Abstract
Context and Objective: Strategies to augment pulsatile GH may be beneficial in patients with excess visceral adiposity, in whom GH secretion is reduced. The objective of this study was to determine the effects of a novel GHRH (GHRH1–44) analog, tesamorelin, on endogenous GH pulsatility and insulin sensitivity in healthy men.
Design, Participants, and Intervention: Thirteen males (mean age 45 ± 3 yr and body mass index 27.3 ± 1.2 kg/m2) received tesamorelin 2 mg sc once daily for 2 wk, with assessment made at baseline, after 2 wk of treatment, and after 2 wk of withdrawal.
Outcome Measures: The primary end point was change in mean overnight GH as determined by overnight frequent sampling. Secondary end points included insulin-stimulated glucose uptake as measured by euglycemic hyperinsulinemic clamp; IGF-I; and GH secretion parameters, including pulse area, pulse frequency, and basal secretion.
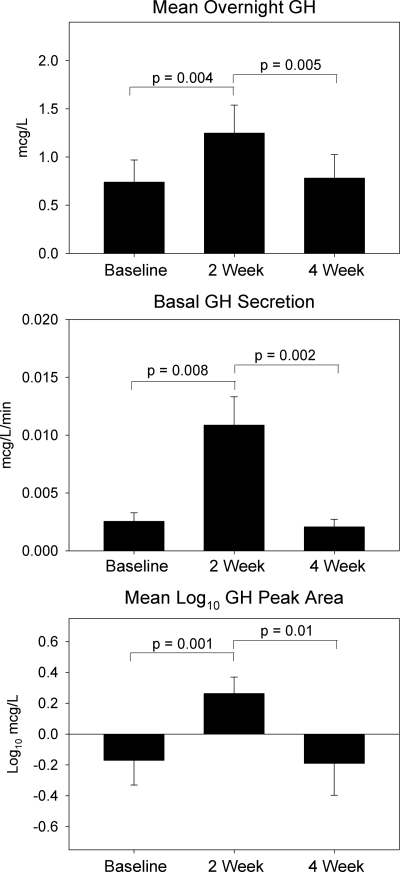
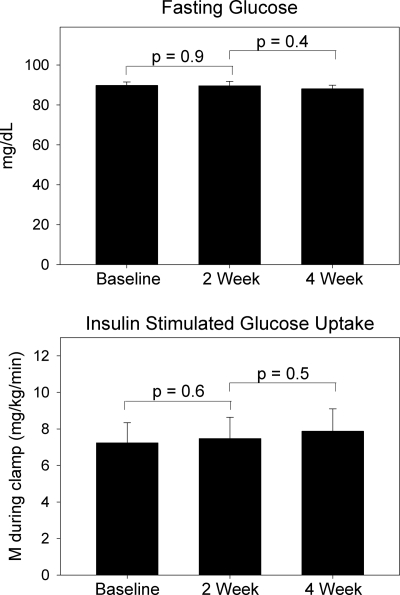
Results: Tesamorelin treatment increased mean overnight GH (change +0.5 ± 0.1 μg/liter, P = 0.004), average log10 GH peak area (change +0.4 ± 0.1 log10 μg/liter, P = 0.001), and basal GH secretion (change +0.008 ± 0.003 μg/liter · min, P = 0.008). IGF-I increased by 181 ± 22 μg/liter (P < 0.0001). Neither fasting glucose (P = 0.93) nor insulin-stimulated glucose uptake (P = 0.61) was significantly affected by tesamorelin.
Conclusions: Once-daily short-term treatment with a GHRH1–44 analog, tesamorelin, augments basal and pulsatile GH secretion. Moreover, although tesamorelin significantly increases IGF-I, peripheral insulin-stimulated glucose uptake appears to be preserved.
A GHRH1-44 analogue augments endogenous GH pulsatile secretion and increases IGF-I without affecting peripheral insulin sensitivity.
GH is secreted in a pulsatile manner, with discrete secretory bursts accounting for 90–95% of total GH release in humans (1,2). Although the physiological importance of pulsatile vs. tonic secretion has yet to be fully elucidated in humans, rodent models demonstrate that many effects of GH depend on its pattern of delivery to peripheral tissues. Hepatic synthesis of coagulation factors and hepatic expression of numerous enzymes differ, depending on whether the liver is exposed to pulsatile or continuous GH (3,4,5,6). Data in humans, although limited, suggest that pulsatile GH may be required to increase lipolysis (7,8), whereas continuous exposure to GH is a more important determinant of IGF-I (7,9,10).
Multiple physiological and pathological states, including aging (11,12), obesity (13), and lipodystrophic conditions with excess visceral obesity, as in HIV-infected patients (14), are associated with reduced GH secretion. Treatment with exogenous recombinant human GH (rhGH) may have beneficial effects in these populations, including improvement of body composition (15,16,17). Treatment with rhGH, however, results in nonpulsatile circulating GH levels and may adversely affect insulin sensitivity (18,19). Given the evidence that a pulsatile pattern of secretion may be important for many of the physiological actions of GH, a strategy to augment physiological pulsatile GH secretion may be desirable and may have less effect on insulin sensitivity than rhGH. In the current study, we investigated the short-term effects of a GHRH analog, tesamorelin, which has been shown to reduce visceral adipose tissue (VAT) in patients with HIV-associated abdominal adiposity (20,21). The effects of tesamorelin on endogenous GH secretion dynamics have not been previously described. We hypothesized that tesamorelin would increase mean overnight GH concentrations primarily by increasing GH pulse area as opposed to altering pulse frequency. Moreover, we hypothesized that this strategy to augment pulsatile secretion would not adversely affect insulin sensitivity as measured by euglycemic hyperinsulinemic clamp.
