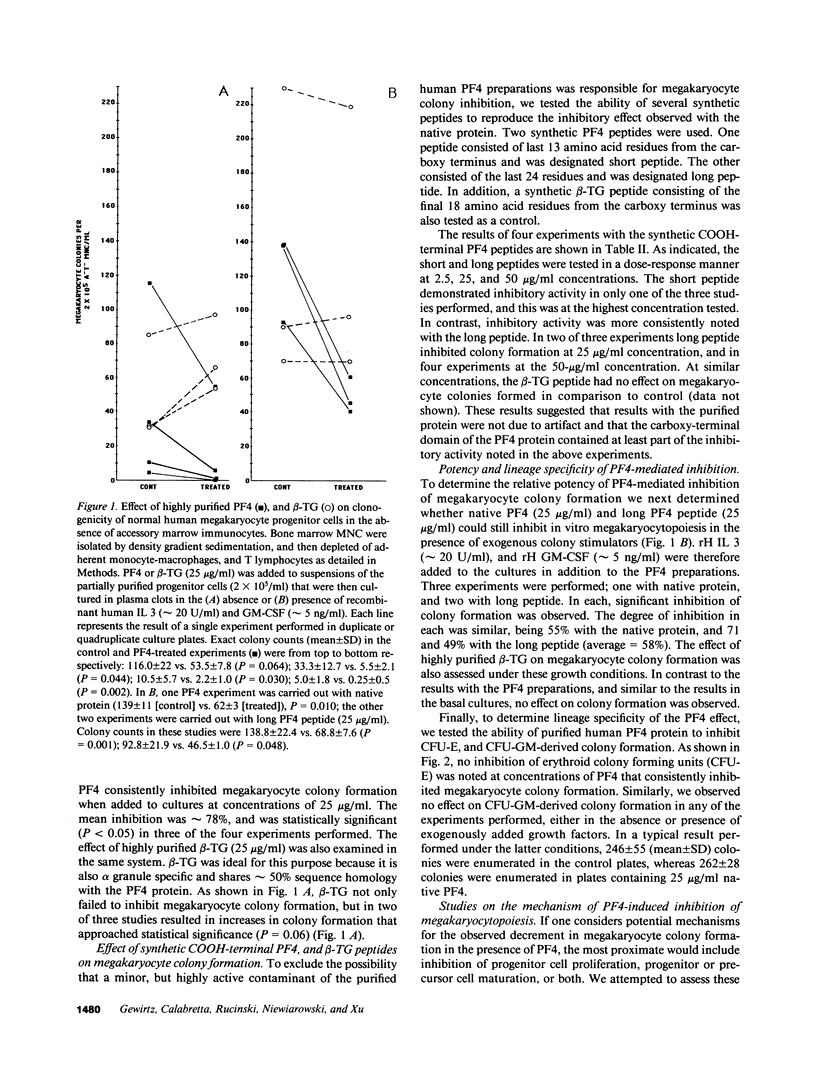
Abstract
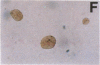
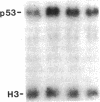
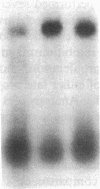
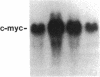
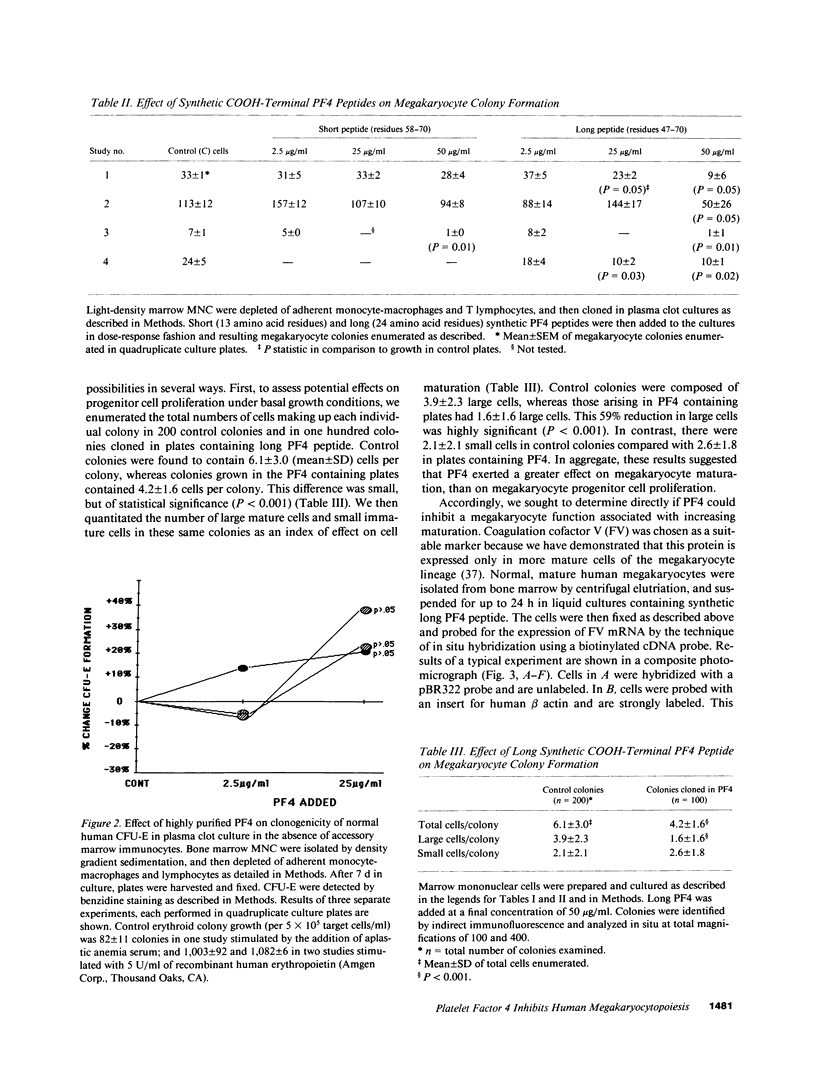
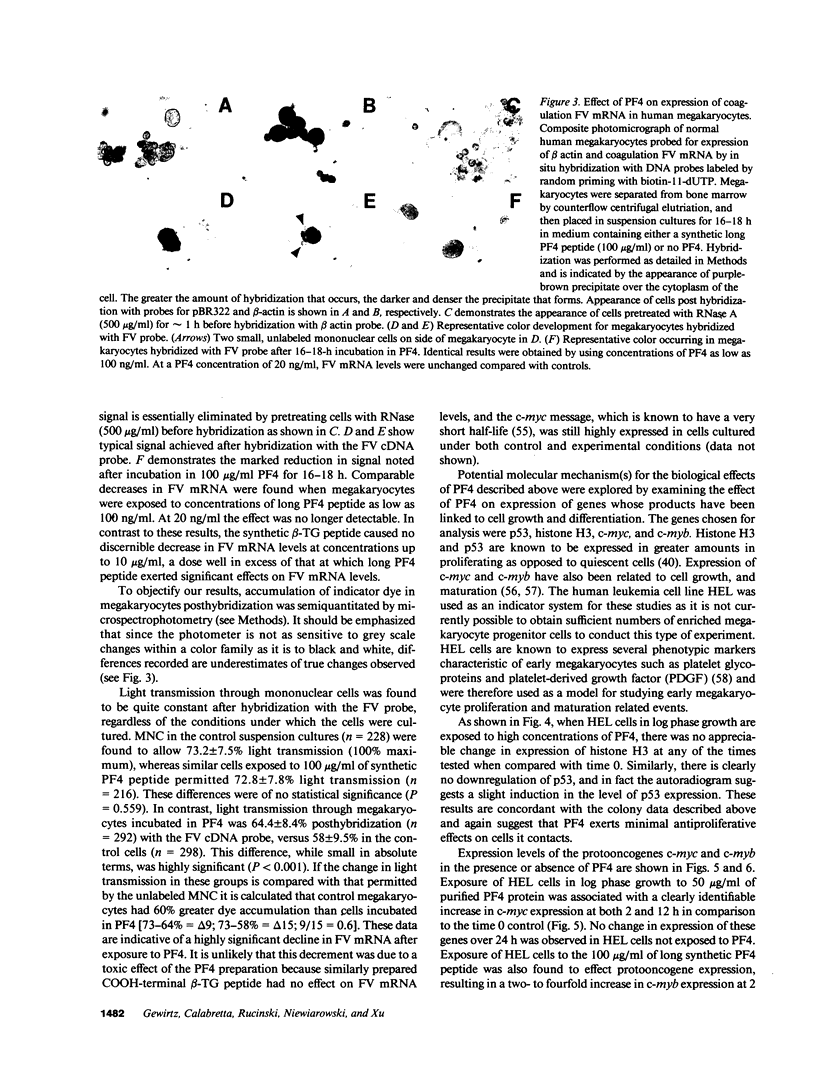
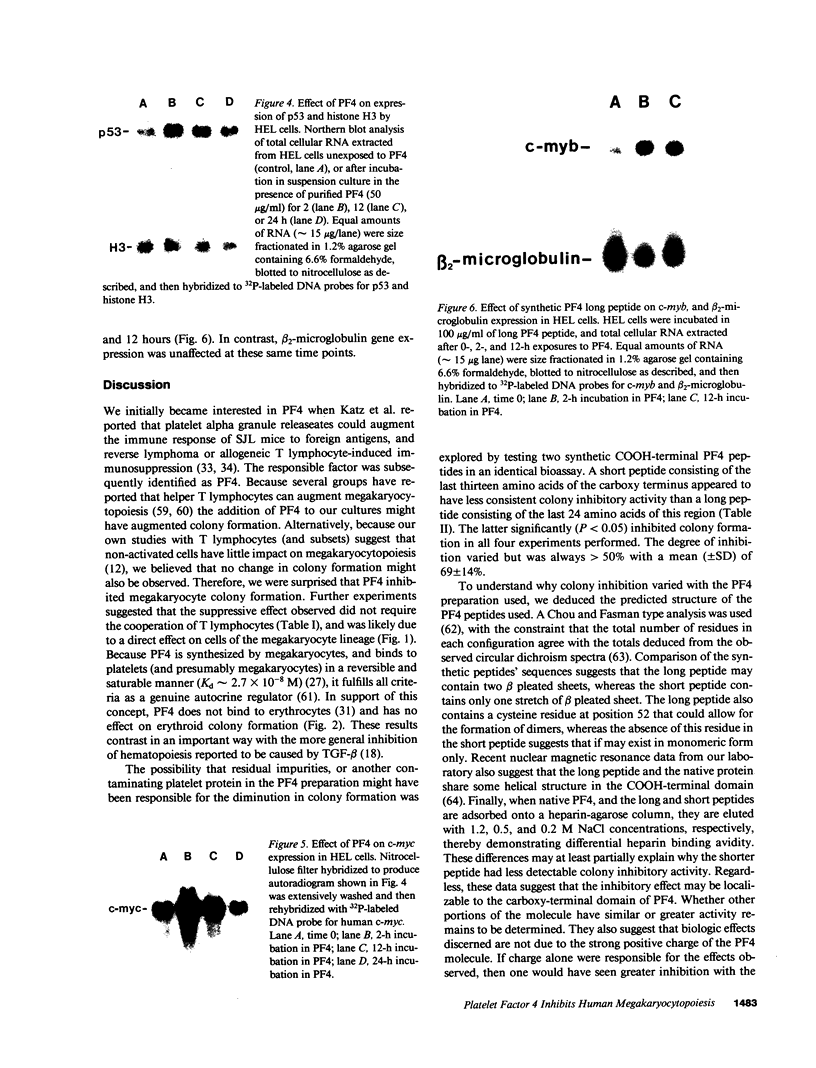
We report that highly purified human platelet factor 4 (PF4) inhibits human megakaryocytopoiesis in vitro. At greater than or equal to 25 micrograms/ml, PF4 inhibited megakaryocyte colony formation approximately 80% in unstimulated cultures, and approximately 58% in cultures containing recombinant human IL 3 and granulocyte-macrophage colony-stimulating factor. Because PF4 (25 micrograms/ml) had no effect on either myeloid or erythroid colony formation lineage specificity of this effect was suggested. A synthetic COOH-terminal PF4 peptide of 24, but not 13 residues, also inhibited megakaryocyte colony formation, whereas a synthetic 18-residue beta-thromboglobulin (beta-TG) peptide and native beta-TG had no such effect when assayed at similar concentrations. The mechanism of PF4-mediated inhibition was investigated. First, we enumerated total cell number, and examined cell maturation in control colonies (n = 200) and colonies (n = 100) that arose in PF4-containing cultures. Total cells per colony did not differ dramatically in the two groups (6.1 +/- 3.0 vs. 4.2 +/- 1.6, respectively), but the numbers of mature large cells per colony was significantly decreased in the presence of PF4 when compared with controls (1.6 +/- 1.5 vs. 3.9 +/- 2.3; P less than 0.001). Second, by using the human leukemia cell line HEL as a model for primitive megakaryocytic cells, we studied the effect of PF4 on cell doubling time, on the expression of both growth-regulated (H3, p53, c-myc,and c-myb), and non-growth-regulated (beta 2-microglobulin) genes. At high concentrations of native PF4 (50 micrograms/ml), no effect on cell doubling time, or H3 or p53 expression was discerned. In contrast, c-myc and c-myb were both upregulated. These results suggested the PF4 inhibited colony formation by impeding cell maturation, as opposed to cell proliferation, perhaps by inducing expression of c-myc and c-myb. The ability of PF4 to inhibit a normal cell maturation function was then tested. Megakaryocytes were incubated in synthetic PF4, or beta-TG peptides for 18 h and effect on Factor V steady-state mRNA levels was determined in 600 