright RJ, Gillman MW 2008 Elevated midpregnancy corticotropin-releasing hormone is associated with prenatal, but not postpartum, maternal depression. J Clin Endocrinol Metab 93:1946–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim IS, Glynn LM, Dunkel-Schetter C, Hobel CJ, Chicz-DeMet A, Sandman CA 2009 Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry 66:162–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA, Dole N, Kaczor D, Herring AH, Siega-Riz AM, Kaufman J, Thorp Jr JM 2005 Probability samples of area births versus clinic populations for reproductive epidemiology studies. Paediatr Perinat Epidemiol 19:315–322 [DOI] [PubMed] [Google Scholar]
- Daniels JL, Savitz DA, Bradley C, Dole N, Evenson KR, Eucker B, Herring A, Siega-Riz AM, Thorp JM 2006 Attitudes toward participation in a pregnancy and child cohort study. Paediatr Perinat Epidemiol 20;260–266 [DOI] [PubMed] [Google Scholar]
- Harville EW, Savitz DA, Dole N, Herring AH, Thorp Jr JM 2009 Stress questionnaires and stress biomarkers during pregnancy. J Womens Health 18:1425–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LS Radloff 1977 The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401 [Google Scholar]
- McDowell I, Newell C 1996 Measuring health, a guide to rating scales and questionnaires. 2nd ed. New York: Oxford University Press [Google Scholar]
- Brouwers EP, van Baar AL, Pop VJ 2001 Does the Edinburgh Postnatal Depression Scale measure anxiety? J Psychosom Res 5:659–663 [DOI] [PubMed] [Google Scholar]
- Matthey S, Henshaw C, Elliott S, Barnett B 2006 Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale—implications for clinical and research practice. Arch Womens Mental Health 9:309–315 [DOI] [PubMed] [Google Scholar]
- Hoffman S, Hatch MC 2000 Depressive symptomatology during pregnancy: evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychol 19:535–543 [PubMed] [Google Scholar]
- Petraglia F, Genazzani AD, Aguzzoli L, Gallinelli A, de Vita D, Caruso A, Genazzani AR 1994 Pulsatile fluctuations of plasma-gonadotropin-releasing hormone and corticotropin-releasing factor levels in healthy pregnant women. Acta Obstet Gynecol Scand 73:284–289 [DOI] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Séguin L, Goulet L, Kahn SR, McNamara H, Genest J, Dassa C, Chen MF, Sharma S, Meaney MJ, Thomson S, Van Uum S, Koren G, Dahhou M, Lamoureux J, Platt RW 2009 Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol 169:1319–1326 [DOI] [PubMed] [Google Scholar]
- Guendelman S, Kosa JL, Pearl M, Graham S, Kharrazi M 2008 Exploring the relationship of second-trimester corticotropin releasing hormone, chronic stress and preterm delivery. J Matern Fetal Neonatal Med 21:788–795 [DOI] [PubMed] [Google Scholar]
- Mancuso RA, Schetter CD, Rini CM, Roesch SC, Hobel CJ 2004 Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosom Med 66:762–769 [DOI] [PubMed] [Google Scholar]
- Holzman C, Jetton J, Siler-Khodr T, Fisher R, Rip T 2001 Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstet Gynecol 97:657–663 [DOI] [PubMed] [Google Scholar]
- Smith R, Smith JI, Shen X, Engel PJ, Bowman ME, McGrath SA, Bisits AM, McElduff P, Giles WB, Smith DW 2009 Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J Clin Endocrinol Metab 94:2066–2074 [DOI] [PubMed] [Google Scholar]