Subjects and Methods
Subject selection
Fifteen healthy men from the Boston area were recruited through the Massachusetts General Hospital (MGH) research volunteer database. Recruitment began in March 2009, and the last subject was randomized in March 2010. The study was approved by the MGH Institutional Review Board, and written, informed consent was obtained from each subject before study procedures.
Inclusion criteria included male sex, age between 18 and 60 yr, and body mass index (BMI) of 20–35 kg/m2. Exclusion criteria included any history of pituitary disease or cranial irradiation as well as use of corticosteroids, gonadal steroids, or antidiabetic agents. In addition, subjects were excluded for any condition for which GH treatment might be contraindicated, including history of malignancy, carpal tunnel syndrome, severe chronic illness, renal disease, liver disease, or prostate-specific antigen greater than 5 ng/ml on screening. No subject had previously received GHRH or GH treatment.
Study design
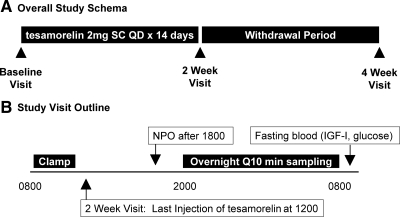
This was a 4-wk, interventional study of an open-label GHRH (1–44) analog, tesamorelin, 2 mg sc daily for 14 d followed by a 14-d withdrawal period. All assessments were conducted after a 12-h fast. After a screening visit to determine eligibility, subjects returned for inpatient assessments of insulin sensitivity and endogenous GH secretion at baseline and 2 wk (after 14 d treatment with tesamorelin) and 4 wk (after 14 d withdrawal from treatment, see Fig. 1A). The time line of the inpatient visits is shown in Fig. 1B. All baseline measurements were taken before administration of tesamorelin. At the 2-wk visit, the euglycemic hyperinsulinemic clamp was performed before the subjects’ last injection of tesamorelin, which was given at approximately 1200 h. Thus, at the 2-wk visit, frequent sampling for GH concentrations began approximately 8 h after the final injection of tesamorelin, and fasting glucose, IGF-I, and free fatty acid values were drawn approximately 20 h after the last injection (Fig. 1B).
Figure 1.
Study schema (A) and schedule of the inpatient assessments conducted at baseline, 2 wk, and 4 wk (B). QD, Every day; NPO, nil per os.
Study drug
GHRH (1–44) (tesamorelin; Theratechnologies, Inc., Montréal, Québec, Canada) was administered at a dose of 2 mg daily × 14 d, injected sc in the abdomen. Tesamorelin is an amidated GHRH (1–44) peptide that is stabilized against dipeptidyl peptidase IV degradation. The measured half-life of tesamorelin after 14 d sc administration in humans is 26–38 min (investigator’s brochure; Theratechnologies). The first dose of the study drug was given at the conclusion of the baseline visit, and the last dose was given after the insulin clamp at the 2-wk visit. Two subjects opted to come to the Clinical Research Center for nurse-administered injections, and the remaining subjects were taught to reconstitute and self-administer the injections at home. Subjects were instructed to administer the study drug in the morning. Subjects were asked to keep injection diaries, and empty vials were returned and counted at the 2-wk visit as a measure of compliance.
Hyperinsulinemic euglycemic clamp
After a 12-h fast, subjects received a primed infusion of 40 mU/m2/min regular insulin for 120 min. The priming dose was 200 mU/m2/min given over the first 2 min. A variable infusion of 20% dextrose maintained plasma glucose concentrations at the euglycemic value of 5 mmol/liter (90 mg/dl). Blood glucose was determined every 5 min using a B-Glucose analyzer (Hemocue, Lake Forest, CA). Insulin samples were collected every 20 min. Insulin stimulated glucose disposal (M) was determined using the method of DeFronzo et al. (22) for the interval between 100 and 120 min. M was corrected for insulin (M/I) and indexed to fat-free mass [M/I per lean body mass (LBM), mg/kg of fat-free mass per minute per microunits per milliliter insulin].
Assessment of GH secretion
Subjects had dinner at 1700 h and began fasting at 1800 h. Blood samples were drawn every 10 min from 2000 until 0740 h to assess endogenous overnight GH secretion. For two patients, data were available at a frequency of every 20 min at each visit. Results were similar, excluding these patients from the data set (data not shown), with no changes in significance; thus, data from all 13 patients who completed the study were included in the primary analysis. GH secretion parameters, including basal secretion, pulse frequency (number of peaks per 12 h), pulse area, and half-life, were determined using the automated deconvolution algorithm AutoDecon (23), which has been well-validated for deconvolution analysis of endogenous GH pulsatility. In a secondary analysis, the Cluster algorithm, which does not use deconvolution methodology, was also used to determine basal secretion, pulse area, and pulse frequency. Pulse data presented below reflect analysis using AutoDecon unless otherwise noted. Due to significant intraindividual variation in pulse area, pulse area is logarithmically transformed using log10, and the mean of the pulse areas (mean log10 GH pulse area) is calculated for each overnight GH profile. For two GH profiles in which three consecutive samples were missing, the last GH value was carried forward ×1 to allow for analysis.
Bionutrition and body composition analysis
Whole-body dual-energy x-ray absorptiometry (DEXA)was performed using a Discovery A densitometer (Hologic, Bedford, MA) to determine total body and regional fat mass. Single-slice computed tomography scans were performed at the L4 pedicle to assess sc and VAT area as previously described (24,25). Subjects had standard anthropometric measurements including waist circumference. Dietary intake, including macronutrient content, was assessed at each visit by 4-d food records analyzed by registered dietitians at the MGH Clinical Research Center.