individual cells by in situ hybridization. beta-TG peptide had no effect on FV mRNA levels, whereas a approximately 60% decrease in expression of Factor V mRNA was found in megakaryocytes exposed to greater than or equal 100 ng/ml synthetic COOH-terminal PF4 peptide. Accordingly, PF4 modulates megakaryocyte maturation in vitro, and may function as a negative autocrine regulator of human megakaryocytopoiesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber A. J., Käser-Glanzmann R., Jakábová M., Lüscher E. F. Characterization of a chondroitin 4 -sulfate proteoglycan carrier for heparin neutralizing activity (platelet factor 4 ) released from human blood platelets. Biochim Biophys Acta. 1972 Dec 29;286(2):312–329. [PubMed] [Google Scholar]
- Beug H., Blundell P. A., Graf T. Reversibility of differentiation and proliferative capacity in avian myelomonocytic cells transformed by tsE26 leukemia virus. Genes Dev. 1987 May;1(3):277–286. doi: 10.1101/gad.1.3.277. [DOI] [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Calabretta B., Kaczmarek L., Selleri L., Torelli G., Ming P. M., Ming S. C., Mercer W. E. Growth-dependent expression of human Mr 53,000 tumor antigen messenger RNA in normal and neoplastic cells. Cancer Res. 1986 Nov;46(11):5738–5742. [PubMed] [Google Scholar]
- Capitanio A. M., Niewiarowski S., Rucinski B., Tuszynski G. P., Cierniewski C. S., Hershock D., Kornecki E. Interaction of platelet factor 4 with human platelets. Biochim Biophys Acta. 1985 Apr 17;839(2):161–173. doi: 10.1016/0304-4165(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Coppola J. A., Cole M. D. Constitutive c-myc oncogene expression blocks mouse erythroleukaemia cell differentiation but not commitment. Nature. 1986 Apr 24;320(6064):760–763. doi: 10.1038/320760a0. [DOI] [PubMed] [Google Scholar]
- Dani C., Blanchard J. M., Piechaczyk M., El Sabouty S., Marty L., Jeanteur P. Extreme instability of myc mRNA in normal and transformed human cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7046–7050. doi: 10.1073/pnas.81.22.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessypris E. N., Gleaton J. H., Sawyer S. T., Armstrong O. L. Suppression of maturation of megakaryocyte colony forming unit in vitro by a platelet-released glycoprotein. J Cell Physiol. 1987 Mar;130(3):361–368. doi: 10.1002/jcp.1041300308. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Keim P. S., Farmer M., Heinrikson R. L. Amino acid sequence of human platelet factor 4. