As the worldwide movement for the improvement of patient safety gains momentum, the subject of drug safety becomes even more prominent. Pharmacovigilance is the science dedicated to reduce the risk of drug-related harms to patients. In India, as elsewhere, the challenges and opportunities are substantial.
The Burden of Drug-related Disease
Drug-related disease causes a considerable burden to healthcare systems around the world. This has been clearly demonstrated in Western countries. A commonly quoted meta analysis performed in the United States indicates that adverse drug reactions (ADRs) were between the 4thand 6thmost common cause of death in 1997.[1] Much less is known about the situation in low-income countries. Published studies on the incidence and cost of drug-related problems in developing countries are few. It is reasonable to assume however that problems associated with drug therapy are more widespread in societies with less rigorous control of drug quality, availability and usage. One published study from Bosappa Memorial Hospital in Mysore[2] confirms that drug-related adverse reactions may be common in an Indian setting. Studies performed in developed countries demonstrate that a majority of drug-related problems are preventable.[3,4] If available instructions on how to use medicines effectively and safely would be adhered to hospitals in India would probably be relieved of hundreds of thousands of patients, and society would save billions of rupees annually, not mentioning the human suffering and the many thousands of fatalities that would be avoided.
The lack of awareness in society about the magnitude of drug-related problems is a mystery. Why is there no public debate on how to reduce the injuries caused by medications like there is for example regarding road traffic accidents. One reason is probably that drug-related injuries are not always obvious, immediate and visible. They often manifest themselves gradually and with symptoms similar to those caused by common diseases, sometimes while patients are at home. The awareness of drug-related problems among professionals, politicians, patients and the general public is probably insufficient to trigger and stimulate a wide discussion on how to reduce them. Is it the case that no player in the healthcare system and supporting industry has an interest in bringing these problems to the fore? Would society in general and healthcare providers in particular lose the confidence of patients if they knew how high the risk of injuries caused by the healthcare system really is?
The Role of Pharmacovigilance
Pharmacovigilance is defined by the WHO as ‘the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other possible drug-related problems’.[5]
The role of pharmacovigilance can be divided into three main areas:
To identify, quantify and document drug-related problems.
To contribute to reduce the risk of drug-related problems in healthcare systems.
To increase knowledge and understanding of factors and mechanisms which are responsible for drug-related injuries.
This wide remit for pharmacovigilance requires interactions with and influence on many stakeholders in society with decision-making powers affecting patient safety. For example:
Politicians at national, regional and local levels
Healthcare administrators
Drug Regulatory Authorities
Pharmaceutical companies
Healthcare professionals (physicians, dentists, pharmacists, nurses, etc.)
Academic institutions
Media representatives
Health insurance companies
Lawyers
Patient groups
The general public including school children
Obviously, this range of stakeholders is in need of information about drug-related risks at different levels in detail and with different content. Pharmacovigilance training can therefore be defined and described in many ways. This text is focusing on the training of graduate professionals with an interest in learning about pharmacovigilance methods, rather than learning about how to use medicines more safely.
The Need for Pharmacovigilance Training
A new national network of pharmacovigilance centres was established in India in 2004. The Central Drugs Standard Control Organization (CDSCO) is coordinating the country-wide pharmacovigilance programmes. It has established a National Pharmacovigilance Advisory Committee to assist with scientific advice on topical drug safety issues. Under the national centre (CDSCO), the Indian pharmacovigilance system is structured like a pyramid with peripheral centres reporting to regional centres which in their turn will report to two zonal centres who are responsible for keeping the main national pharmacovigilance databases. The original structure had some 50 pharmacovigilance centres involved, geographically distributed over the whole of India.
To be effective, the introduction of a new network, with the aim of collecting information from the healthcare system not previously requested by authorities, would need an ambitious training programme to be associated with it. Professionals charged with the task of motivating their clinical colleagues to fill in adverse reaction reports, process reports, analyze them, draw conclusions and provide feedback have to receive at least a minimum of training in the basic principles of pharmacovigilance in order to be successful in their new role. This is particularly true in a situation where pharmacovigilance centres are located in academic institutions not directly interacting with patient care or clinical practice. The need for training is continuous. There will be a certain level of staff turnover in such a large system and the need for specialist training in various sub-functions of pharmacovigilance is constant.
In many disease-oriented Public Health Programmes (PHP), large amounts of medicines or vaccines are distributed in the population. PHPs often operate separately and in isolation from the normal healthcare system that focuses on patient care. It is rare for the programme manuals to contain any aspect of drug safety follow-up when treatment outcomes are being measured. This has to change. In several kinds of PHBs, e.g., malaria, HIV/AIDS and immunization, new medicines and vaccines are distributed to populations which have never been used before. There is an evident need for an upgrading of pharmacovigilance activities in many PHBs and this requires training in methodology and data analysis.
The recent introduction of a legal requirement (schedule Y amendment) for pharmaceutical companies to submit suspected ADRs from clinical trials performed in India to the regulatory authority have created a new demand for pharmacovigilance competence in industry. Major companies are establishing their own units for post-marketing safety surveillance, whereas smaller companies are likely to turn to contract research organizations (CROs) to assist them with pharmacovigilance activities. The major multinational pharmaceutical companies are also carrying out an increasing number of their phase III and phase IV clinical trials in India, either managed by themselves or by CROs. This trend drives another demand for greater understanding about adverse reactions and methods for their study. This is further increased by the requirement in ICH countries for companies to submit risk management plans in connection with submission of application for marketing authorization of new medicines. Pharmaceutical industry is required to have a life-cycle approach to their products and patient safety issues associated with them.