Laboratory methods
Fasting glucose was measured using standard methodology at the MGH clinical laboratory. Insulin was measured using the paramagnetic particle, chemiluminescent Access immunoassay system (Beckman Coulter, Chaska, MN), with a sensitivity of 0.03 μU/ml and a precision of 3–5.6%. GH was measured using the paramagnetic particle, chemiluminescent Beckman Access ultrasensitive human GH assay (Beckman Coulter), with an intraassay variation of 1.90–2.78% and an interassay variation of 1.77–2.65% at concentrations of 2–10 μg/liter. At a concentration of 0.005 μg/liter, intraassay coefficient of variation (CV) is 6.4%, and the interassay CV is 5.8%. The effective analytic sensitivity of the human GH assay is 0.01 μg/liter. Serum IGF-I levels were measured with Immulite 2000 (Siemens Healthcare Diagnostics, Deerfield, IL), with an analytical sensitivity of 20 μg/liter, the intraassay CV ranging from 2.3 to 3.9%, and the interassay CV ranging from 3.7 to 8.1%. Free fatty acid (FFA) concentrations were measured with colorimetric assay (ZenBio, Research Triangle Park, NC), with an intraassay variability of 4.96% and an interassay variability of 10.93%.
Statistical analysis
To assess the effects of tesamorelin, changes between baseline, 2-wk (after treatment), and 4 wk (after withdrawal) visits were analyzed using two-tailed paired t testing. Statistical analysis was performed using JMP 5.0.1.2 (SAS Institute, Cary, NC). Statistical significance was defined as P < 0.05. Results are mean ± sem unless otherwise stated. A subanalysis was performed among patients with BMI greater than 25 kg/m2 (n = 8).
Results
Of 15 men recruited for the study, two discontinued for personal reasons after the baseline visit. Data from these subjects were not included in analyses. The remaining 13 subjects successfully completed the protocol. Compliance with the study drug, confirmed by vial count, was excellent at 98%.
Baseline characteristics
Mean age of the subjects was 45 ± 3 yr, and mean BMI was 27.3 ± 1.2 kg/m2 (Table 1). In univariate analysis, VAT was negatively associated with mean overnight GH (r = −0.71, P = 0.007), basal GH secretion (r = −0.65, P = 0.02), and mean log10 GH peak area (r = −0.72, P = 0.006). There was no association between VAT and GH half-life or number of GH peaks. Similarly, BMI was related to mean overnight GH (r = −0.72, P = 0.006), basal GH secretion (r = −0.69, P = 0.009), and mean log10 peak GH area (r = −0.77, P = 0.002) and showed no relationship with GH half-life or number of GH peaks. As expected, insulin-stimulated glucose uptake was negatively associated with both VAT (r = −0.76, P = 0.003) and BMI (r = −0.76, P = 0.003) at baseline.
Table 1.
Baseline characteristics
| Entire cohort (n = 13) | BMI >25 kg/m2 (n = 8) | |
|---|---|---|
| Age (yr) | 45 ± 3 | 48 ± 4 |
| Race [n (%)] | ||
| White | 10 (77%) | 7 (88%) |
| African American | 2 (15%) | 1 (12%) |
| Other | 1 (8%) | 0 |
| BMI (kg/m2) | 27.3 ± 1.2 | 30.1 ± 1.1 |
| Waist circumference (cm) | 95 ± 4 | 102 ± 4 |
| VAT area (cm2) | 110 ± 20 | 148 ± 21 |
| Fat by DEXA (%) | 21 ± 2 | 25 ± 2 |
Values are mean ± sem.
Effects of tesamorelin on endogenous GH secretion and IGF-I
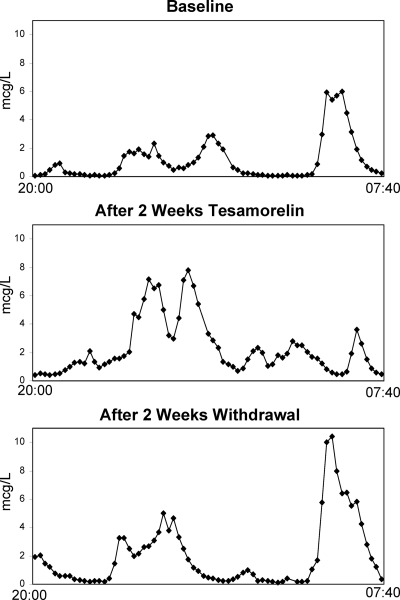
GH pulse parameters at the baseline, 2-wk, and 4-wk visits are shown in Table 2 for the entire cohort. Tesamorelin treatment for 14 d increased mean overnight GH concentration by 0.5 ± 0.1 μg/liter (P = 0.004, Table 2 and Fig. 2). Likewise, GH area under the curve increased by 366 ± 105 μg/liter per 12 h (P = 0.005). The overall increase in GH secretion was comprised of both increased basal GH secretion (+0.008 ± 0.003 μg/liter · min increase, P = 0.008) and increased average pulse area, with an increase in mean log10 GH pulse area of 0.4 ± 0.1 log10 μg/liter (P = 0.001), as shown in Fig. 2. Nonlogarithmically transformed pulse area data were also analyzed to provide clinical context; in this analysis, mean pulse area increased from 2.2 ± 0.6 to 3.4 ± 0.7 μg/liter after tesamorelin (P = 0.002). In deconvolution analysis performed by AutoDecon (23), there was no significant change in number of GH pulses (P = 0.68) or GH half-life (P = 0.60) after tesamorelin administration. Maximum overnight GH value also did not change (P = 0.98), suggesting that tesamorelin increased pulse area without significantly affecting maximal GH secretory capacity. GH secretion parameters returned to baseline after 2 wk of tesamorelin withdrawal, with the exception of GH half-life, which appeared to be longer at 4 wk compared with baseline (+3 ± 1 min, P = 0.01). There were no other significant differences between baseline and 4-wk GH secretion characteristics. A representative subject’s GH profiles from baseline, 2 wk, and 4 wk are shown in Fig. 3.
Table 2.