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2256–2258. doi: 10.1073/pnas.74.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrovsky E., Kuehl W. M., Hollis G. F., Kirsch I. R., Bender T. P., Segal S. Expression of a transfected human c-myc oncogene inhibits differentiation of a mouse erythroleukaemia cell line. Nature. 1986 Aug 21;322(6081):748–750. doi: 10.1038/322748a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Files J. C., Malpass T. W., Yee E. K., Ritchie J. L., Harker L. A. Studies of human plate alpha-granule release in vivo. Blood. 1981 Sep;58(3):607–618. [PubMed] [Google Scholar]
- Geissler D., Lu L., Bruno E., Yang H. H., Broxmeyer H. E., Hoffman R. The influence of T lymphocyte subsets and humoral factors on colony formation by human bone marrow and blood megakaryocyte progenitor cells in vitro. J Immunol. 1986 Oct 15;137(8):2508–2513. [PubMed] [Google Scholar]
- Gewirtz A. M., Bruno E., Elwell J., Hoffman R. In vitro studies of megakaryocytopoiesis in thrombocytotic disorders of man. Blood. 1983 Feb;61(2):384–389. [PubMed] [Google Scholar]
- Gewirtz A. M., Hoffman R. Transitory hypomegakaryocytic thrombocytopenia: aetiological association with ethanol abuse and implications regarding regulation of human megakaryocytopoiesis. Br J Haematol. 1986 Feb;62(2):333–344. doi: 10.1111/j.1365-2141.1986.tb02937.x. [DOI] [PubMed] [Google Scholar]
- Gewirtz A. M. Human megakaryocytopoiesis. Semin Hematol. 1986 Jan;23(1):27–42. [PubMed] [Google Scholar]
- Gewirtz A. M., Keefer M., Doshi K., Annamalai A. E., Chiu H. C., Colman R. W. Biology of human megakaryocyte factor V. Blood. 1986 Jun;67(6):1639–1648. [PubMed] [Google Scholar]
- Gewirtz A. M., Sacchetti M. K., Bien R., Barry W. E. Cell-mediated suppression of megakaryocytopoiesis in acquired amegakaryocytic thrombocytopenic purpura. Blood. 1986 Sep;68(3):619–626. [PubMed] [Google Scholar]
- Gewirtz A. M., Xu W. Y., Mangan K. F. Role of natural killer cells, in comparison with T lymphocytes and monocytes, in the regulation of normal human megakaryocytopoiesis in vitro. J Immunol. 1987 Nov 1;139(9):2915–2924. [PubMed] [Google Scholar]