Training in Pharmacovigilance
Pharmacovigilance is a relatively new and small science. It is not a well-established academic specialism. Current curricula in the training programmes of professions such as clinical medicine, clinical pharmacy, clinical pharmacology or medical biology do not cover all the skills needed in pharmacovigilance. Very few universities in the world offer specific courses in pharmacovigilance. One reason might be that pharmacovigilance spans a wide range of subjects, e.g., pharmacology, epidemiology, clinical medicine, data management, drug legislation and communication, and they do not easily fit within the competence area of any of the existing academic departments. It is not to be expected that pharmacovigilance will be taught as an academic subject at any Indian university in the near future.
The International Society of Pharmacovigilance (ISoP) is active in providing specialized ad hoc training courses in pharmacovigilance. Also, the Indian chapter, the Society of Pharmacovigilance India (SoPI), could become a provider of regular pharmacovigilance training. SoPI is still a very small organization, however, and would need a stronger base to provide a credible and effective training service for the country as a whole.
With a growing demand for pharmacovigilance training in India, it is likely that various private initiatives will be taken to meet this demand and profit from it. Although there are knowledgeable and experienced pharmacovigilance experts in India, they are still very few. Even if the existing experts spent all their time on pharmacovigilance teaching, they would still not be able to satisfy the need. Some providers of pharmacovigilance training might be forced to bring in pharmacovigilance experts from abroad or rely on a faculty of less-qualified and less-experienced tutors. Bringing foreign experts to India contributes seriously to high tuition fees, and the foreign experts might not be very familiar with the context in which patient safety monitoring has to operate in India. It will be tempting for providers of pharmacovigilance training courses to include persons with limited pharmacovigilance expertise and experience in their faculty. For professionals wanting pharmacovigilance training, it may become very important to critically assess the quality of the training being offered by various course providers.
Pharmacovigilance Training - The Content
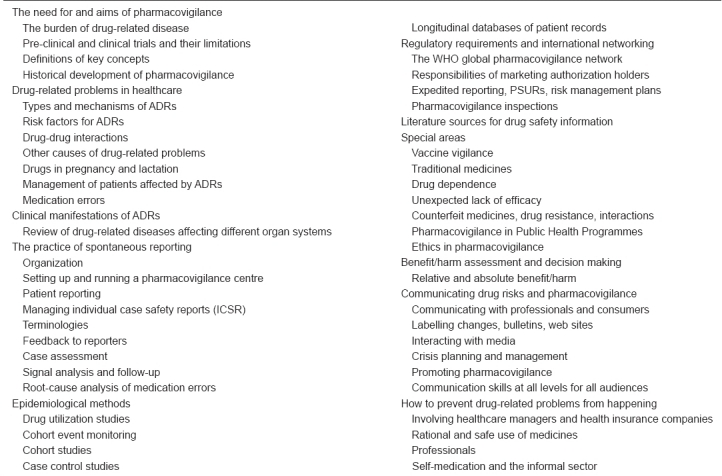
All training has to be adapted to the needs of the trainees. Since there are many different stakeholders in society with a need for and an interest in pharmacovigilance, training has to be offered at different levels, with different content and given for different length of time. In basic curricula for health professionals, a few hours of drug safety teaching is all that can be hoped for. Health professionals in active service, caring for patients in healthcare, might need a couple of days of training on ADR mechanisms, reporting requirements and patient management. Pharmacovigilance professionals involved in data collection, processing and analysis should have at least two weeks of basic pharmacovigilance training. Apart from this, specific in-depth training in special sub-areas has to be provided. Table 1 provides a framework of topics that should be covered in a comprehensive pharmacovigilance training programme. The borderlines of pharmacovigilance are not well defined and there are aspects of a patient's well-being during drug treatment that can extend beyond this framework. Any pharmacovigilance programme being offered is likely to focus on a sub-set of these topics. It is very rare for any one individual to acquire full proficiency in all the aspects of pharmacovigilance and patient safety monitoring. Because of this fact, it is essential that experts in the various sub-specialist areas work together for the protection of patient safety.
Table 1.
Outline of a general pharmacovigilance training programme
Conclusion
Pharmacovigilance is a demanding science offering great opportunities for reducing harm to patients and costs to healthcare systems. From small beginnings, with the right knowledge and skills, pharmacovigilance can make an important contribution to the health of the nation.
References
- 1.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 2.Ramesh M, Pandit J, Parthasarathi G. Pharmacoepidemiol. Drug Saf. 2003;12:687–92. doi: 10.1002/pds.871. [DOI] [PubMed] [Google Scholar]
- 3.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–9. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winterstein AG, Sauer BC, Hepler CD, Poole C. Preventable drug-related hospital admissions. Ann Pharmacother. 2002;36 (7-8):1238–48. doi: 10.1345/aph.1A225. [DOI] [PubMed] [Google Scholar]
- 5.The importance of pharmacovigilance. Geneva: World Health Organization; 2002. Anonymous. [Google Scholar]