Effects of tesamorelin on GH pulse parameters (deconvolution analysis) and insulin sensitivity
| Entire cohort (n = 13)
|
BMI >25 kg/m2 (n = 8)
|
|||||
|---|---|---|---|---|---|---|
| Baseline | 2 wk | 4 wk | Baseline | 2 wk | 4 wk | |
| GH secretion | ||||||
| Mean overnight GH (μg/liter) | 0.7 ± 0.2 | 1.2 ± 0.3a | 0.8 ± 0.2 | 0.2 ± 0.1 | 0.6 ± 0.2 | 0.3 ± 0.1 |
| Basal secretion (μg/liter · min) | 0.003 ± 0.001 | 0.011 ± 0.002b | 0.002 ± 0.001 | 0.001 ± 0.0003 | 0.011 ± 0.003b | 0.001 ± 0.0002 |
| GH half-life (min) | 13.6 ± 0.8 | 14.9 ± 2.5 | 16.3 ± 1.3b | 12.7 ± 0.8 | 12.8 ± 3.4 | 15.1 ± 1.4 |
| GH peaks (n per 12 h) | 10 ± 1 | 10 ± 1 | 8 ± 1 | 10 ± 1 | 10 ± 1 | 9 ± 1 |
| GH AUC (μg/liter per 12 h) | 531 ± 166 | 897 ± 211a | 549 ± 170 | 172 ± 42 | 423 ± 136 | 180 ± 48 |
| Mean log10 GH peak area (log10 μg/liter) | −0.17 ± 0.16 | 0.26 ± 0.11a | −0.19 ± 0.21 | −0.53 ± 0.14 | 0.02 ± 0.08b | −0.65 ± 0.19 |
| GH maximum (μg/liter) | 4.2 ± 1.0 | 4.2 ± 1.1 | 4.2 ± 1.0 | 1.8 ± 0.5 | 2.7 ± 1.4 | 1.8 ± 0.6 |
| GH nadir (μg/liter) | 0.05 ± 0.02 | 0.28 ± 0.10b | 0.05 ± 0.02 | 0.02 ± 0.002 | 0.13 ± 0.04b | 0.02 ± 0.01 |
| IGF-I (μg/liter) | 148 ± 15 | 329 ± 28a | 161 ± 13 | 138 ± 15 | 308 ± 34a | 150 ± 14 |
| Insulin sensitivity | ||||||
| Fasting glucose (mg/dl) | 90 ± 2 | 90 ± 2 | 88 ± 2 | 92 ± 2 | 93 ± 2 | 91 ± 2 |
| M (mg/kg · min) | 7.2 ± 1.1 | 7.5 ± 1.2 | 7.9 ± 1.2 | 5.3 ± 0.8 | 5.7 ± 0.7 | 5.5 ± 1.0 |
| M/I per LBM (mg/kg LBM per μU/ml insulin per min) | 0.17 ± 0.03 | 0.19 ± 0.03 | 0.22 ± 0.05 | 0.13 ± 0.02 | 0.14 ± 0.04 | 0.13 ± 0.04 |
| HOMA-IR | 1.3 ± 0.3 | 2.0 ± 0.6 | 1.2 ± 0.4 | 1.7 ± 0.4 | 2.8 ± 0.8 | 1.7 ± 0.5 |
Values are mean ± sem. M is insulin-stimulated glucose uptake calculated between 100 and 120 min of the euglycemic hyperinsulinemic clamp procedure. M/I per LBM is insulin-stimulated glucose uptake indexed to fat-free mass and divided by serum insulin concentration. GH pulse parameters determined by the automated deconvolution algorithm AutoDecon. AUC, Area under the curve.
P < 0.005 compared with baseline by paired t test.
P < 0.05 compared with baseline by paired t test.
Figure 2.
Effects of tesamorelin on mean overnight GH, GH basal secretion, and mean log10 GH pulse area. Error bars represent sem.
Figure 3.
A representative GH profile at baseline, after 2 wk of tesamorelin treatment, and after 2 wk of withdrawal (4 wk visit).
Analysis using the Cluster algorithm confirmed significant increases in basal GH secretion and GH pulse area after tesamorelin treatment (Table 3). In Cluster analysis, however, the number of overnight GH pulses also significantly increased after tesamorelin (Table 3).
Table 3.
Effects of tesamorelin on GH pulse parameters as determined by cluster analysis
| Entire cohort (n = 13)
|
BMI >25 kg/m2 (n = 8)
|
|||||
|---|---|---|---|---|---|---|
| Baseline | 2 wk | 4 wk | Baseline | 2 wk | 4 wk | |
| Basal secretion (μg/liter · min) | 0.018 ± 0.007 | 0.041 ± 0.011a | 0.020 ± 0.008 | 0.006 ± 0.002 | 0.017 ± 0.005 | 0.006 ± 0.001 |
| GH peaks (n per 12 h) | 5 ± 0 | 7 ± 0a | 5 ± 0 | 5 ± 1 | 7 ± 1a | 5 ± 1 |
| Mean log10 GH peak area (log10 μg/liter) | −0.40 ± 0.16 | −0.09 ± 0.15a | −0.39 ± 0.24 | −0.69 ± 0.19 | −0.43 ± 0.14 | −0.70 ± 0.20 |
Values are mean ± sem. GH pulse parameters were determined by Cluster analysis.
P < 0.05 compared withy baseline by paired t test.
IGF-I increased significantly after tesamorelin treatment (+181 ± 22 μg/liter, P < 0.0001) and subsequently decreased after 2 wk of withdrawal to values that did not significantly differ from baseline (Table 2).
Effects of tesamorelin on insulin sensitivity and FFA
As shown in Table 2 and Fig. 4, there were no significant changes in fasting glucose (P = 0.93) or insulin-stimulated glucose uptake (P = 0.61) as determined by euglycemic hyperinsulinemic clamp. Results were similar when insulin-stimulated glucose uptake was adjusted for insulin level and indexed to LBM (P = 0.62, Table 2). Homeostasis model assessment insulin resistance index (HOMA-IR) did not significantly change, although there was a trend toward increased HOMA-IR with tesamorelin treatment (P = 0.08, Table 2). Fasting FFA did not change after tesamorelin treatment (428 ± 46 μm at baseline vs. 392 ± 33 μm at 2 wk, P = 0.37 by paired t test).