- Gowda S. D., Koler R. D., Bagby G. C., Jr Regulation of C-myc expression during growth and differentiation of normal and leukemic human myeloid progenitor cells. J Clin Invest. 1986 Jan;77(1):271–278. doi: 10.1172/JCI112287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S. M., Kuter D. J., Rosenberg R. D. In vitro stimulation of megakaryocyte maturation by megakaryocyte stimulatory factor. J Biol Chem. 1987 Mar 5;262(7):3269–3277. [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Desensitisation of cultured glial cells to epidermal growth factor by receptor down-regulation. Nature. 1979 Nov 22;282(5737):419–420. doi: 10.1038/282419a0. [DOI] [PubMed] [Google Scholar]
- Hermodson M., Schmer G., Kurachi K. Isolation, crystallization, and primary amino acid sequence of human platelet factor 4. J Biol Chem. 1977 Sep 25;252(18):6276–6279. [PubMed] [Google Scholar]
- Hill R., Levin J. Partial purification of thrombopoietin using lectin chromatography. Exp Hematol. 1986 Sep;14(8):752–759. [PubMed] [Google Scholar]
- Hiti-Harper J., Wohl H., Harper E. Platelet factor 4: an inhibitor of collagenase. Science. 1978 Mar 3;199(4332):991–992. doi: 10.1126/science.203038. [DOI] [PubMed] [Google Scholar]
- Hoffman R., Yang H. H., Bruno E., Straneva J. E. Purification and partial characterization of a megakaryocyte colony-stimulating factor from human plasma. J Clin Invest. 1985 Apr;75(4):1174–1182. doi: 10.1172/JCI111813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T., Koziol J. A., Burstein S. A. Human recombinant erythropoietin promotes differentiation of murine megakaryocytes in vitro. J Clin Invest. 1987 Jan;79(1):286–289. doi: 10.1172/JCI112796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T., Miller S. L., Burstein S. A. Type beta transforming growth factor is a potent inhibitor of murine megakaryocytopoiesis in vitro. Blood. 1987 Jun;69(6):1737–1741. [PubMed] [Google Scholar]
- Kane W. H., Davie E. W. Cloning of a cDNA coding for human factor V, a blood coagulation factor homologous to factor VIII and ceruloplasmin. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6800–6804. doi: 10.1073/pnas.83.18.6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanz L., Löhr G. W., Fauser A. A. Lymphokine(s) from isolated T lymphocyte subpopulations support multilineage hematopoietic colony and megakaryocytic colony formation. Blood. 1986 Nov;68(5):991–995. [PubMed] [Google Scholar]
- Katz I. R., Hoffmann M. K., Zucker M. B., Bell M. K., Thorbecke G. J. A platelet-derived immunoregulatory serum factor with T cell affinity. J Immunol. 1985 May;134(5):3199–3203. [PubMed] [Google Scholar]
- Katz I. R., Thorbecke G. J., Bell M. K., Yin J. Z., Clarke D., Zucker M. B. Protease-induced immunoregulatory activity of platelet factor 4. Proc Natl Acad Sci U S A. 1986 May;83(10):3491–3495. doi: 10.1073/pnas.83.10.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K., O'Hara P. J., Berkner K., Segal G. M., Hagen F. S., Adamson J. W. Genomic cloning, characterization, and multilineage growth-promoting activity of human granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3101–3105. doi: 10.1073/pnas.83.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating M. T., Williams L. T. Autocrine stimulation of intracellular PDGF receptors in v-sis-transformed cells. Science. 1988 Feb 19;239(4842):914–916. doi: 10.1126/science.2829358. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kimura H., Burstein S. A., Thorning D., Powell J. S., Harker L. A., Fialkow P. J., Adamson J. W. Human megakaryocytic progenitors (CFU-M) assayed in methylcellulose: physical characteristics and requirements for growth. J Cell Physiol. 1984 Jan;118(1):87–96. doi: 10.1002/jcp.1041180115. [DOI] [PubMed] [Google Scholar]
- Lachman H. M., Skoultchi A. I. Expression of c-myc changes during differentiation of mouse erythroleukaemia cells. Nature. 1984 Aug 16;310(5978):592–594. doi: 10.1038/310592a0. [DOI] [PubMed] [Google Scholar]
- Leary A. G., Yang Y. C., Clark S. C., Gasson J. C., Golde D. W., Ogawa M. Recombinant gibbon interleukin 3 supports formation of human multilineage colonies and blast cell colonies in culture: comparison with recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1987 Nov;70(5):1343–1348. [PubMed] [Google Scholar]
- Levine S. P., Wohl H. Human platelet factor 4: Purification and characterization by affinity chromatography. Purification of human platelet factor 4. J Biol Chem. 1976 Jan 25;251(2):324–328. [PubMed] [Google Scholar]
- Lonky S. A., Wohl H. Stimulation of human leukocyte elastase by platelet factor 4. Physiologic, morphologic, and biochemical effects on hamster lungs in vitro. J Clin Invest. 1981 Mar;67(3):817–826. doi: 10.1172/JCI110099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscalzo J., Melnick B., Handin R. I. The interaction of platelet factor four and glycosaminoglycans. Arch Biochem Biophys. 1985 Jul;240(1):446–455. doi: 10.1016/0003-9861(85)90049-9. [DOI] [PubMed] [Google Scholar]
- Majello B., Kenyon L. C., Dalla-Favera R. Human c-myb protooncogene: nucleotide sequence of cDNA and organization of the genomic locus. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9636–9640. doi: 10.1073/pnas.83.24.9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- Mazur E. M., Hoffman R., Chasis J., Marchesi S., Bruno E. Immunofluorescent identification of human megakaryocyte colonies using an antiplatelet glycoprotein antiserum. Blood. 1981 Feb;57(2):277–286. [PubMed] [Google Scholar]