Figure 4.
Effects of tesamorelin on fasting glucose and insulin-stimulated glucose uptake (M) as measured by euglycemic hyperinsulinemic clamp. Error bars represent sem.
Body composition and dietary intake
As expected, given the short duration of the study, there were no significant changes in subjects’ BMI, iliac waist circumference, or percent body fat as measured by DEXA during the study (data not shown). There were also no significant changes in caloric or macronutrient intake over the course of the study as measured by the 4-d food diary (data not shown).
Subanalysis in subjects with BMI greater than 25 kg/m2 (n = 8)
Because individuals with excess visceral adiposity are likely to be overweight or obese, the subgroup of subjects with BMI greater than 25 kg/m2 was examined separately. This subgroup was similar in age to the larger group but had an average BMI of 30.1 ± 1.1 kg/m2 (Table 1). GH pulse parameters for this subgroup at the baseline, 2-wk, and 4-wk visits are shown in Table 2. After 14 d of tesamorelin treatment, mean overnight GH concentration tended to increase (+0.4 ± 0.2 μg/liter, P = 0.09), and significant increases were seen in GH basal secretion (+0.010 ± 0.003 μg/liter · min, P = 0.01) and log10 mean pulse area (+0.5 ± 0.2 log10 μg/liter, P = 0.009). As in the entire cohort, there were no significant changes in half-life or pulse frequency (Table 2). All GH secretion parameters returned to baseline after 2 wk of treatment withdrawal. IGF-I increased significantly following treatment, (+171 ± 29 μg/liter, P = 0.0006) and subsequently decreased after 2 wk of withdrawal to values that did not significantly differ from baseline. There were no significant changes in fasting glucose (P = 0.85) or insulin-stimulated glucose uptake (P = 0.29) after 2 wk of treatment. HOMA-IR did not significantly change (P = 0.11, Table 2).
Discussion
In this study we demonstrate that once-daily sc administration of a GHRH (1–44) analog, tesamorelin, augments GH secretion by increasing both basal secretion and GH pulse area, without changing pulse frequency as assessed by deconvolution analysis. Moreover, although mean GH concentrations and IGF-I levels increase to a significant degree, we did not observe changes in insulin-stimulated glucose uptake like those that might be expected with rhGH administration (18,19). The above holds true, even among those with significantly increased BMI at baseline.
GHRH analogs have shown benefit in numerous conditions, including aging (26,27), HIV-lipodystrophy (20,28), and childhood short stature (29,30). Subjects in the current study were healthy and male to avoid confounding by gender in the assessment of GHRH effects on GH pulsatility. We recruited subjects who were on average overweight to assess the baseline relationship of BMI and VAT to GH pulse parameters in this population. At baseline, both VAT and BMI were significantly inversely related to multiple parameters of GH secretion, including mean overnight GH, basal GH secretion, and GH pulse area, but neither was associated with GH pulse frequency. This is in keeping with previous observations that overall GH secretion is reduced but pulse frequency unchanged in states of obesity or visceral adiposity (14,31) and likewise that fasting or weight loss increases GH secretion without altering pulse frequency (32,33).
In a subanalysis, we evaluated the effects of tesamorelin in patients specifically with overweight and obesity, in whom baseline BMI was increased. In this group, tesamorelin significantly increased GH pulse area and basal GH secretion, although changes were of a smaller absolute magnitude than those among the whole group, which included a greater range of BMIs. These data suggest that even though GH pulse parameters are reduced in the overweight and obese in association with excess VAT, stimulation with an exogenous GHRH analog (tesamorelin) for 2 wk can increase critical parameters of GH pulsatility and raise IGF-I to a clinically significant level in this group.
Although the efficacy of GHRH analogs is limited in states of true pituitary GH deficiency, GHRH may be a beneficial strategy to physiologically augment GH secretion in conditions with excess visceral fat, including specific lipodystrophic conditions, e.g. among patients with HIV receiving highly active antiretroviral therapy, and, as seen in this study, non-HIV-infected patients with more generalized obesity. In these conditions, pituitary somatotroph function is intact, but pulsatile GH secretion is reduced due to physiological factors related to excess abdominal fat (14,34). Evidence that this is a functional deficiency related to weight and nutritional status derives from studies in which GH secretion normalizes in obese patients after significant weight loss (33). For conditions of obesity and particularly of excess VAT accumulation, GHRH analogs may have potential advantages over rhGH. First, unlike exogenous rhGH, GHRH administration preserves the negative feedback of IGF-I on pituitary GH secretion, which may limit side effects of GH excess. Second, as previously shown with other GHRH compounds (26), and now shown with a novel GHRH (1–44) analog, tesamorelin, in a study that was large enough to allow us to specifically examine effects in obese patients per se, GHRH administration preserves the pulsatile pattern of endogenous GH secretion.
Although further investigation into the importance of the secretory pattern of GH in humans is needed, data strongly suggest that pulsatile secretion is required for some of the physiologic effects of GH. In vitro studies demonstrate that a critical GH responsive transcription factor, signal transducer and activator of transcription-5b, is maximally activated by pulsatile GH delivery, whereas continuous GH delivery decreases signal transducer and activator of transcription-5b activation to 10–20% of the maximal level (35,36). In humans, Jaffe et al. (9) using pulsatile and continuous GH delivery paradigms to administer equivalent doses of rhGH, demonstrate that continuous GH increases IGF-I to a greater degree than pulsatile administration, whereas pulsatile GH has greater effects on markers of bone turnover. In addition, the activity of P-450 cytochromes, CYP1A2 and CYP3A4, differs according to pattern of GH administration, with the former decreasing to a greater degree in response to pulsatile GH and the latter increasing in response to continuous GH but decreasing with pulsatile GH (9). Further studies are necessary to determine the effects of tesamorelin on transcription factors and cytochrome markers of GH action.