- McDonald T. P., Cottrell M., Clift R., Khouri J. A., Long M. D. Studies on the purification of thrombopoietin from kidney cell culture medium. J Lab Clin Med. 1985 Aug;106(2):162–174. [PubMed] [Google Scholar]
- Messner H. A., Jamal N., Izaguirre C. The growth of large megakaryocyte colonies from human bone marrow. J Cell Physiol Suppl. 1982;1:45–51. doi: 10.1002/jcp.1041130410. [DOI] [PubMed] [Google Scholar]
- Mitjavila M. T., Vinci G., Villeval J. L., Kieffer N., Henri A., Testa U., Breton-Gorius J., Vainchenker W. Human platelet alpha granules contain a nonspecific inhibitor of megakaryocyte colony formation: its relationship to type beta transforming growth factor (TGF-beta). J Cell Physiol. 1988 Jan;134(1):93–100. doi: 10.1002/jcp.1041340111. [DOI] [PubMed] [Google Scholar]
- Nagasawa T., Sakurai T., Kashiwagi H., Abe T. Cell-mediated amegakaryocytic thrombocytopenia associated with systemic lupus erythematosus. Blood. 1986 Feb;67(2):479–483. [PubMed] [Google Scholar]
- Ness S. A., Beug H., Graf T. v-myb dominance over v-myc in doubly transformed chick myelomonocytic cells. Cell. 1987 Oct 9;51(1):41–50. doi: 10.1016/0092-8674(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Osterman D. G., Griffin G. L., Senior R. M., Kaiser E. T., Deuel T. F. The carboxyl-terminal tridecapeptide of platelet factor 4 is a potent chemotactic agent for monocytes. Biochem Biophys Res Commun. 1982 Jul 16;107(1):130–135. doi: 10.1016/0006-291x(82)91679-5. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Raines E., Collins S., Nakamoto B., Tweeddale M., Ross R. Constitutive and inducible secretion of platelet-derived growth factor analogs by human leukemic cell lines coexpressing erythroid and megakaryocytic markers. J Clin Invest. 1987 Mar;79(3):859–866. doi: 10.1172/JCI112895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochownik E. V., Kukowska J. Deregulated expression of c-myc by murine erythroleukaemia cells prevents differentiation. 1986 Aug 28-Sep 3Nature. 322(6082):848–850. doi: 10.1038/322848a0. [DOI] [PubMed] [Google Scholar]
- Rucinski B., Knight L. C., Niewiarowski S. Clearance of human platelet factor 4 by liver and kidney: its alteration by heparin. Am J Physiol. 1986 Oct;251(4 Pt 2):H800–H807. doi: 10.1152/ajpheart.1986.251.4.H800. [DOI] [PubMed] [Google Scholar]