In this study we used AutoDecon (23) in our primary analysis because we wanted to assess the effects of tesamorelin on pituitary GH secretion using deconvolution analysis to define and differentiate discrete pituitary secretory episodes from background basal secretion. However, this technique may not assess biological action in the periphery, which may relate to the specific pattern of peaks and troughs better detected by a pulse detection algorithm. For this reason we reanalyzed our data using Cluster in a secondary analysis, confirming the result obtained using deconvolution analysis. Significant changes in basal and peak GH secretion were seen, similar to that shown by deconvolution analysis. Using Cluster, fewer peaks were detected, and an increase in number of peaks was noted after tesamorelin treatment. Tesamorelin clearly augments basal and peak GH secretion, confirmed by two independent methods and may increase GH pulsatility. Further studies are needed in this regard. Calculated basal secretion using AutoDecon (23) is below the minimum detectable concentration of the assay for some subjects, as the program approximates a best-fit theoretical curve to the data. The calculation of pulse area by AutoDecon does not include and is independent of any changes in the basal secretion area.
The GHRH analog tested in the current short-term physiology study has shown significant beneficial effects to decrease VAT in individuals with HIV-associated abdominal fat accumulation, without clinically meaningful changes in long-term glucose homeostasis over 12 months (20,21,37). However, the effects of tesamorelin on short-term glucose homeostasis using euglycemic hyperinsulinemic clamp to assess effects on peripheral insulin sensitivity were not previously known. Our data demonstrate that insulin-stimulated glucose uptake did not significantly change nor show any trend toward decrease after 2 wk of tesamorelin administration. Importantly, adverse effects of rhGH on insulin sensitivity are usually greatest at the beginning of treatment (15,38), suggesting that a treatment period of 2 wk should be optimal to observe changes in insulin sensitivity. Due to the size of our cohort, however, we cannot rule out the possibility of type 2 error. HOMA-IR did show a trend toward increase in our study, although changes were not statistically significant. Differential effects of tesamorelin on these indices may reflect differential effects of short-term tesamorelin on hepatic vs. peripheral insulin sensitivity. Further long-term studies will be needed to investigate this possibility.
The half-life of tesamorelin is short, estimated at 26–38 min, yet effects to stimulate pituitary secretion were clearly seen after 2 wk of once-daily dosing, with assessment of overnight GH starting 8 h after the last dose. Although the increases in GH pulsatility may have been due to the last dose of GHRH administered per se, we performed the study over 2 wk because prior pharmacokinetic studies demonstrate achievement of steady-state increases in IGF-I after 2 wk of administration. Why tesamorelin is able to increase GH pulsatility when given once a day, despite its short half-life, is unknown but may reflect dissociation time from the GHRH receptor, longer biological half-life at the pituitary, or unknown factors. Further studies are needed to better understand the biological effects of GHRH (1–44) on pituitary GH secretion.
Tesamorelin administration in the current short-term, physiology study was well tolerated, with no serious adverse events related to treatment. Definitive studies on safety require larger study periods and greater number of subjects, and these issues were not the intent of the current study. Larger and longer studies might be useful to evaluate the effects of GH augmentation with tesamorelin on metabolic end points such as lipid metabolism, body composition including ectopic accumulation of fat in liver and muscle, subclinical inflammation, and carotid intima-media thickness, all of which have shown an inverse association with GH dynamics (39,40,41,42).
Overall, we demonstrate that short-term use of a GHRH (1–44) analog, tesamorelin, augments endogenous GH pulsatility and increases IGF-I without apparent changes in insulin-stimulated glucose uptake among healthy men, including those with overweight and obesity. To the extent that reduced GH is associated with increased cardiovascular risk indices in obesity (40,42,43), augmentation of GH using a GHRH strategy may prove beneficial to enhance pulsatile GH secretion in this population. Our data suggest that pituitary GH secretion is diminished in overweight and obese patients in association with excess VAT, but pituitary GH reserve is sufficiently intact to respond to GHRH administration at the dose given, at least over the short term. Further studies of the physiological effects of this compound on women are necessary. In addition, longer-term studies may be useful to assess the efficacy and safety of GHRH to augment GH secretion in obesity.
Acknowledgments
We gratefully acknowledge the Massachusetts General Hospital bionutrition and nursing staffs, the research volunteers for their participation in the study, and Theratechnologies Inc. for providing the study drug, tesamorelin. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant R01DK063639 (to S.G.), and NIH Grants M01-RR-01066 and 1 UL1 RR025758–01, Harvard Clinical and Translational Science Center from the National Center for Research Resources. NIH funding was also provided through Grants T32 HD052961-03, F32 DK080642-02, and K23 DK089910-01 (to T.L.S.) and Grant K24 DK064545-08 (to S.K.G.). The study drug (tesamorelin) was donated by Theratechnologies, Inc.
Disclosure Summary: T.L.S., C.Y.C., K.L.B., and H.M. have nothing to disclose. S.G. has served as a consultant for Theratechnologies, Inc., and EMD Serono.
First Published Online October 13, 2010
Abbreviations: BMI, Body mass index; CV, coefficient of variation; DEXA, dual-energy x-ray absorptiometry; FFA, free fatty acid; HOMA-IR, homeostasis model assessment insulin resistance index; LBM, lean body mass; M, glucose disposal; MGH, Massachusetts General Hospital; M/I, M corrected for insulin; rhGH, recombinant human GH; VAT, visceral adipose tissue.
References
- Reutens AT, Veldhuis JD, Hoffman DM, Leung KC, Ho KK 1996 A highly sensitive growth hormone (GH) enzyme-linked immunosorbent assay uncovers increased contribution of a tonic mode of GH secretion in adults with organic GH deficiency. J Clin Endocrinol Metab 81:1591–1597 [DOI] [PubMed] [Google Scholar]
- Hartman ML, Faria AC, Vance ML, Johnson ML, Thorner MO, Veldhuis JD 1991 Temporal structure of in vivo growth hormone secretory events in humans. Am J Physiol 260:E101–E110 [DOI] [PubMed] [Google Scholar]
- Mode A, Gustafsson JA, Jansson JO, Edén S, Isaksson O 1982 Association between plasma level of growth hormone and sex differentiation of hepatic steroid metabolism in the rat. Endocrinology 111:1692–1697 [DOI] [PubMed] [Google Scholar]
- Gustafsson JA, Edén S, Eneroth P, Hökfelt T, Isaksson O, Jansson JO, Mode A, Norstedt G 1983 Regulation of sexually dimorphic hepatic steroid metabolism by the somatostatin-growth hormone axis. J Steroid Biochem 19:691–698 [DOI] [PubMed] [Google Scholar]
- Waxman DJ, O'Connor C 2006 Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20:2613–2629 [DOI] [PubMed] [Google Scholar]
- Wong JH, Dukes J, Levy RE, Sos B, Mason SE, Fong TS, Weiss EJ 2008 Sex differences in thrombosis in mice are mediated by sex-specific growth hormone secretion patterns. J Clin Invest 118:2969–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surya S, Horowitz JF, Goldenberg N, Sakharova A, Harber M, Cornford AS, Symons K, Barkan AL 2009 The pattern of growth hormone delivery to peripheral tissues determines insulin-like growth factor-1 and lipolytic responses in obese subjects. J Clin Endocrinol Metab 94:2828–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo E, Danou F, Persson M, Miles JM 1996 Effects of pulsatile delivery of basal growth hormone on lipolysis in humans. Am J Physiol 271:E123–E126 [DOI] [PubMed] [Google Scholar]
- Jaffe CA, Turgeon DK, Lown K, Demott-Friberg R, Watkins PB 2002 Growth hormone secretion pattern is an independent regulator of growth hormone actions in humans. Am J Physiol Endocrinol Metab 283:E1008–E1015 [DOI] [PubMed] [Google Scholar]
- Faje AT, Barkan AL 2010 Basal, but not pulsatile, growth hormone secretion determines the ambient circulating levels of insulin-like growth factor-I. J Clin Endocrinol Metab 95:2486–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KY, Evans WS, Blizzard RM, Veldhuis JD, Merriam GR, Samojlik E, Furlanetto R, Rogol AD, Kaiser DL, Thorner MO 1987 Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab 64:51–58 [DOI] [PubMed] [Google Scholar]
- Zadik Z, Chalew SA, McCarter Jr RJ, Meistas M, Kowarski AA 1985 The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab 60:513–516 [DOI] [PubMed] [Google Scholar]
- Iranmanesh A, Lizarralde G, Veldhuis JD 1991 Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) ceretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab 73:1081–1088 [DOI] [PubMed] [Google Scholar]
- Koutkia P, Meininger G, Canavan B, Breu J, Grinspoon S 2004 Metabolic regulation of growth hormone by free fatty acids, somatostatin, and ghrelin in HIV-lipodystrophy. Am J Physiol Endocrinol Metab 286:E296–E303 [DOI] [PubMed] [Google Scholar]
- Johannsson G, Mårin P, Lönn L, Ottosson M, Stenlöf K, Björntorp P, Sjöström L, Bengtsson BA 1997 Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab 82:727–734 [DOI] [PubMed] [Google Scholar]
- Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE 1990 Effects of human growth hormone in men over 60 years old. N Engl J Med 323:1–6 [DOI] [PubMed] [Google Scholar]
- Lo J, You SM, Canavan B, Liebau J, Beltrani G, Koutkia P, Hemphill L, Lee H, Grinspoon S 2008 Low-dose physiological growth hormone in patients with HIV and abdominal fat accumulation: a randomized controlled trial. JAMA 300:509–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz D, Klassen GA, Zierler KL 1965 Effect of human growth hormone on muscle and adipose tissue metabolism in the forearm of man. J Clin Invest 44:51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov L, Schmitz O, Jørgensen JO, Arnfred J, Abildgaard N, Christiansen JS, Alberti KG, Orskov H 1989 Influence of growth hormone on glucose-induced glucose uptake in normal men as assessed by the hyperglycemic clamp technique. J Clin Endocrinol Metab 68:276–282 [DOI] [PubMed] [Google Scholar]
- Falutz J, Allas S, Blot K, Potvin D, Kotler D, Somero M, Berger D, Brown S, Richmond G, Fessel J, Turner R, Grinspoon S 2007 Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med 357:2359–2370 [DOI] [PubMed] [Google Scholar]
- Falutz J, Allas S, Mamputu JC, Potvin D, Kotler D, Somero M, Berger D, Brown S, Richmond G, Fessel J, Turner R, Grinspoon S 2008 Long-term safety and effects of tesamorelin, a growth hormone-releasing factor analogue, in HIV patients with abdominal fat accumulation. Aids 22:1719–1728 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R 1979 Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- Johnson ML, Pipes L, Veldhuis PP, Farhy LS, Nass R, Thorner MO, Evans WS 2009 AutoDecon: a robust numerical method for the quantification of pulsatile events. Methods Enzymol 454:367–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE 1982 Assessment of abdominal fat content by computed tomography. Am J Clin Nutr 36:172–177 [DOI] [PubMed] [Google Scholar]