- Rucinski B., Niewiarowski S., James P., Walz D. A., Budzynski A. Z. Antiheparin proteins secreted by human platelets. purification, characterization, and radioimmunoassay. Blood. 1979 Jan;53(1):47–62. [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Huang J. S., Walz D. A., Deuel T. F. Chemotactic activity of platelet alpha granule proteins for fibroblasts. J Cell Biol. 1983 Feb;96(2):382–385. doi: 10.1083/jcb.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra F., Lichtler A., Marashi F., Rickles R., Van Dyke T., Clark S., Wells J., Stein G., Stein J. Organization of human histone genes. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1795–1799. doi: 10.1073/pnas.79.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayrien G., Rosenberg R. D. Purification and properties of a megakaryocyte stimulatory factor present both in the serum-free conditioned medium of human embryonic kidney cells and in thrombocytopenic plasma. J Biol Chem. 1987 Mar 5;262(7):3262–3268. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torelli G., Selleri L., Donelli A., Ferrari S., Emilia G., Venturelli D., Moretti L., Torelli U. Activation of c-myb expression by phytohemagglutinin stimulation in normal human T lymphocytes. Mol Cell Biol. 1985 Oct;5(10):2874–2877. doi: 10.1128/mcb.5.10.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchenker W., Chapman J., Deschamps J. F., Vinci G., Bouguet J., Titeux M., Breton-Gorius J. Normal human serum contains a factor(s) capable of inhibiting megakaryocyte colony formation. Exp Hematol. 1982 Sep;10(8):650–660. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt R., Stanton L. W., Marcu K. B., Gallo R. C., Croce C. M., Rovera G. Nucleotide sequence of cloned cDNA of human c-myc oncogene. Nature. 1983 Jun 23;303(5919):725–728. doi: 10.1038/303725a0. [DOI] [PubMed] [Google Scholar]
- Westin E. H., Gallo R. C., Arya S. K., Eva A., Souza L. M., Baluda M. A., Aaronson S. A., Wong-Staal F. Differential expression of the amv gene in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2194–2198. doi: 10.1073/pnas.79.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakut-Houri R., Bienz-Tadmor B., Givol D., Oren M. Human p53 cellular tumor antigen: cDNA sequence and expression in COS cells. EMBO J. 1985 May;4(5):1251–1255. doi: 10.1002/j.1460-2075.1985.tb03768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]