- Stanley TL, Joy T, Hadigan CM, Liebau JG, Makimura H, Chen CY, Thomas BJ, Weise SB, Robbins GK, Grinspoon SK 2009 Effects of switching from lopinavir/ritonavir to atazanavir/ritonavir on muscle glucose uptake and visceral fat in HIV-infected patients. Aids 23:1349–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas E, Harman SM, Piñeyro MA, Roberson R, Blackman MR 1992 Growth hormone (GH)-releasing hormone-(1–29) twice daily reverses the decreased GH and insulin-like growth factor-I levels in old men. J Clin Endocrinol Metab 75:530–535 [DOI] [PubMed] [Google Scholar]
- Vittone J, Blackman MR, Busby-Whitehead J, Tsiao C, Stewart KJ, Tobin J, Stevens T, Bellantoni MF, Rogers MA, Baumann G, Roth J, Harman SM, Spencer RG 1997 Effects of single nightly injections of growth hormone-releasing hormone (GHRH 1–29) in healthy elderly men. Metabolism 46:89–96 [DOI] [PubMed] [Google Scholar]
- Koutkia P, Canavan B, Breu J, Torriani M, Kissko J, Grinspoon S 2004 Growth hormone-releasing hormone in HIV-infected men with lipodystrophy: a randomized, controlled trial. JAMA 292:210–218 [DOI] [PubMed] [Google Scholar]
- Gelato MC, Ross JL, Malozowski S, Pescovitz OH, Skerda M, Cassorla F, Loriaux DL, Merriam GR 1985 Effects of pulsatile administration of growth hormone (GH)-releasing hormone on short term linear growth in children with GH deficiency. J Clin Endocrinol Metab 61:444–450 [DOI] [PubMed] [Google Scholar]
- Low LC, Wang C, Cheung PT, Ho P, Lam KS, Young RT, Yeung CY, Ling N 1988 Long term pulsatile growth hormone (GH)-releasing hormone therapy in children with GH deficiency. J Clin Endocrinol Metab 66:611–617 [DOI] [PubMed] [Google Scholar]
- Riedel M, Hoeft B, Blum WF, von zur Mühlen A, Brabant G 1995 Pulsatile growth hormone secretion in normal-weight and obese men: differential metabolic regulation during energy restriction. Metabolism 44:605–610 [DOI] [PubMed] [Google Scholar]
- Kasa-Vubu JZ, Barkan A, Olton P, Meckmongkol T, Carlson NE, Foster CM 2002 Incomplete modified fast in obese early pubertal girls leads to an increase in 24-hour growth hormone concentration and a lessening of the circadian pattern in leptin. J Clin Endocrinol Metab 87:1885–1893 [DOI] [PubMed] [Google Scholar]
- Rasmussen MH, Hvidberg A, Juul A, Main KM, Gotfredsen A, Skakkebaek NE, Hilsted J, Skakkebae NE 1995 Massive weight loss restores 24-hour growth hormone release profiles and serum insulin-like growth factor-I levels in obese subjects. J Clin Endocrinol Metab 80:1407–1415 [DOI] [PubMed] [Google Scholar]
- Cordido F, Peino R, Peñalva A, Alvarez CV, Casanueva FF, Dieguez C 1996 Impaired growth hormone secretion in obese subjects is partially reversed by acipimox-mediated plasma free fatty acid depression. J Clin Endocrinol Metab 81:914–918 [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Ram PA, Park SH, Choi HK 1995 Intermittent plasma growth hormone triggers tyrosine phosphorylation and nuclear translocation of a liver-expressed, Stat 5-related DNA binding protein. Proposed role as an intracellular regulator of male-specific liver gene transcription. J Biol Chem 270:13262–13270 [DOI] [PubMed] [Google Scholar]
- Gebert CA, Park SH, Waxman DJ 1999 Down-regulation of liver JAK2-STAT5b signaling by the female plasma pattern of continuous growth hormone stimulation. Mol Endocrinol 13:213–227 [DOI] [PubMed] [Google Scholar]
- Falutz J, Potvin D, Mamputu JC, Assaad H, Zoltowska M, Michaud SE, Berger D, Somero M, Moyle G, Brown S, Martorell C, Turner R, Grinspoon S 2010 Effects of tesamorelin, a growth hormone-releasing factor, in HIV-infected patients with abdominal fat accumulation: a randomized placebo-controlled trial with a safety extension. J Acquir Immune Defic Syndr 53:311–322 [DOI] [PubMed] [Google Scholar]
- Bramnert M, Segerlantz M, Laurila E, Daugaard JR, Manhem P, Groop L 2003 Growth hormone replacement therapy induces insulin resistance by activating the glucose-fatty acid cycle. J Clin Endocrinol Metab 88:1455–1463 [DOI] [PubMed] [Google Scholar]
- Bredella MA, Torriani M, Thomas BJ, Ghomi RH, Brick DJ, Gerweck AV, Miller KK 2009 Peak growth hormone-releasing hormone-arginine-stimulated growth hormone is inversely associated with intramyocellular and intrahepatic lipid content in premenopausal women with obesity. J Clin Endocrinol Metab 94:3995–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz AL, Yamamoto A, Hemphill L, Miller KK 2008 Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J Clin Endocrinol Metab 93:2507–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller L, Norrelund H, Jessen N, Flyvbjerg A, Pedersen SB, Gaylinn BD, Liu J, Thorner MO, Moller N, Lunde Jorgensen JO 2009 Impact of growth hormone receptor blockade on substrate metabolism during fasting in healthy subjects. J Clin Endocrinol Metab 94:4524–4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makimura H, Stanley T, Mun D, Chen C, Wei J, Connelly JM, Hemphill LC, Grinspoon SK 2009 Reduced growth hormone secretion is associated with increased carotid intima-media thickness in obesity. J Clin Endocrinol Metab 94:5131–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KK, Biller BM, Lipman JG, Bradwin G, Rifai N, Klibanski A 2005 Truncal adiposity, relative growth hormone deficiency, and cardiovascular risk. J Clin Endocrinol Metab 90:768–774 [DOI] [PubMed] [Google Scholar]